Effect of canopy structures and their steric interactions on CO2 sorption behavior of liquid-like nanoparticle organic hybrid materials†
Abstract
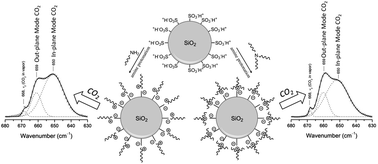
Liquid-like NOHMs with different grafting densities of polymeric canopy were synthesized to evaluate their solvating properties as CO2 solvents. The in situ ATR FT-IR study of NOHMs with linear and branched canopies revealed distinct CO2 capture and corresponding swelling behaviors. These observations suggested that the entropic contribution for CO2 sorption in NOHMs can be tuned via the canopy design.

 Please wait while we load your content...
Please wait while we load your content...