Preparation of helical fibers from cellulose–cuprammonium solution based on liquid rope coiling
Baoquan Jiaa,
Li Yub,
Feiya Fua,
Lingyan Lia,
Jinping Zhou*a and
Lina Zhanga
aDepartment of Chemistry, Wuhan University, Wuhan 430072, China. E-mail: zhoujp325@whu.edu.cn
bSchool of Medicine and Pharmaceutics, Jiangnan University, Wuxi 214122, Jiangsu Province, China
First published on 20th January 2014
Abstract
A new method for large-scale mimicking of the helical structure of plant tendrils from cellulose solution was proposed based on liquid rope coiling of viscous fluid. In the spinning process, cellulose–cuprammonium solution was extruded from a certain height above a mobile coagulating bath. Helical structure was shown to form spontaneously at the surface of the coagulating bath as a result of buckling instability, and regenerated cellulose helical fiber was obtained after treatment with acid and air-drying. Spinning parameters, such as spinning height, flow rate and moving speed of the coagulating bath were examined in relation to the size and structure of the helical fiber. The microstructure of the helical fiber was further investigated by SEM. The diameters of the fiber and helix were found in the range of 100–400 and 300–700 μm, respectively. The helical fibers demonstrated high elongation at break and elasticity within a certain range of strain, which were attributed to the coiling structure. The microporous feature of the regenerated cellulose helical fiber shows potential as a helical scaffold and template for inorganic materials.
1. Introduction
Many of the unique and exquisite shapes and structures demonstrated in nature are still beyond the current capacity of manufacturing technology. Further exploration into such natural phenomena can provide compelling insights for science and technology.1–3 Helical structures are very common in both animals and plants and can be seen in trumpet shell and spiral tendrils. Extended helical or spiral structures can be also found in micro and nano areas, e.g. spiral vessels in vascular plants, abalone shells, DNA etc.2–5 These fascinating structures have attracted much research interest with particular focus on the underlying mechanisms and possible applications.6–10 Helical structures are present in nearly every part of our life. These interesting structures have been applied in various fields, including microelectronics, micro-electro-mechanical systems, electromagnetic waves or cosmic rays absorbers, microfluidics, optics, catalysis, sensor and smart systems.11–13Periodic buckling usually occurs when a thin stream of viscous fluid is poured onto a surface from a certain height. As a result, the viscous stream exhibits a beautiful helical structure near the surface, which spontaneously appears following the pouring process. Barnes et al. termed this mechanical phenomenon of fluid “liquid rope coiling”, and it has since been studied in detail theoretically and experimentally.14–16 The liquid rope coiling process is initiated through the competition between axial compression and bending in the fluid rope.17 Therefore, liquid rope coiling is comparable to the coiling of an elastic rope falling onto a surface. The liquid rope coiling phenomenon is greatly influenced by liquid density, viscosity, flow rate, gravity, rope size and height.17 The periodic coiling of viscous stream on a surface has been used in several areas, including food processing, polymer processing, geophysics, etc.18 Several works have recently presented the preparation of micro helical structures based on the same buckling instability using electrospinning.13,19–21 Based on these previous applications, it should also be feasible to spin helical fibers using the periodic coiling effect through a traditional wet spinning process.
As the most abundant natural biomass, cellulose is only surpassed by polyester as the most popular source for mass-produced fibers. Regenerated cellulose (RC) filaments are unique among man-made fibers as the substance to use a natural polymer directly. With the depletion of fossil-based resources, the renewable natural resource shows many advantages over petroleum-based polymers.22 In recent decades, the application of RC filaments has not just limited in traditional fields (textile and clothes), but also in other fields such as antibacterial, electronic, magnetic and filtration materials.23–27 In this work, the helical structure of tendrils was mimicked through a simple spinning process analog to wet spinning. Helical RC fibers were obtained from a cellulose–cuprammonium solution based on the liquid rope coiling effect of a viscous stream. The relationship between the spinning parameters and the sizes of the fiber and the coiling frequency were studied. Tensile strength, elongation and elasticity of the helical fibers were also tested. The potential as helical template for inorganic materials was presented. The results may prove valuable for the study of liquid rope coiling process and further application in helical materials.
