Solution-processed Cu2ZnSnS4 absorbers prepared by appropriate inclusion and removal of thiourea for thin film solar cells†
Si-Nae Park‡
a,
Shi-Joon Sung‡a,
Dae-Ho Sona,
Dae-Hwan Kima,
Mungunshagai Gansukhb,
Hyeonsik Cheongb and
Jin-Kyu Kang*a
aAdvanced Convergence Research Center, Daegu Gyeongbuk Institute of Science & Technology, 223 Sang-Ri, Hyeonpung-myeon, Dalseong-gun, Daegu 711-873, Republic of Korea. E-mail: apollon@dgist.ac.kr; Fax: +82 53 785 3739; Tel: +82 53 785 3700
bDepartment of Physics, Sogang University, 35 Baekbeom-ro, Mapo-gu, Seoul 121-742, Republic of Korea
First published on 16th January 2014
Abstract
We studied how to effectively add/remove organic chemicals to/from CZTS precursor thin films to prepare uniform CZTS thin films that show optimal properties. We used multi-functional thiourea, as both a stabiliser and a source of sulphur, to prepare the precursor solutions. This is because it forms complexes with metal chlorides, which stabilise the CZTS precursor solutions and enable CZTS thin films to be spin-coated onto substrates thereby enabling fabrication of CZTS absorbers. However, the excess thiourea, required to stabilise the CZTS precursor solutions, induced the formation of a ZnS secondary phase in the CZTS thin films, which deteriorated the photovoltaic properties of the CZTS solar cells. We therefore pre-annealed the thin films to inhibit ZnS formation. We used the thiourea-stabilized CZTS precursor solutions and simple solution processing techniques to prepare CZTS precursor thin films, and optimized the pre-annealing temperature to fabricate CZTS solar cells that showed 5.29% efficiency.
Introduction
Cu2ZnSnS4 (CZTS) has recently attracted a great amount of interest as a potential material for fabricating a low-cost, low-environmental-impact absorber layers for solar cells. Absorber layers require a direct bandgap energy of ∼1.5 eV and an optical absorption coefficient of around 105 cm−1 so that they can be used for photovoltaic applications. The elements that comprise CZTS are abundant and CZTS is nontoxic; thus, it is the ideal candidate to replace CuInxGa1−xSe2 (CIGS) and CdTe in solar cells because the elements that comprise CIGS and CdTe are scarce and because these materials themselves are toxic.1–4 The high cost of indium, gallium, and tellurium limits their potential use for the large-scale fabrication of photovoltaic devices. A number of methods for fabricating CZTS thin films have been developed, including vacuum-based methods such as co-evaporation and radio frequency (RF) sputtering.5–7 The typical vacuum-based process of producing CZTS absorbers onto an appropriate substrate is very promising for fabricating high-efficiency CZTS solar cells. However, vacuum-based deposition methods generally show relatively slow throughput, low material use, and considerable energy consumption.9 Therefore, solution-based deposition is becoming an attractive alternative to vacuum-based deposition because it is simple and is suitable for depositing thin films onto large areas, which is an important criterion for achieving low-cost, high-throughput manufacturing. Various solution-based deposition methods such as spin-coating,10 spray pyrolysis,11 electrochemical deposition,12 and paste coating13 are used to fabricate various photovoltaic devices.CZTSSe thin film solar cells that show 11.1% power conversion efficiency have recently been produced by spin-coating a hydrazine solution onto an appropriate substrate.14 However, the complete procedure needs to be performed in a glove box because hydrazine is highly toxic, very unstable, and flammable. Thus, solution-based deposition methods that involve the use of non-toxic organic solvents such as alcohols or dimethyl sulfoxide (DMSO) are actively being researched.15,16 In many of these organic-solution-based methods, metal acetate, chloride and nitrates are dissolved in an organic solvent, to which organic binders are subsequently added in order to secure suitable rheological properties.9 Securing the rheological properties of CZTS precursor solutions is crucial to fabricating high-quality CZTS solar cells from solution-processed CZTS absorbers. However, these organic binders remain even in sulfurized CZTS thin films. In addition, acetate and nitrate chemicals inevitably contain carbon, nitrogen, and oxygen, which are also difficult to completely remove from CZTS thin films and which could degrade the quality of the films. Therefore, CZTS thin films fabricated by the organic-solution-based methods would inevitably contain residual amounts of carbon and oxygen, which might degrade the photovoltaic properties of the films. Especially also CZTS thin films would most probably contain residual carbon because organic solvents and organic binders are carbon-rich. The carbon residue acts as a high resistor, increasing the series resistance of the photovoltaic cell.17 Further, large amounts of residual carbon form a separate carbon interlayer between crystallized CZTS and Mo layers, the presence of which causes the CZTS thin film to adhere poorly at the Mo interface.8,13 Therefore, most CZTS thin films prepared using methods of organic solution processing cannot be used to produce high-efficiency solar cells.
