Oxone-mediated oxidative carbon-heteroatom bond cleavage: synthesis of benzoxazinones from benzoxazoles with α-oxocarboxylic acids†
Hua Wanga,
Hua Yanga,
Yiping Lib and
Xin-Hua Duan*a
aDepartment of Chemistry, School of Science and MOE Key Laboratory for Nonequilibrium Synthesis and Modulation of Condensed Matter, Xi'an Jiaotong University, Xi'an 710049, China. E-mail: duanxh@mail.xjtu.edu.cn
bDepartment of Pharmacy, College of Medicine, Xi'an Jiaotong University, Xi'an 710061, China
First published on 20th January 2014
Abstract
A metal-free oxidative cleavage of benzoxazoles using Oxone as an oxidant has been developed. The in situ formed o-aminophenol has been proved to react successfully with α-oxocarboxylic acids affording the benzoxazinones in moderate to good yields.
Benzoxazoles are important heterocycles and versatile intermediates in the preparation of a wide variety of biologically and industrially valuable organic compounds.1 In particular, the catalytic direct C–H bond cleavage of benzoxazoles followed by the C–C and C-heteroatom bond formation with various electrophiles or nucleophiles have received significant attention in recent years.2 Furthermore, the carbon-heteroatom bond cleavage of benzoxazoles is also an important fundamental transformation in organic chemistry. For example, Lown has reported an efficient cleavage of benzoxazoles to o-hydroxy-N-substituted anilines in the presence of sodium borohydride/acetic acid.3a Other methods were also available for the break of C-heteroatom bond of benzoxazoles under acidic conditions.3b,c More recently, the Pt-catalyzed oxidative cleavage of benzoxazoles to the corresponding aminophenols has also been reported.4 However, there have been no reports concerning a tandem oxidative cleavage of benzoxazoles for the formation of more valuable heterocycles to date.
Oxone is usually environmentally benign and easily handled oxidant that has been used extensively in organic synthesis.5 For example, the ability of Oxone has been demonstrated in the oxidative cleavage of alkenes or alkynes to the corresponding carboxylic acids and ketones.6 Oxone is also well known for its oxidative cleavage of dicarbonyls and α-hydroxyketones to carboxylic acids or α-keto esters.7 Compared to the carbon–carbon bond cleavage, Oxone is less commonly employed in carbon-heteroatom bond cleavage reactions.5 In continuation of our interest in developing oxidative tandem processes for the construction of heterocycles8 and the oxidative coupling reactions of α-oxocarboxylic acids.9 We envisioned that the use of Oxone as an oxidant for the oxidative cleavage of benzoxazoles followed by coupling with α-oxocarboxylic acids might be a useful method for the synthesis of more valuable heterocycles.10 Herein, we report a facile protocol for the tandem oxidative cleavage/coupling of benzoxazoles with α-oxocarboxylic acids in the presence of Oxone providing the benzoxazinones which are important structural motifs in biological medicinal chemistry and material chemistry.11
Our investigation started with the coupling of 5-methylbenzoxazole 1a with phenylglyoxylic acid 2a using 1.0 equiv. Oxone as sole oxidant (Table 1). After screening a variety of solvents, it was found that the 5% DMSO/diglyme was the best solvent giving the 6-methyl-3-phenylbenzoxazinone 3a in 80% isolated yield (entries 1–6). To our delight, simply changing the ratio of 1a and 2a from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, the yield of 3a could be further improved to 90% (entry 7). It should be noted that the reaction did not occur in the absence of Oxone (entry 8). A series of oxidants showed less effective or ineffective (entries 9–12).
