Green up-conversion luminescence of Yb3+-Er3+ co-doped CaLa2ZnO5 for optically temperature sensing†
Abstract
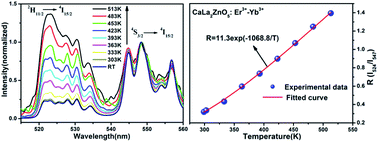
A class of Yb3+/Er3+ ion co-doped CaLa2ZnO5 (CLZ) novel up-conversion (UC) phosphors were synthesized by a sol–gel method. The crystal structure and the phase composition of the samples were characterized by X-ray diffraction (XRD). Under 980 nm excitation, UC spectra are composed of three prominent emission bands centered at 524, 547 and 672 nm originating from 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 electronic transitions of Er3+ ion in green and red regions, respectively. The UC spectra as a function of the concentration of the dopants were also investigated to determine the optimal composition. A possible UC mechanism in Er3+/Yb3+ co-doped CLZ is proposed in light of the dependence of emission intensities on pumping power. The photoluminescence lifetimes of the green band at 547 nm and the red band at 672 nm were also measured. The temperature dependence of fluorescence intensity ratio (FIR) of the two green UC emission bands peaked at 524 and 547 nm was studied in the range of 298–513 K under an excitation of 980 nm produced by a diode laser, and the maximum sensitivity was approximately 0.0059 K−1. Results suggest that the Yb3+/Er3+ co-doped CLZ phosphor is an efficient UC phosphor and a promising candidate for optical temperature sensors.

 Please wait while we load your content...
Please wait while we load your content...