We developed a non-toxic solvent-based method of fabricating CZTS thin films without producing a carbon interlayer. Metal chlorides, such as copper, zinc, and tin chlorides, were used as the precursors for CZTS in order to minimise the amount of residual carbon in the CZTS thin films. Thiourea, which is a versatile metal-ion complexing agent, was used both as a source of sulphur and to stabilise the CZTS precursor solutions, and no other organic binder was added to the CZTS precursor solutions, which were prepared by dissolving each of the metal chlorides and thiourea in an ethanol-based solvent. The CZTS precursor thin films were subsequently fabricated by spin-coating the precursor solutions onto molybdenum-coated soda-lime glass substrates. Using metal chlorides and thiourea as components for the precursor solutions enabled us to spin-coat the CZTS thin films without forming a carbon interlayer. Thiourea stabilises aqueous metal precursor solutions because it can co-ordinate with various metal ions to form metal–thiourea complexes, which enables precipitate-free CZTS precursor solutions to be easily prepared.18 Further, it is unnecessary to use an additional source of sulphur in the precursor solutions because thiourea is a good source of sulphur. However, the amount of thiourea required in order to prepare the CZTS precursor solutions was above the stoichiometric ratio, and the excess sulphur in the sulphur-overdosed CZTS precursor thin films induced the formation of a ZnS secondary phase in them. The CZTS precursor thin films were pre-annealed at various temperatures to prevent the ZnS secondary phase from forming and were subsequently sulphurised. The amount of excess sulphur in the precursor thin films was diminished by controlling the pre-annealing temperature, effectively inhibiting the formation of the ZnS secondary phase in the CZTS thin films. Moreover, the pre-annealing temperature was also an important factor in controlling the morphology, chemical composition, and photovoltaic properties of the CZTS thin films, which might be attributed to the different thermal degradation temperatures of the thiourea and metal chlorides in the CZTS precursor solutions. We fabricated solution-processed Cu-poor, Zn-rich CZTS absorbers by optimally pre-annealing the CZTS precursor thin films and used the absorbers to fabricate CZTS solar cells that showed 5.29% efficiency.
Experimental
The CZTS precursor thin films were spin-coated onto Mo-coated soda-lime glass substrates. Copper(II) chloride dihydrate (CuCl2·H2O) (99%, Aldrich, 0.9 M), zinc chloride (ZnCl2) (97%, Aldrich, 0.7 M), tin(II) chloride (SnCl2), (98%, Aldrich, 0.5 M), and thiourea (99%, Aldrich, 4 M) were used as the source chemicals for the precursor solutions. They were completely dissolved in a mixture of deionised water and ethanol to produce the precursor solutions (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.78
0.78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.56
0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.44), which were subsequently spin-coated onto molybdenum-coated soda-lime glass substrates at 5000 rpm for 30 s. The spin-coated CZTS precursor thin films were then baked on a hotplate in atmospheric condition at 250, 300, 350, or 400 °C for 5 min to pre-anneal them. The CZTS precursor thin films were spin-coated and pre-annealed twice more to produce suitably thick films, which were then sulphurised at atmospheric pressure in a two-zone tubular quartz furnace. The sulphur evaporated at 300 °C, and the sample zone was heated to 550 °C for 30 min. Ar carrier gas with 200 sccm at atmospheric pressure was used in the quartz furnace to facilitate the flow of sulphur vapour, and both heating zones were naturally cooled to room temperature. Chemical bath deposition was used to cover the obtained CZTS thin films with a 40 nm-thick CdS buffer layer, and RF sputtering was used to sequentially deposit a 50 nm-thick intrinsic ZnO layer and a 300 nm-thick Al-doped ZnO layer on top of the buffer layer in order to fabricate solar cells. Thermal evaporation was then used to deposit a 500 nm-thick Al collection grid on top of the device.