1.5, the yield of 3a could be further improved to 90% (entry 7). It should be noted that the reaction did not occur in the absence of Oxone (entry 8). A series of oxidants showed less effective or ineffective (entries 9–12).
| Entry | Oxidant (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol, 1.0 equiv.), 2a (0.2 mmol, 1.0 equiv.), oxidant (1.0 equiv.), solvent (2.0 mL), 120 °C, 12 h.b Yield of isolated product.c N.r. = No reaction.d 1a (0.2 mmol, 1.0 equiv.), 2a (0.3 mmol, 1.5 equiv.). | |||
| 1 | Oxone (1.0) | DMF | Trace |
| 2 | Oxone (1.0) | DCE | N.r.c |
| 3 | Oxone (1.0) | DME | N.r.c |
| 4 | Oxone (1.0) | CH3CN | N.r.c |
| 5 | Oxone (1.0) | Diglyme | 78 |
| 6 | Oxone (1.0) | 5% DMSO/Diglyme | 80 |
| 7 | Oxone (1.0) | 5% DMSO/Diglyme | 90d |
| 8 | — | 5% DMSO/Diglyme | N.r.c |
| 9 | (NH4)2S2O8 (1.0) | 5% DMSO/Diglyme | 26 |
| 10 | K2S2O8 (1.0) | 5% DMSO/Diglyme | 23 |
| 11 | BQ (1.0) | 5% DMSO/Diglyme | N.r.c |
| 12 | PhI(OAc)2 (1.0) | 5% DMSO/Diglyme | Trace |
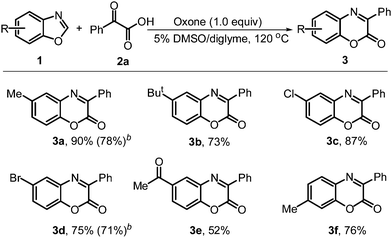
Under the optimized reaction conditions, a variety of benzoxazoles 1 were examined for this tandem oxidative coupling reaction (Table 2). Benzoxazoles with 5-substituted electron-rich and -withdrawing groups are all effectively engaged in this reaction (3a–e). It is noteworthy that bromo-substituted benzoxazole 1d was tolerated well and provided the corresponding product 3d in moderate yield, which could be used for further transformation. The reaction of 6-methylbenzoxazole with 2a also worked well to give the product 3f in 76% yield. It should be noted that the reaction could also be scaled up to 2 mmol, the corresponding products 3a and 3d, were obtained in good yields.
Various substituted α-oxocarboxylic acids were then tested for this oxidative cleavage/coupling reaction under the optimized conditions. The results are summarized in Table 3. As shown in Table 3, this reaction proceeded well with a wide range of p-substituted phenylglyoxylic acids and gave the corresponding benzoxazinones in moderate to good yields (4b–g). Notably, when bromo or iodo group was included in phenylglyoxylic acid, the corresponding products 4f and 4g were also obtained in 76% and 71% yields, respectively. The results were significant because the halo groups retained in the benzoxazinones provide opportunities for further transformation under transition metal catalytic systems. On the other hand, substrates bearing an ortho-substituent such as o-methyl phenylglyoxylic acid 2h furnished lower yield, probably due to the steric hindrance. Besides the desired product 4h (41%), the byproduct o-methylbenzaldehyde was also isolated in 28% yield in this reaction. When α-naphthyloxoacetic acid was employed, only a trace amount of the desired product 4i was detected and the major product was determined as the α-naphthaldehyde.12 While, for β-naphthyloxoacetic acid, the corresponding product 4j was obtained in good yield. These results indicated that the tandem oxidative reaction was hampered by steric hindrance. Hetero- and aliphatic α-oxocarboxylic acids, such as 2-thienylglyoxylic acid and pyruvic acid, resulted in the desired products in somewhat lower yields (4k and 4l).
To gain better insight into the mechanism, some control experiments were conducted under the standard reaction conditions. When benzoxazole 1g was subjected to the optimized reaction conditions (eqn 1), o-aminophenol 5 was detected, which indicated that benzoxazole could undergo the carbon-heteroatom bond cleavage in the presence of Oxone.4,5 On the other hand, the reaction of o-aminophenol 5 with phenylglyoxylic acid 2a gave the benzoxazinone 4a in 46% isolated yield (eqn 2), suggesting that aminophenol should be an intermediate in this reaction. On the basis of the above observations, a tentative mechanism is proposed in Scheme 1. o-Aminophenol 5 is formed via oxidative carbon-heteroatom bond cleavage of benzoxazole under Oxone. A subsequent standard esterification reaction followed by condensation provided the benzoxazinone product.