2. Experimental section
2.1. Materials
Cotton linter pulp was provided by Hubei Chemical Fiber Group Ltd. (Xiangyang, China). CuSO4·5H2O, tetraethyl orthosilicate (TEOS), FeCl2 and NaOH of analytical grade and aqueous ammonia were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), and used without further purification.2.2. Preparation of helical fibers
Cellulose–cuprammonium solution (8 wt%) was prepared in accordance with previous method,28,29 and the kinematic viscosity (ν) at 25 °C was determined to be 0.016 m2 s−1. The spinning process is illustrated in Scheme 1. The cellulose solution was inhaled into a syringe, where the inner diameter of the spinneret was 2.28 mm. A flow pump (Longer Precision Pump Co., Ltd., China) was used to supply cellulose solution at a constant speed (7–10 mL min−1). A movable coagulating bath loaded with 10 wt% NaOH solution was then pulled by an adjustable-speed rotator. The spinning height was the distance between the syringe tip and the surface of coagulating bath. The process was performed wholly under room temperature (25 °C). The coagulated fiber was obtained as the cellulose–cuprammonium solution immersed in NaOH solution. Regenerated cellulose (RC) fiber was subsequently obtained by immersing the coagulated fiber in 5 wt% H2SO4 solution for 5–10 min and then washed with distilled water. The helical coagulated and RC fibers were air-dried at room temperature. | ||
| Scheme 1 Schematic diagram of spinning helical regenerated cellulose fibers (a) on the solid surface and (b) in the movable coagulating bath. | ||
The CuO helical fiber was obtained by calcination of the coagulated fiber at 500 °C for 1 h. For the SiO2 and Fe2O3 helical fibers, in situ synthesis was carried out firstly. RC helical fiber in wet state was immersed in Fe2+ solution (0.5 M) for 3–5 h and then treated with NaOH solution (1 M) for 5–10 min. After washed with distilled water, the fiber was air-dried. To prepare cellulose/SiO2 composite fiber, the wet RC fiber was treated with ethanol to remove water and then soaked in TEOS for 10 h. Afterwards, the fiber was transferred to ammonium hydroxide and kept for 20 min. It was air-dried after washing with water and ethanol. The SiO2 and Fe2O3 helical fibers were obtained after calcination at 500 °C for 1 h.
2.3. Characterization
The viscosity of the spinning solution at 25 °C was tested by an ARES RFSIII rheometer (TA Instruments, USA) with couttee cups at the shear rate of 0.1 s−1. The density of cellulose–cuprammonium solution was determined as 1.28 g cm−3 with a density bottle at 20 °C. Mechanical properties of the RC helical fibers in dry state were measured on a universal testing machine (CMT6350, Shenzhen SANS Test Machine Co., Ltd., Shenzhen, China).Photographs of the liquid rope and the RC, CuO, SiO2 and Fe2O3 helical fibers were obtained using a digital camera. The liquid rope coiling process in the stationary coagulating bath were documented using films recorded by a digital camera at 25 frames per second (E-PL5, OLYMPUS, Japan). The coiling frequency (Ω) was measured by counting frames. SEM images of the fibers were taken on a VEGA 3 LMU (TESCAN, Czech) scanning electron microscope. The samples for SEM observation were sputtered with gold.
3. Results and discussion
The liquid jet formed when the cellulose–cuprammonium solution was extruded from the determined height. The flow rate (Q) and height (H) were vital in determining the size and behavior of the liquid stream. To confirm the flow rate and height for spinning, different flow rates (3–10 mL min−1) were tested at a height of 50 cm, while the diameter of the stream was measured at different positions. Due to the capillary instability of viscous thread, continuous liquid rope of the cellulose solution could be only maintained at Q > 6 mL min−1.30 Fig. 1 shows the diameters of the liquid rope (2a1) at different distance from the spinning orifice (Q = 7 mL min−1). It can be seen that the width of the liquid rope thinned due to gravity and inertial stretching but remained a continuous stream as a result of viscosity. The phenomenon of extrusion swelling could be observed around the orifice. With increasing distance, the diameter of the slender stream first decreased dramatically and began to flatten when it was greater than ∼10 cm. Moreover, the diameter could be thickened by increasing flow rate and viscosity.31 | ||
| Fig. 1 Liquid rope diameters of the cellulose–cuprammonium solution at different distances from the orifice. (Q = 7 mL min−1). | ||
The investigation on the liquid rope coiling in a coagulating bath was initiated with a stationary coagulating bath. Fig. 2 displays the situation of the cellulose solution liquid jet in NaOH solution within 1 s. The first frame is the one before the liquid jet fell into the bath. After the straight liquid jet fell into the coagulating bath, buckling could be observed. The liquid rope clearly deformed due to the gravity, inertia and the counterforce of liquid surface. As with the solid surface, folding was the first to occur.32 After about 200 ms, folding turned into coiling. During the coiling, the cellulose filament surface was rapidly coagulated by the NaOH solution. The helical structure could thus be preserved instead of collapsing. However, helical fibers could not be produced massively in a stationary coagulating bath because the fibers would float in the NaOH solution for some time during coagulation. The liquid stream would directly fall onto the earlier fibers. Serious fiber adhesion or collapse was to be expected in this situation and only a short part of the helical structure could be maintained.