4.44), which were subsequently spin-coated onto molybdenum-coated soda-lime glass substrates at 5000 rpm for 30 s. The spin-coated CZTS precursor thin films were then baked on a hotplate in atmospheric condition at 250, 300, 350, or 400 °C for 5 min to pre-anneal them. The CZTS precursor thin films were spin-coated and pre-annealed twice more to produce suitably thick films, which were then sulphurised at atmospheric pressure in a two-zone tubular quartz furnace. The sulphur evaporated at 300 °C, and the sample zone was heated to 550 °C for 30 min. Ar carrier gas with 200 sccm at atmospheric pressure was used in the quartz furnace to facilitate the flow of sulphur vapour, and both heating zones were naturally cooled to room temperature. Chemical bath deposition was used to cover the obtained CZTS thin films with a 40 nm-thick CdS buffer layer, and RF sputtering was used to sequentially deposit a 50 nm-thick intrinsic ZnO layer and a 300 nm-thick Al-doped ZnO layer on top of the buffer layer in order to fabricate solar cells. Thermal evaporation was then used to deposit a 500 nm-thick Al collection grid on top of the device.
We analysed the CZTS thin films with various instruments to determine how the pre-annealing temperature affected the chemical composition and morphology of the films and the photovoltaic properties of the CZTS solar cells. The surface morphology and chemical composition of the CZTS thin films were observed using scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) (Hitachi, SU 8020). The crystalline structure of the CZTS thin films was observed using X-ray diffraction (XRD) (Bruker) and Raman spectroscopy. The photovoltaic properties of the solar cells were characterised using a Keithley 2400 source meter unit and a solar simulator (Newport 69907) to simulate 1.5 AM solar irradiation.
Results and discussion
SEM was used to examine the surface morphologies of the pre-annealed and sulphurised crystalline CZTS thin films in order to determine how the pre-annealing temperature affected the surface morphology of the thin films (Fig. 1). Fig. 1(a) shows SEM images of the surface morphologies of the CZTS thin films pre-annealed in the range 250–400 °C. The precursor thin films pre-annealed at higher temperatures showed different surface morphologies than those pre-annealed at lower ones. Unlike conventional precursor thin films used to fabricate inorganic thin films, none of the pre-annealed CZTS precursor thin films showed a smooth, flat surface morphology. Further, the CZTS precursor thin films pre-annealed at 350 and 400 °C contained many nano-pores, which might be related with removing the thiourea and metal chlorides during pre-annealing. Heat activates the loss of thiourea and metal chlorides, and the nano-cavities formed when the materials are removed could transform into more nano-pores in the precursor thin films pre-annealed above 300 °C. The pre-annealing-induced change in the surface morphology of the CZTS precursor thin films might be connected to the final sulphurised crystalline CZTS thin films.Fig. 1(b) shows SEM images of the surface morphologies of the crystalline sulphurised CZTS thin films. All of the sulphurised CZTS thin films showed clusters of CZTS nano-grains, which is different from the surface morphologies of CZTS thin films prepared using vacuum-based deposition. The CZTS grains became increasingly clear with increasing pre-annealing temperature, which might be attributed to the different amounts of carbon residue in the CZTS precursor thin films. The CZTS nano-grains showed well-connected, densely packed structures despite the higher porosity of the CZTS thin films. In general, the lower efficiency of thin film solar cells is due to the recombination of electrons and holes at grain boundaries. However, in the case of CZTS, it has previously been reported that the grain boundaries in CZTS thin films are the key to achieving highly efficient solar cells.19 The grain boundaries in the CZTS thin films provide a current pathway for carriers, which increases the collection of minority carriers and thereby induces higher currents and efficiencies in CZTS solar cells.
The CZTS thin films uniformly covered the substrates, and the films were around 1.5 μm thick, as shown in the cross-sectional SEM images (Fig. 1(c)). None of the films showed a carbon interlayer between the molybdenum and absorber layers, which increased the series resistance of the device and thus limited the efficiency of the solar cell.17,20 In order to clarify the existence of a carbon interlayer in CZTS thin films, we measured Auger Electron Spectroscopy (AES) of a CZTS thin film with 350 °C pre-annealing (Fig. S1†). AES showed that the distribution of carbon in a CZTS thin film was uniform and there was no evidence of a carbon interlayer. The absence of a carbon interlayer might be attributed to using thiourea and metal chlorides, which minimised the amount of carbon in the precursor solutions, and to pre-annealing, which effectively removed the carbon residues from the thin films. These results suggest that using solution processing with precursor solutions prepared from thiourea and metal chlorides might be an effective method of preparing high-quality CZTS thin films that do not have an additional carbon interlayer.