In summary, we have demonstrated an interesting tandem oxidative cleavage/coupling process of benzoxazoles with α-oxocarboxylic acids to the benzoxazinones using Oxone as a sole oxidant under metal-free conditions. The method is compatible with a wide range of easily available reactants and reagents.
Acknowledgements
Financial support from National Natural Science Foundation of China (no. 21102110) and the Fundamental Research Funds of the Central Universities (no. 2011jdhz28) are greatly appreciated.Notes and references
- For some recent selected examples, see: (a) S.-T. Huang, I.-J. Hsei and C. Chen, Bioorg. Med. Chem., 2006, 14, 6106 CrossRef CAS PubMed; (b) M. H. Potashman, J. Bready, A. Coxon, T. M. DeMelfi, Jr, L. DiPietro, N. Doerr, D. Elbaum, J. Estrada, P. Gallant, J. Germain, Y. Gu, J.-C. Harmange, S. A. Kaufman, R. Kendall, J. L. Kim, G. N. Kumar, A. M. Long, S. Neervannan, V. F. Patel, A. Polverino, P. Rose, S. Van der Plas, D. Whittington, R. Zanon and H. Zhao, J. Med. Chem., 2007, 50, 4351 CrossRef CAS PubMed; (c) M. L. McKee and S. M. Kerwin, Bioorg. Med. Chem., 2008, 16, 1775 CrossRef CAS PubMed; (d) E. H. Sessions, Y. Yin, T. D. Bannister, A. Weiser, E. Griffin, J. Pocas, M. D. Cameron, C. Ruiz, L. Lin, S. C. Schürer, T. Schröter, P. LoGrasso and Y. Feng, Bioorg. Med. Chem. Lett., 2008, 18, 6390 CrossRef CAS PubMed; (e) S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. G. Stevens, C. S. Matthews, T. D. Bradshaw and A. D. Westwell, J. Med. Chem., 2008, 51, 5135 CrossRef CAS PubMed; (f) S. M. Johnson, S. Connelly, I. A. Wilson and J. W. Kelly, J. Med. Chem., 2008, 51, 260 CrossRef CAS PubMed.
- For some recent selected reviews, see: (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (b) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (c) X. Chen, K. M. Engle, D. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (d) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (e) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem. - Eur. J., 2010, 16, 2654 CrossRef CAS PubMed; (f) K. Hirano and M. Miura, Synlett, 2011, 294 CAS.
- (a) B. Yadagiri and J. W. Lown, Synth. Commun., 1990, 20, 175 CrossRef CAS; (b) S. Fujita, J. Org. Chem., 1983, 48, 177 CrossRef CAS; (c) F. Bruyneel, E. Enaud, L. Billottet, S. Vanhulle and J. Marchand-Brynaert, Eur. J. Org. Chem., 2008, 72 CrossRef CAS.
- B. Laksmi, K. N. Shivananda, Puttaswamy, K. N. Mahendra, N. M. M. Gowda and R. V. Jagadeesh, Chem. Eng. J., 2010, 163, 403 CrossRef CAS PubMed.
- H. Hussain, I. R. Green and I. Ahmed, Chem. Rev., 2013, 113, 3329 CrossRef CAS PubMed.
- (a) B. R. Travis, R. S. Narayan and B. Borhan, J. Am. Chem. Soc., 2002, 124, 3824 CrossRef CAS PubMed; (b) K. Lee, Y.-H. Kim, S. B. Han, H. Kang, S. Park, W. S. Seo, J. T. Park, B. Kim and S. Chang, J. Am. Chem. Soc., 2003, 125, 6844 CrossRef CAS PubMed; (c) D. Yang, F. Chen, Z.-M. Dong and D.-W. Zhang, J. Org. Chem., 2004, 69, 2221 CrossRef CAS PubMed; (d) P. P. Thottumkara and T. K. Vinod, Org. Lett., 2010, 12, 5640 CrossRef CAS PubMed.
