Exploring the solid state properties of enzymatic poly(amine-co-ester) terpolymers to expand their applications in gene transfection†
Irina Voevodinaa,
Mariastella Scandola*a,
Junwei Zhangb and
Zhaozhong Jiang*c
aUniversity of Bologna, Department of Chemistry G. Ciamician, INSTM UdR Bologna, via Selmi 2, 40126 Bologna, Italy. E-mail: mariastella.scandola@unibo.it; irina.voevodina@unibo.it; Tel: +39 0512099577
bDepartment of Chemical and Environmental Engineering, Yale University, 55 Prospect Street, New Haven, Connecticut 06511, USA. E-mail: junwei.zhang@yale.edu
cMolecular Innovations Center, Yale University, 600 West Campus Drive, West Haven, Connecticut 06516, USA. E-mail: zhaozhong.jiang@yale.edu; Tel: +1-203-737-3262
First published on 21st January 2014
Abstract
Polymers bearing amino functional groups are an important class of materials capable of serving as non-viral carriers for DNA delivery to living cells. In this work biodegradable poly(amine-co-ester) terpolymers were synthesized via ring-opening and polycondensation copolymerization of lactone (ε-caprolactone (CL), ω-dodecalactone, ω-pentadecalactone (PDL), and ω-hexadecalactone) with diethyl sebacate (DES) and N-methyldiethanolamine (MDEA) in diphenyl ether, catalyzed by Candida antarctica lipase B (CALB). All lactone-DES-MDEA terpolymers had random distributions of lactone, sebacate, MDEA repeat units in the polymer chains. PDL-DES-MDEA terpolymers were studied in the composition range from 21 to 90 mol% PDL whereas the terpolymers with other lactones were investigated at a single composition (80 mol% lactone). DSC and WAXS analyses showed that all investigated terpolymers crystallize in their respective homopolylactone crystal lattice. Terpolymers with large lactones and a high lactone content melt well above room temperature and are hard solids, whereas terpolymers with small lactones (e.g. CL) or with a low lactone content melt below/around ambient temperature and are waxy/gluey materials. Given the importance of hydrophobicity in influencing gene delivery, water contact angle measurements were carried out on lactone-DES-MDEA terpolymers showing that it is possible to tune the hydrophilic-to-hydrophobic balance by varying polymer composition and size of lactone units. To demonstrate the feasibility of using solid terpolymers as nanocarriers for DNA delivery, PDL-DES-MDEA copolymers with 65–90% PDL were successfully transformed into free-standing nanoparticles with average particle size ranging from 163 to 175 nm. Our preliminary results showed that LucDNA-loaded nanoparticles of the terpolymer with 65% PDL were effective for luciferase gene transfection of HEK293 cells.
1. Introduction
Cationic polymers bearing amino functional groups are an important class of materials capable of serving as non-viral carriers for DNA delivery to living cells, and have been previously employed for various gene transfection applications.1–5 Such polymers include poly(ethyleneimine),6 poly(4-hydroxy-L-proline ester) (PHP),7,8 poly[α-(4-aminobutyl)-L-glycolic acid] (PAGA),9,10 poly(β-amino esters) (PBAE),11,12 poly(amine-co-esters),13 poly(L-lysine),14 chitosan,15 poly(dimethylaminoethyl methacrylate),16 and poly(trimethylaminoethyl methacrylate).17 Among these materials, polyesters containing tertiary amino substituents, such as poly(amine-co-esters) and PBAEs, are particularly attractive due to their biodegradability, low cytotoxicity, and outstanding gene transfection efficacy.18,19Recently, poly(lactone-co-sebacate-co-N-methyldiethyleneamine) terpolymers were synthesized via copolymerization of lactones with diethyl sebacate (DES) and N-methyldiethanolamine (MDEA) using Candida antarctica lipase B (CALB) as the catalyst.20 These poly(amine-co-ester) terpolymers were prepared by Jiang (a coauthor of this article) based on his earlier work showing that CALB efficiently catalyzes copolymerization of lactone with dialkyl diester and diol to form aliphatic polyesters21 and the lipase is also highly tolerant of tertiary amino functional groups during the copolymerization of diester with amino-substituted diol to form poly(amine-co-esters).13 The resultant lactone-DES-MDEA terpolymers are extraordinary non-viral gene vectors capable of delivering therapeutic genes to inhibit tumor growth in mice.20 The physical properties of these poly(amine-co-ester) terpolymers depend on both size and content of the lactone units in the terpolymer. In general, the terpolymers with a large lactone and a high lactone content tend to be solids at ambient temperature, and those with a small lactone and a low lactone content remain as liquids or waxy materials. The liquid lactone-DES-MDEA terpolymers were soluble in polar organic solvents (e.g., DMSO) and were successfully used in aqueous medium to form nanosized polyplexes with DNA via self-assembly by electrostatic interactions between positively charged terpolymers and negatively charged DNA. The formed polyplexes are highly potent in transfecting various types of living cells.20 On the other hand, because of their low solubility in polar organic solvents, the large number of solid lactone-DES-MDEA terpolymers are not capable of forming nanosized polyplexes with DNA using conventional methods, and so far have not been evaluated for gene transfection applications. A possible mean of utilizing the solid terpolymers is to transform the bulk polymer samples to solid nanoparticles for encapsulation and delivery of DNA. For this purpose, it is essential to elucidate and understand the solid state properties of the lactone-DES-MDEA terpolymers. This article reports the results on solid state characterization of the copolymers, including measurement and analysis of the copolymer thermal stability, crystalline properties, and selected mechanical properties. To demonstrate the feasibility of using the solid terpolymers as nanocarriers for DNA delivery, several representative terpolymer samples were transformed to free-standing nanoparticles and gene transfection of living cells by DNA-loaded terpolymer nanoparticles was preliminarily evaluated. Furthermore, the composition-dependent hydrophobicity of the terpolymers was quantitatively investigated by measuring water contact angle on the terpolymer films. Early studies show that, as observed in other cationic polymers,22 hydrophobicity plays a crucial role in influencing the gene transfection efficiency of the poly(amine-co-esters).20 However, quantitative analysis on the terpolymer hydrophobicity has not previously been performed.