 | ||
| Fig. 2 Photographs of the liquid rope coiling of cellulose–cuprammonium solution in the aqueous NaOH bath at different time. (H = 15 cm, Q = 10 mL min−1). | ||
To achieve large-scale production of helical fibers, a movable coagulating bath was used as shown in Scheme 1. The coagulating bath was equipped with wheels and a cable connected to an adjustable-speed rotator to pull the bath. The moving bath was shown to be able to separate the rings and preserve the helix. The moving coagulant bath could carry the newly coagulated helical fiber away in case of collapse and adhesion. Through this method, continuous helical fibers were successfully prepared. To the best of our knowledge, this should be one of the simplest methods of mimicking the helical structure of plant tendrils. The helical RC fibers are created directly from cellulose solution via the wet spinning technique.
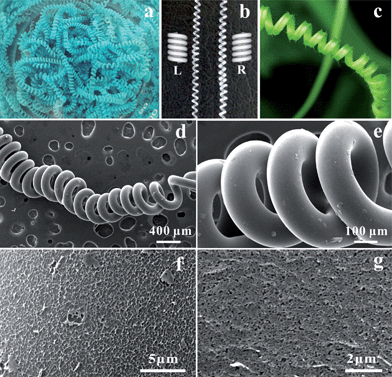
Fig. 3 displays the photographs and SEM images of the coagulated and RC helical fibers after air-drying and freeze-drying. The exquisite blue helical rings (coagulated fibers) were immersed in water to wash out the alkaline and ammonia. After treatment with H2SO4 solution, RC fibers with helical structure were obtained. Both right- and left-handed helices were found among the as-spun fibers (Fig. 3b). The length of the helical fiber was basically determined by the size of coagulating bath. The helical shape was comparable to that of a plant tendril (Fig. 3c). Smooth surface and helical structure of the air-dried cylinders were also observed in the SEM images (Fig. 3d and e).
Effects of the spinning parameters on the size of the RC helical fiber were examined. Fig. 4 displays the diameter of RC fiber with different spinning height (H) and flow rate (Q). As the liquid rope became thinner with larger H, the diameter of the fibers decreased. This is in consistence with the tapering liquid slender (Fig. 1). Moreover, increasing flow rate resulted in slightly thicker fibers. RC fibers were found to shrink during the air-drying process compared to the wet fibers. The air-dried fiber diameters were in the range of 100–400 μm. Fig. 5 displays the diameter of the helix (D) with different H, Q and moving speed of the coagulating bath (V). The size of the helix was in close relation to the spinning height (Fig. 5a and b), which decreased as the height increased, i.e. D decreased with the smaller fiber size.16 D also became shrunk because of air-drying. D values of the air-dried fibers in this work were about 300–700 μm. It was observed that increasing flow rate resulted in an expanded helix diameter (Fig. 5c) which is relevant with the thicker fiber. D was diminished when the coagulating bath increased in speed (Fig. 5d). This can be attributed to the stretching force of the moving bath in the horizontal direction. The helical spring was stretched longer in the axial direction by the moving liquid and the width of the helical ring was thus diminished. In general, the size of the RC helix strongly depended on the spinning height and was influenced by flow rate and moving speed of the coagulating bath. The helical pitch (P) of the RC fiber was equal to the length of the helical fiber divided by number of the ring. And finally the equation was deduced as follows:
| P = LfV/ΩLb, | (1) |
 | ||
| Fig. 4 Effects of the (a) spinning height (H) and (b) flow rate (Q) on the fiber diameter in wet and dry states. | ||
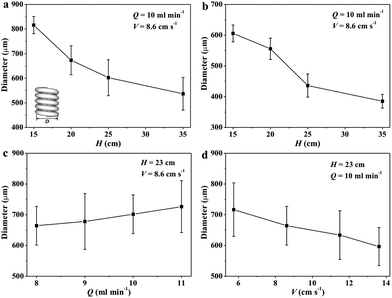 | ||
| Fig. 5 Effects of the (a and b) spinning height (H), (c) flow rate (Q) and (d) moving speed (V) of the coagulating bath on the diameter of the helical ring in (a, c and d) wet and (b) dry states. | ||