The chemical composition of CZTS thin films is a very important factor in determining the efficiency of kesterite-structured thin film solar cells.21 The low thermal stability of the metal chlorides used in our precursor solutions enables the metal chlorides to be eliminated from the CZTS precursor thin films during pre-annealing; therefore, pre-annealing the films at high temperature could considerably affect the chemical composition of the sulphurised CZTS thin films. EDS was used to analyse the sulphurised CZTS thin films pre-annealed at various temperatures in order to determine how the pre-annealing temperature affected the chemical composition of the films. Fig. 2(a) shows the composition of the various elements in the sulphurised CZTS thin films plotted as functions of pre-annealing temperature. The relative Cu/(Zn + Sn), Zn/Sn, and S/(Cu + Zn + Sn) ratios showed different trends with increasing pre-annealing temperature, which might be attributed to the different thermal behaviours of the chemicals in precursor thin films. The Cu/(Zn + Sn) ratio slightly increased while the Zn/Sn ratio significantly increased with increasing pre-annealing temperature. Unlike the Cu/(Zn + Sn) and Zn/Sn ratios, the S/(Cu + Zn + Sn) ratio decreased with increasing pre-annealing temperature.
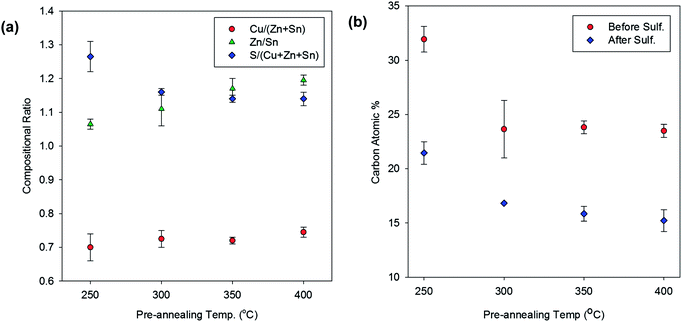 | ||
| Fig. 2 (a) Compositional ratio of elements in sulphurized CZTS thin films and (b) carbon atomic percent of precursor and sulphurized CZTS as a function of pre-annealing temperature. | ||
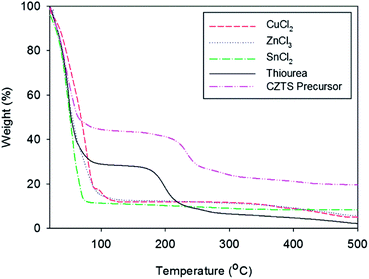
Coated precursor thin films are usually pre-annealed at low temperature to eliminate the solvents used in conventional methods of solution-processing. However, the CZTS precursor thin films were pre-annealed at relatively high temperatures in our work; thus, some of the thiourea and metal chlorides could be also eliminated from the precursor films during pre-annealing. The SEM images of the surface morphologies of the CZTS precursor thin films pre-annealed at various temperatures (Fig. 1) also show that the high-temperature-pre-annealing-induced loss of thiourea and metal chlorides from the CZTS precursor thin films is closely related with the nano-porous morphology of the films. Thermogravimetric analysis (TGA) was performed on the metal chlorides to quantify the amount of organic chemicals and metal chlorides lost in the precursor solutions during pre-annealing (Fig. 3). The results of the TGA analysis show that most of the thiourea weight was lost from the precursor thin film pre-annealed below 300 °C, which is consistent with the obvious decrease in the S/(Cu + Zn + Sn) ratio for the precursor thin film pre-annealed at 300 °C. Slightly more thiourea weight was gradually lost with increasing temperature from the precursor thin films pre-annealed above 300 °C. Further, most of the SnCl2 weight was lost below 330 °C, while the CuCl2 and ZnCl2 weight loss started at 300 °C and continued until 500 °C. This result implies that although pre-annealing readily affects the composition of Sn in the precursor thin films, it has little effects on the compositions of Cu and Zn in the precursor thin films. Accordingly, the different tendencies of the relative Cu/(Zn + Sn) and Zn/Sn ratios with increasing pre-annealing temperature is closely related with the different thermal degradation behaviours of thiourea and metal chlorides. Cu-poor, Zn-rich CZTS thin films generally show higher p-type conductivity and conversion efficiency than Cu-rich, Zn-poor ones because more Cu vacancies form in Cu-poor thin films, which produces shallow acceptors in the CZTS layer, while the substitution of Cu at Zn sites is suppressed in Zn-rich thin films, which produces relatively deep acceptors.21,22 The pre-annealing temperature considerably affected the relative ratio of Zn/Sn in our solution-processed CZTS thin films; thus, Zn-rich CZTS thin films might be easily fabricated by controlling the pre-annealing temperature of the CZTS precursor films.
