Controlled synthesis and characterization of iron oxide nanostructures with potential applications for gas sensors and the environment†
Abstract
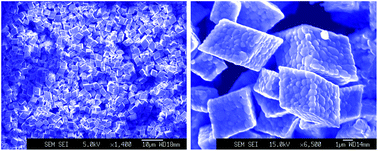
In the present research, large iron oxide microparticles with large sizes in the range of 1–5 μm have been facilely synthesized by a modified polyol method with NaBH4 as a versatile strong reducing agent. We found that the highly homogeneous iron oxide microparticles' novel structure is the best pure crystal phase of α-Fe2O3 in terms of polyhedral morphology and shape in existence. There are no diffraction peaks of other crystal phases from impurities in α-Fe2O3 microparticle products in the crystal growth. Interestingly, a new method of heat treatment or atomic surface deformation allowed for the discovery of a new large α-Fe2O3 structure with controlled specific α-Fe2O3 oxide grains in the crystal structure. The severe surface deformation of sharp, polyhedral, large α-Fe2O3 microparticles under a sintering treatment was found to give un-sharp, polyhedral large α-Fe2O3 microparticles with specific grains and boundaries.

 Please wait while we load your content...
Please wait while we load your content...