Although the size of the helical fiber was smaller as the spinning height increased, the spinning process became more instable. When the spinning height was raised to more than 30 cm (Q = 10 mL min−1), disordered region occurred obviously as shown in Fig. 6b and c. The instability became more serious with increasing height. Fiber diameters of the disordered region were showing difference from the helical parts according to the pictures, i.e. the size fluctuation of liquid rope was obvious. The size fluctuation of the liquid rope was usually caused by capillary instability and became more serious with increasing spinning height.33 The variation of liquid rope size would greatly affect the coiling process (diameter of helix and coiling frequency) and further produce the defects.16 Therefore, the capillary instability should account for the defects at higher H. Moreover, the falling slender would be more susceptible to the ambient when it became thinner. This might be the other reason causing the irregularity.
 | ||
| Fig. 6 Photographs of the helical RC fibers spun at H = (a) 25 cm, (b) 30 cm and (c) 40 cm (Q = 10 mL min−1 and V = 8.6 cm s−1). | ||
Coiling frequency of liquid coiling is vital for the study of liquid rope coiling. The coiling frequency is mainly decided by the balance of viscous, gravitational and inertial forces in the coiling process, which control the motion of the liquid rope. It has been reported that four distinct regimes (viscous, gravitational, inertio-gravitational and inertial) are present, representing different balances among the three forces.16 These regimes are divided by the dimensionless height (Ĥ):
| Ĥ = H (g/ν2)1/3 | (2) |
| ΩI ∼ (Q4/νa110)1/3. | (3) |
The coiling frequency results were obtained by counting the frames at different heights. The scaling law for frequency was obtained by combining the experimental results and inertial coiling law, where Ω ≈ 0.019ΩI (Fig. 7). At a height of 9.5–20 cm, the experimental results were very close to the theoretical prediction. However, it was a little higher than expected at H = 25 cm. The video-taking speed was 25 frames per second, which might cause larger deviations at high coiling frequency. Moreover, the scaling law is based on a solid surface and may not wholly apply to a liquid bath, especially at a higher coiling frequency.
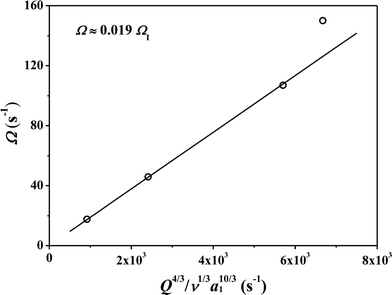 | ||
| Fig. 7 Comparison of theoretical model (line) with experimental results (circle) at different spinning heights. | ||
Fig. 8 shows the mechanical properties of the helical RC fibers. The tensile strength of the fibers was determined to be around 21 MPa. It is noteworthy that the elongation at break can be up to ∼160%. This was attributed to the helical structure that was known as an efficient conformation for saving space. The RC fiber was screwy instead of being put up straight after fracture (Fig. 8a). It was because that the ends of the helical fiber were immobilized during the tensile test and twisting force along the fiber could not be released. The deformation of the helical RC fiber was irreversible due to the poor elasticity. However, elasticity within the linear strain range (less than 4%) was tested under the maximum force of 0.1 N and the curve was shown in Fig. 8b. The strain of the spring coil changed along with the stretching force. And the strain returned to original state when the force decreased to zero. The helical RC fiber lacks elasticity under a large deformation, but the diameter and pitch of the helix can be adjusted by stretching to a required degree based on the poor elasticity (inset of Fig. 8a).