Minimising the amount of carbon residue in solution-processed CZTS thin films is a critical factor in determining the quality of the films because carbon residues in CZTS thin films disturb the growth of CZTS crystals and act as a capacitor, increasing the amount of electrical resistance in CZTS solar cells. The cross-sectional SEM images of the CZTS thin films (Fig. 1(c)) show that no carbon layer had formed between the Mo and CZTS layers in any of the thin films, which is common for solution-processed CZTS thin films. No additional organic binders (such as monoethanolamine (MEA), ethyl cellulose, or other viscous organic chemicals), which are difficult to completely eliminate from CZTS thin films annealed at high temperatures, were used to prepare our CZTS precursor solutions. Instead, thiourea played an important role as a binder in our precursor solutions, and the precursor solutions were spin-coated onto substrates to prepare the CZTS thin films without any problems. However, although no additional carbon layer had formed between the Mo and CZTS layers, the CZTS thin films still contained some residual carbon. Therefore, we used EDS to measure the atomic ratio of carbon in the pre-annealed and sulphurised CZTS thin films in order to determine the amount of carbon residue in the CZTS absorbers (Fig. 2(b)). All of the sulphurised CZTS thin films showed considerably less carbon content, about 10 at%, than the pre-annealed ones because the films were annealed at high temperature during sulphurisation. Further, the amount of residual carbon in the CZTS thin films was closely related with the pre-annealing temperature. The amount of residual carbon in the sulphurised CZTS thin films significantly decreased in the film pre-annealed at 300 °C and remained constant in the films pre-annealed up to 400 °C. The results of the TGA analysis show that most of the thiourea was eliminated from the CZTS thin film pre-annealed below 300 °C; thus, the larger amount of carbon residue lost from the CZTS thin films pre-annealed at 300 °C might be attributed to the thermal loss of thiourea from the CZTS precursor thin films. However, the relatively large amount of carbon residue in the CZTS thin films pre-annealed at 250 °C could negatively affect the quality of CZTS absorbers and the electrical properties of CZTS solar cells.
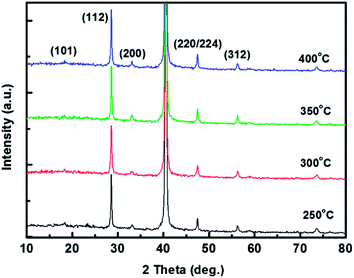
The crystallinity of the kesterite phases in CZTS absorbers is an important factor in determining the electrical properties of solar cells fabricated using CZTS thin films. X-ray diffraction (XRD) was thus used to measure the crystallinity of the phases in the sulphurised CZTS thin films. Fig. 4 shows the XRD patterns for the CZTS films pre-annealed at various temperatures. The XRD patterns for the sulphurised CZTS thin films showed peaks at 18.3, 28.5, 33.0, 47.4, and 56.3°, corresponding to the single-phase kesterite CZTS structure (Joint Committee on Powder Diffraction Standards, JCPDS 26-0575). The (112) peak is the most intense, indicating that the crystalline structure of the CZTS was oriented in the (112) direction. These peaks clearly indicate that the CZTS showed a kesterite structure, which is consistent with previously reported data in the literature.23,24 All the sulphurised CZTS thin films grew well and showed good crystallinity.
