Synthesis of metal nanoparticle@graphene hydrogel composites by substrate-enhanced electroless deposition and their application in electrochemical sensors†
Abstract
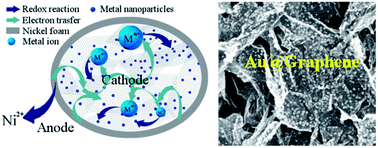
In this paper, a green and facile method based on substrate-enhanced electroless deposition is designed for the fabrication of three-dimensional (3D) metal nanoparticle@graphene hydrogel (MNP@GHG) composites. A galvanic cell was constructed by inducing nickel foam as the substrate of GHG, to enhance the deposition of MNPs via galvanic cell reaction. Various MNPs with redox potential higher than that of Ni, including Au, Pt, Pd and Cu, were successfully deposited onto GHG. The produced gold nanoparticles/GHG composite showed good electrocatalytic activity and was used to fabricate an amperometric sensor towards uric acid with good sensitivity.

 Please wait while we load your content...
Please wait while we load your content...