Porous structure of RC products was usually expected when cellulose–cuprammonium was coagulated in NaOH solution.35 As shown in Fig. 3f and g, RC helical fibers display homogeneous microporous structure in wet state, and the pore size was about 80 nm. This porous feature could be of great use in synthesis or loading nanoparticles, drugs etc. These pores are also natural cages for dispersing the nanoparticles from aggregation.25,36 In this work, we prepared CuO, Fe2O3 and SiO2 helical fibers by calcination of the coagulated helical fiber (CuO) and the in situ synthesized helical fibers (SiO2 and Fe2O3), respectively. Fig. 9 shows the photographs and SEM images of the inorganic helical fibers. The free-standing helical fibers were composed of CuO, SiO2 and Fe2O3, respectively. They preserved the helical structure of RC helical fiber (H = 25 cm) after pyrolysis of cellulose at 500 °C. The inorganic fibers exhibited good mechanical strength and could be carefully lifted with tweezers. Therefore, the inorganic helical fibers can be widely used in various fields. The helical RC fiber is a promising template for preparing helical inorganic materials.
 | ||
| Fig. 9 Photographs and SEM images of (a and b) CuO, (c and d) SiO2 and (e and f) Fe2O3 helical fibers. (Scale bar: 100 μm). | ||
4. Conclusions
In summary, a new method for preparing regenerated cellulose fibers with a periodically helical structure has been proposed via a simple spinning process. The as-spun helical fibers reproduced the shape of plant tendrils using cellulose, the main component of plants, based on “liquid rope coiling” effect. Right- and left-handed directions of the helical regenerated cellulose fibers occurred randomly. The size of helical fibers demonstrated close relation with spinning parameters: increased spinning height and decreased flow rate were shown to decrease the fiber diameter, and the helix diameter decreased with the finer fiber and faster moving speed of the coagulant bath. The frequency of coiling increased with increasing spinning height, which was consistent with the previously reported theoretical prediction. As a result of the helical structure, the regenerated cellulose fiber showed an outstanding elongation and elasticity at certain range of strain. With the porous structure, the helical cellulose fiber could be a good candidate for nanoparticle scaffold or helical template. Helical inorganic fibers could be prepared after pyrolysis of cellulose matrix. The helical regenerated cellulose fibers will provide explorations into the applications of liquid rope coiling and the preparation of helical structures.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (51273151), the Program for New Century Excellent Talents in University (NCET-11-0415), National Basic Research Program of China (973 Program, 2010CB732203) and Fundamental Research Funds for the Central Universities.References
- T. Sun, G. Qing, B. Su and L. Jiang, Chem. Soc. Rev., 2011, 40, 2909–2921 RSC
.
- K. Kamata, S. Suzuki, M. Ohtsuka, M. Nakagawa, T. Iyoda and A. Yamada, Adv. Mater., 2011, 23, 5509–5513 CrossRef CAS PubMed
.
- Z. R. Tian, J. A. Voigt, J. Liu, B. Mckenzie and M. J. Mcdermott, J. Am. Chem. Soc., 2002, 124, 12954–12955 CrossRef CAS PubMed
.
- S. Han, M. Lee and Y. Lim, Biomacromolecules, 2013, 14, 1594–1599 CrossRef CAS PubMed
.
- Y. Cao, J. Xie, B. Liu, L. Han and S. Che, Chem. Commun., 2013, 49, 1097–1099 RSC
.
- A. Aggeli, I. Nyrkova, M. Bell, R. Harding, L. Carrick, T. McLeish, A. Semenov and N. Boden, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11857–11862 CrossRef CAS PubMed
.
- D. Farrar, K. Ren, D. Cheng, S. Kim, W. Moon, W. L. Wilson, J. E. West and S. M. Yu, Adv. Mater., 2011, 23, 3954–3958 CrossRef CAS PubMed
.
- K. Pagel, S. C. Wagner, K. Samedov, H. von Berlepsch, C. Böttcher and B. Koksch, J. Am. Chem. Soc., 2006, 128, 2196–2197 CrossRef CAS PubMed
.