The XRD patterns suggest that our method of solution processing resulted in the formation of a kesterite CZTS absorber. Further, although the CZTS absorber could also contain secondary phases such as ZnS, Cu2S, and SnS because the region of single-phase kesterite CZTS is quite narrow, it is impossible to distinguish the secondary phases from the CZTS phases on the basis of XRD patterns, which have limited sensitivity to secondary phases.25 Therefore, we used Raman spectroscopy to distinguish between the CZTS phases of the kesterite structure and the secondary phases of the CZTS absorbers. It is essential to use resonant conditions for Raman spectroscopy in order to accurately detect the secondary phases of CZTS absorbers.26 We used two light sources: a 514.5 nm-wavelength Ar+ laser and a 325 nm-wavelength He–Cd laser to measure the Raman spectra. Fig. 5(a) shows the Raman spectra measured using the 514.5 nm laser for the CZTS thin films pre-annealed at various temperatures. The spectra for all of the CZTS thin films show two main Raman peaks at 287.6 and 337.4 cm−1, corresponding to the main A1 mode characteristic for the CZTS phase, which is consistent with the corresponding XRD pattern. These results suggest that our method of solution processing resulted in the formation of a kesterite-structured CZTS phase. Fig. 5(b) shows the Raman spectra measured using the 325 nm laser for the CZTS thin films pre-annealed at various temperatures. The spectrum for the CZTS thin film pre-annealed at 250 °C showed distinctive Raman peaks at 343.6, 694.2, and 1041.8 cm−1, which originate from the first-, second-, and third-order peaks characteristic of the ZnS phase.26 The spectrum also shows shoulder peaks at 287.6 and 337.4 cm−1, which are associated with the CZTS phase. Although the intensity of the peaks associated with ZnS rapidly diminished with increasing pre-annealing temperature, the intensity of the shoulder peaks associated with the CZTS phase did not change.
The formation of the ZnS secondary phase in the CZTS thin films might be closely related with the amount of residual thiourea in the CZTS precursor thin films. The relative compositional ratio of thiourea to metal chlorides in our precursor solutions was about 1.9, which is higher than the S/(Cu + Zn + Sn) ratio for the CZTS thin films. Overdosing the CZTS precursor solutions with thiourea is inevitable in order to prepare precipitate-free solutions. However, excess thiourea could react with ZnCl2, unnecessarily forming the secondary ZnS phase. Accordingly, it is essential to effectively eliminate excess thiourea from the CZTS thin films, and the pre-annealing temperature is a key factor in minimising the amount of thiourea in the CZTS precursor thin films. The Raman spectrum for the CZTS precursor thin film pre-annealed at 250 °C shows intense peaks associated with the ZnS secondary phase because 250 °C was not sufficiently hot to remove thiourea from the precursor thin films. The Raman spectra for the CZTS precursor thin films pre-annealed above 300 °C, on the other hand, shows weak peaks associated with the ZnS secondary phase, which might be because pre-annealing the films above 300 °C removed a sufficient amount of thiourea from them. These results suggest that the pre-annealing temperature is closely related with inhibiting the ZnS secondary phase from forming in CZTS thin films and that high-temperature pre-annealing is indispensable for preparing single-phase kesterite-structured CZTS. In order to verify the presence of carbon residue in CZTS thin films, we also investigated amorphous carbon peaks in wider range (200–2100 nm), but there was no peak of amorphous carbon in Raman spectrum (Fig. S2†).
The chemical composition and crystalline structure of the CZTS thin films, which are closely related with the electrical properties of CZTS solar cells, can be controlled by controlling the temperature at which the CZTS precursor thin films are pre-annealed. To elucidate in detail how the pre-annealing temperature affects the electrical properties of CZTS solar cells, we used the CZTS thin films as absorbers to fabricate solar cells and measured the current density–voltage (J–V) curves for cells exposed to simulated AM 1.5 (1000 W m−2) solar light. Table 1 shows the photovoltaic properties of the CZTS solar cells fabricated using the absorbers prepared from the CZTS thin films pre-annealed at various temperatures. The CZTS solar cell fabricated using the absorbers prepared from the CZTS thin films pre-annealed at 250 °C showed poor photovoltaic properties, while those fabricated using the absorbers prepared from the CZTS thin films pre-annealed above 300 °C all showed similar device characteristics such as FF, Rs, and Rsh. The poor performance of the CZTS solar cell fabricated using the absorbers prepared from the CZTS thin films pre-annealed at 250 °C might be attributed to the carbon residues due to the insufficient removal of thiourea from precursor thin films and undesirable formation of ZnS secondary phase in the CZTS absorber. However, the temperature at which the CZTS precursor thin films were pre-annealed considerably affected the Voc and Jsc of the solar cells fabricated using the absorbers prepared from the CZTS thin films pre-annealed above 300 °C; that is, the temperature at which these precursor films were pre-annealed was directly correlated with the efficiency of the CZTS solar cells. Voc and Jsc both showed maximum values for the precursor film pre-annealed at 350 °C, which might be closely related with the chemical composition of the CZTS absorbers. The results of the EDS analysis of the CZTS thin films pre-annealed at various temperatures show that the Cu/(Zn + Sn) and Zn/Sn ratios gradually increase with increasing pre-annealing temperature. The CZTS thin film pre-annealed at 350 °C showed Cu/(Zn + Sn) = 0.72 and Zn/Sn = 1.17, which might be the optimal chemical composition for fabricating high-efficiency CZTS solar cells. Fig. 6 shows the J–V curves measured under simulated AM 1.5 (1000 W m−2) solar illumination for the CZTS solar cell fabricated using the absorbers prepared from the CZTS thin films pre-annealed at 350 °C. The CZTS solar cell exhibited an open-circuit voltage of 558 mV, a short-circuit current of 18.53 mA cm−2, a fill factor of 51.19%, and a conversion efficiency of 5.29%. In order to investigate the light response of CZTS devices, we measured Incident Photo-to-Current Efficiency (IPCE) of CZTS thin film solar cell pre-annealed at 350 °C (Fig. S3†). This solar cell showed maximum 78.8% external quantum efficiency (EQE) for a photon wavelength at 600 nm.