- (a) S. W. Ashford and K. C. Grega, J. Org. Chem., 2001, 66, 1523 CrossRef CAS; (b) J. Yan, B. R. Travis and B. Borhan, J. Org. Chem., 2004, 69, 9299 CrossRef CAS PubMed; (c) J. Yu, J. Cui and C. Zhang, Eur. J. Org. Chem., 2010, 7020 CrossRef CAS; (d) A. Stergiou, A. Bariotaki, D. Kalaitzakis and I. Smonou, J. Org. Chem., 2013, 78, 7268 CrossRef CAS PubMed.
- (a) Y. Meng, L.-N. Guo, H. Wang and X.-H. Duan, Chem. Commun., 2013, 49, 7540 RSC; (b) S.-L. Zhou, L.-N. Guo, H. Wang and X.-H. Duan, Chem. - Eur. J., 2013, 19, 12970 CrossRef CAS PubMed; (c) H. Wang, L.-N. Guo and X.-H. Duan, Chem. Commun., 2013, 49, 10370 RSC; (d) H. Wang, L.-N. Guo and X.-H. Duan, Org. Lett., 2013, 15, 5254 CrossRef CAS PubMed.
- (a) H. Wang, L.-N. Guo and X.-H. Duan, Org. Lett., 2012, 14, 4358 CrossRef CAS PubMed; (b) S. Zhang, L.-N. Guo, H. Wang and X.-H. Duan, Org. Biomol. Chem., 2013, 11, 4308 RSC; (c) H. Wang, L.-N. Guo and X.-H. Duan, Org. Biomol. Chem., 2013, 11, 4573 RSC; (d) H. Wang, L.-N. Guo and X.-H. Duan, Adv. Synth. Catal., 2013, 355, 2222 CrossRef CAS.
- Z. Yang, X. Chen, S. Wang, J. Liu, K. Xie, A. Wang and Z. Tan, J. Org. Chem., 2012, 77, 7086 CrossRef CAS PubMed.
- For some recent selected examples, see: (a) R. B. Moffett, J. Med. Chem., 1966, 9, 475 CrossRef CAS; (b) F. Chioccara, E. Ponsiglione, G. Prota and R. H. Thomson, Tetrahedron, 1976, 32, 2033 CrossRef CAS; (c) F. Chioccara, F. D. Gala, E. Novellino and G. J. Prota, Heterocycl. Chem., 1977, 14, 773 CrossRef CAS; (d) M.-T. Le Bris, J. Heterocycl. Chem., 1984, 21, 551 CrossRef CAS; (e) J. C. Powers, J. L. Asgian, Ö. D. Ekici and K. E. James, Chem. Rev., 2002, 102, 4639 CrossRef CAS PubMed; (f) F. Bihel and J.-L. Kraus, Org. Biomol. Chem., 2003, 1, 793 RSC; (g) Y.-G. Zhou, P.-Y. Yang and X.-W. Han, J. Org. Chem., 2005, 70, 1679 CrossRef CAS PubMed; (h) J. Ilaš, P. Š. Anderluh, M. S. Dolenc and D. Kikelj, Tetrahedron, 2005, 61, 7325 CrossRef PubMed; (i) M. Pietsch and M. Gütschow, J. Med. Chem., 2005, 48, 8270 CrossRef CAS PubMed; (j) F. A. Macías, D. Marín, A. Oliveros-Bastidas and J. M. G. Molinillo, Nat. Prod. Rep., 2009, 26, 478 RSC.
- α-Oxocarboxylic acids were readily decarboxylated to form aldehyde under mild conditions, see: T. Cohen and H. Song II, J. Am. Chem. Soc., 1965, 87, 3780 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47660j |
| This journal is © The Royal Society of Chemistry 2014 |