- S. J. Gerbode, J. R. Puzey, A. G. McCormick and L. Mahadevan, Science, 2012, 337, 1087–1091 CrossRef CAS PubMed
.
- O. Giraldo, M. Marquez, S. L. Brock, S. L. Suib, H. Hillhouse and M. Tsapatsis, J. Am. Chem. Soc., 2000, 122, 12158–12163 CrossRef CAS
.
- Y. Qin, Z. Zhang and Z. Cui, Carbon, 2004, 42, 1917–1922 CrossRef CAS PubMed
.
- K. Mukhopadhyay, K. Ram, D. Lal, G. N. Mathur and K. Rao, Carbon, 2005, 43, 2400–2402 CrossRef CAS PubMed
.
- S. P. Shariatpanahi, A. Iraji zad, I. Abdollahzadeh, R. Shirsavar, D. Bonn and R. Ejtehadi, Soft Matter, 2011, 7, 10548–10551 RSC
.
- G. Barnes and R. Woodcock, Am. J. Phys., 1958, 26, 205 CrossRef
.
- G. Barnes and J. Mackenzie, Am. J. Phys., 1959, 27, 112 CrossRef
.
- N. M. Ribe, M. Habibi and D. Bonn, Annu. Rev. Fluid Mech., 2012, 44, 249–266 CrossRef
.
- L. Mahadevan, W. S. Ryu and A. D. T. Samuel, Nature, 1998, 392, 140 CrossRef CAS PubMed
.
- N. M. Ribe, Proc. R. Soc. London, Ser. A, 2004, 460, 3223–3239 CrossRef
.
- T. Han, D. H. Reneker and A. L. Yarin, Polymer, 2007, 48, 6064–6076 CrossRef CAS PubMed
.
- H.-Y. Kim, M. Lee, K. J. Park, S. Kim and L. Mahadevan, Nano Lett., 2010, 10, 2138–2140 CrossRef CAS PubMed
.
- M. Godinho, J. Canejo, L. Pinto, J. Borges and P. Teixeira, Soft Matter, 2009, 5, 2772–2776 RSC
.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed
.
- D. Roy, J. S. Knapp, J. T. Guthrie and S. Perrier, Biomacromolecules, 2008, 9, 91–99 CrossRef CAS PubMed
.
- L. Dall'Acqua, C. Tonin, R. Peila, F. Ferrero and M. Catellani, Synth. Met., 2004, 146, 213–221 CrossRef CAS PubMed
.
- S. Liu, L. Zhang, J. Zhou and R. Wu, J. Phys. Chem. C, 2008, 112, 4538–4544 CAS
.
- F. Aloulou, S. Boufi and J. Labidi, Sep. Purif. Technol., 2006, 52, 332–342 CrossRef CAS PubMed
.
- N. Sun, R. P. Swatloski, M. L. Maxim, M. Rahman, A. G. Harland, A. Haque, S. K. Spear, D. T. Daly and R. D. Rogers, J. Mater. Chem., 2008, 18, 283–290 RSC
.
- B. Jia, Y. Mei, L. Cheng, J. Zhou and L. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 2897–2902 CAS
.
- B. Jia, Y. Dong, J. Zhou and L. Zhang, J. Mater. Chem. C, 2014, 2, 524–529 RSC
.
- C. Pozrikidis, J. Eng. Math., 1999, 36, 255–275 CrossRef
.
- M. Maleki, M. Habibi, R. Golestanian, N. M. Ribe and D. Bonn, Phys. Rev. Lett., 2004, 93, 214502 CrossRef CAS
.
- M. Habibi, Y. Rahmani, D. Bonn and N. M. Ribe, Phys. Rev. Lett., 2010, 104, 074301 CrossRef CAS
.
- B. Cheong and T. Howes, Chem. Eng. Sci., 2004, 59, 2145–2157 CrossRef CAS PubMed
.
- L. Mahadevan, W. S. Ryu and A. D. T. Samuel, Nature, 2000, 403, 502 CrossRef CAS
.
- M. Inamoto, I. Miyamoto, T. Hongo, M. Iwata and K. Okajima, Polym. J., 1996, 28, 507–512 CrossRef CAS
.
- J. He, T. Kunitake and A. Nakao, Chem. Mater., 2003, 15, 4401–4406 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2014 |