| Pre-annealing temp. (°C) | Voc (mV) | Jsc (mA cm−2) | FF (%) | Eff. (%) | RS (Ω) | Rsh (Ω) |
|---|---|---|---|---|---|---|
| 250 | 245 | 4.24 | 33.14 | 0.34 | 94.82 | 521.2 |
| 300 | 500 | 14.59 | 51.50 | 3.83 | 25.50 | 1424.6 |
| 350 | 558 | 18.53 | 51.19 | 5.29 | 34.10 | 1680.0 |
| 400 | 440 | 15.67 | 49.80 | 3.45 | 15.50 | 507.0 |
In order to elucidate the effect of pre-annealing temperature on the electrical characteristics of CZTS devices, we studied the defect state of CZTS devices using admittance spectroscopy.27 Admittance spectroscopy involves measuring the junction capacitance as a function of frequency and temperature T. From the first derivative of the capacitance, inflection frequency wt can be obtained, which is correlated as following equation;
| ωt(T) = 2ξ0T2 exp(−EA/kT) |
 | ||
| Fig. 7 The Arrhenius plots of CZTS thin film solar cells with different pre-annealing temperature showing the calculated activation energies of the defect level. | ||
Conclusions
We used thiourea as a multi-functional component in CZTS precursor solutions and simple spin-coating to fabricate high-quality CZTS thin films. No additional carbon layer was formed between the Mo layer and the CZTS absorber because we did not use any organic chemicals except thiourea to prepare the CZTS precursor solutions. However, excess thiourea had to be added to stabilise the CZTS precursor solutions, which led to the undesirable formation of a ZnS secondary phase in the sulphurised CZTS thin films. The unsulphured CZTS precursor thin films were pre-annealed at various temperatures to eliminate the excess sulphur in the thin films. The formation of the ZnS secondary phase depended on the difference between the temperatures at which the precursor thin films were pre-annealed and that at which the thiourea thermally degraded, which significantly affected the photovoltaic properties of the CZTS solar cells. Furthermore, the morphology and chemical composition of the CZTS thin films was also closely related with the pre-annealing temperature, which might be attributed to the different thermal degradation behaviours of the thiourea and metal chlorides in the CZTS precursor solutions. Simple spin-coating was used to prepare Cu-poor, Zn-rich CZTS thin films by optimising the pre-annealing temperature of the CZTS precursor thin films, and the Cu-poor, Zn-rich CZTS thin films were used to fabricate absorbers for a CZTS solar cell that showed 5.29% efficiency.Acknowledgements
This work was supported by the DGIST R&D Programs of the Ministry of Education, Science and Technology of Korea (13-BD-05) and the New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy (no. 20123010010130).Notes and references
- J. J. Scragg, P. J. Dale, L. M. Peter, G. Zoppi and I. Forbes, Phys. Status Solidi B, 2008, 245, 1772 CrossRef CAS
.
- S. C. Riha, B. A. Parkinson and A. L. Prieto, J. Am. Chem. Soc., 2009, 131, 12054 CrossRef CAS PubMed
.
- T. Tanaka, T. Nagatomo, D. Kawasaki, M. Nishio, Q. Guo, A. Wakahara, A. Yoshida and H. Ogawa, J. Phys. Chem. Solids, 2005, 66, 1978 CrossRef CAS PubMed
.
- H. Katagiri, Thin Solid Films, 2005, 480–481, 426 CrossRef CAS PubMed
.
- H. Katagiri, K. Jimbo, W. S. Maw, K. Oishi, M. Yamazaki, H. Araki and A. Takeuchi, Thin Solid Films, 2009, 517, 2455 CrossRef CAS PubMed
.
- H. Katagiri, K. Jimbo, S. Yamada, T. Kamimura, W. S. Maw, T. Fukano, T. Ito and T. Motohiro, Appl. Phys. Express, 2008, 1, 041201 CrossRef
.
- B. Shin, O. Gunawan, Y. Zhu, N. A. Bojarczuk, S. J. Chey and S. Guha, Progress in Photovoltaics: Research and Applications, 2013, 21, 72 CrossRef CAS
.
- S. J. Ahn, C. W. Kim, J. H. Yun, J. H. Gwak, S. H. Jeong, B. H. Ryu and K. H. Yoon, J. Phys. Chem. C, 2010, 114, 8108 CAS
.
- S. E. Habas, H. A. S. Platt, M. F. A. M. van Hest and D. S. Ginley, Chem. Rev., 2010, 110, 6571 CrossRef CAS PubMed
.
- K. Tanaka, Y. Fukui, N. Moritake and H. Uchiki, Sol. Energy Mater. Sol. Cells, 2011, 95, 838 CrossRef CAS PubMed
.
- N. Kamoun, H. Bouzouita and B. Rezig, Thin Solid Films, 2007, 515, 5949 CrossRef CAS PubMed
.
- S. Ahmed, D. B. Reuter, O. Gunawan, L. Guo, L. T. Romankiw and H. Deligianni, Adv. Energy Mater., 2012, 2, 253 CrossRef CAS
.
- M. Kaelin, D. Rudmann, F. Kurdesau, H. Zogg, T. Meyer and A. N. Tiwari, Thin Solid Films, 2005, 480–481, 486 CrossRef CAS PubMed
.
- T. K. Todorv, J. Tang, S. Bag, O. Gunawan, T. Gokmen, Y. Zhu and D. B. Mitzi, Adv. Energy Mater., 2013, 3, 34 CrossRef
.
- K. H. Woo, Y. W. Kim and J. H. Moon, Energy Environ. Sci., 2012, 5, 5340 CAS
.
- W. Ki and H. W. Hillhouse, Adv. Energy Mater., 2011, 1, 732 CrossRef CAS
.
- E. J. Lee, S. J. Park, J. W. Cho, J. H. Gwak, M. K. Oh and B. K. Min, Sol. Energy Mater. Sol. Cells, 2011, 95, 2928 CrossRef CAS PubMed
.
- Y. Chen, X. He, X. Zhao, M. Song and X. Gu, Mater. Sci. Eng., B, 2007, 139, 88 CrossRef CAS PubMed
.
- J. B. Li, V. Chawla and B. M. Clemens, Adv. Mater., 2012, 24, 720 CrossRef CAS PubMed
.
- G. M. Ilari, C. M. Fella, C. Ziegler, A. R. Uhl, Y. E. Romanyuk and A. N. Tiwari, Sol. Energy Mater. Sol. Cells, 2012, 104, 125 CrossRef CAS PubMed
.
- K. Tanaka, Y. Fukui, N. Moritake and H. Uchiki, Sol. Energy. Mater. Sol. Cells, 2011, 95, 838 CrossRef CAS PubMed
.
- S. Chen, X. G. Gong, A. Walsh and S. H. Wei, Appl. Phys. Lett., 2010, 96, 021902 CrossRef PubMed
.
- P. A. Fernandes, P. M. P. Salomé and A. F. da Cunha, J. Alloys Compd., 2011, 509, 7600 CrossRef CAS PubMed
.
- P. A. Fernandes, P. M. P. Salomé and A. F. da Cunha, Thin Solid Films, 2009, 517, 2519 CrossRef CAS PubMed
.
- P. J. Dale, K. Hoenes, J. J. Scragg and S. Siebentritt, 34th IEEE Photovoltaic Specialist Conference, Philadelphia, 2009 Search PubMed
.
- X. Fontané, L. Calvo-Barrio, V. Izquierdo-Roca, E. Saucedo, A. Pérez-Rodriguez, J. R. Morante, D. M. Berg, P. J. Dale and S. Siebentritt, Appl. Phys. Lett., 2011, 98, 181905 CrossRef PubMed
.
- E. Kask, T. Raadik, M. Grossberg, R. Josepson and J. Krustok, Energy Procedia, 2011, 10, 261 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45441j |
| ‡ These two authors have equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2014 |