2. Experimental section
2.1. Materials
ε-Caprolactone (CL, 99%), 12-dodecalactone (DDL, 98%), ω-pentadecalactone (PDL, 98%), 16-hexadecalactone (HDL, 97%), diethyl sebacate (DES, 98%), N-methyldiethanolamine (MDEA, 99+%), and diphenyl ether (99%) were purchased from Aldrich Chemical Co. and were used as received. Immobilized Candida antarctica lipase B (CALB) supported on acrylic resin or Novozym 435, chloroform (HPLC grade), dichloromethane (99+%), hexane (97+%), methanol (98%), and chloroform-d were also obtained from Aldrich Chemical Co. The lipase catalyst was dried at 50 °C under 2.0 mmHg for 20 h prior to use. Human embryonic kidney 293 (HEK293) cells were obtained from American Type Culture Collection (ATCC) and maintained in DMEM medium (Invitrogen) containing 10% fetal bovine serum, 100 units per mL penicillin and 100 μg mL−1 streptomycin at 37 °C under a 5% CO2 humidified atmosphere.2.2. Instrumental methods
1H and 13C NMR spectra were recorded on a Bruker AVANCE 500 spectrometer. The chemical shifts reported were referenced to internal tetramethylsilane (0.00 ppm) or to the solvent resonance at the appropriate frequency. The number and weight average molecular weights (Mn and Mw, respectively) of polymers were measured by gel permeation chromatography (GPC) using a Waters HPLC system equipped with a model 1515 isocratic pump, a 717 plus autosampler, and a 2414 refractive index (RI) detector with Waters Styragel columns HT6E and HT2 in series. Empower II GPC software was used for running the GPC instrument and for calculations. Both the Styragel columns and the RI detector were heated and maintained at 40 °C temperature during sample analysis. Chloroform was used as the eluent at a flow rate of 1.0 mL min−1. Sample concentrations of 2 mg mL−1 and injection volumes of 100 μL were used. Polymer molecular weights were determined based on a conventional calibration curve generated by narrow polydispersity polystyrene standards from Aldrich Chemical Co. Thermogravimetric measurements were carried out using a TA-TGA 2950 instrument. The analyses were performed at 10 °C min−1 from room temperature to 600 °C under nitrogen flow. The released volatile products were directly transferred to a quadrupole mass spectrometer (Balzers ThermoStar GSD 300T; temperature setting of the interface = 200 °C, mass range = 10–300 amu, CH-tron detector = 1400 V). Differential Scanning Calorimetry (DSC) measurements were performed using a TA DSC-Q100 apparatus, equipped with a Liquid Nitrogen Cooling System (LNCS) accessory in a helium atmosphere. DSC scans were run at 20 °C min−1 from −100 to 150 °C. Controlled cooling at 10 °C min−1 was applied between scans. Crystallization temperature (Tc) and melting temperature (Tm) were taken at the peak maximum of exotherm and endotherm, respectively. In the presence of multiple peaks, the temperature of the main peak was taken as Tc or Tm unless otherwise specified. Wide angle X-ray diffraction measurements (WAXS) were carried out at room temperature with a PANalytical X'Pert PRO diffractometer equipped with an X'Celerator detector (for ultrafast data collection). A Cu anode was used as X-ray source (K radiation: λα = 0.15418 nm, 40 KV, 40 mA) and 1/4° divergence slit was used to collect the data in the 2θ range of 3–60° (step of 0.1°, counting time 60 s per step). An Instron 4465 tensile testing machine (gauge length = 30 mm, crosshead speed = 0.2 mm min−1) was used for the mechanical testing of rectangular specimens (5 mm wide) die-cut from compression-molded sheets (0.2 mm thick). Static water contact angle measurements were performed on samples coated on glass slides by means of a Laurell (WS-650-23NPP) spin coater under ambient conditions. The contact angle experiments were performed with an optical contact angle and surface tension meter KSV's CAM 100 (KSV, Espoo, Finland). Milli-Q water was used for measurements. The water drop profile images were collected every 1 s in a time range of 0–60 s. Optical contact angle and pendant drop surface tension software was used for image processing. A minimum of five drops per sample were analyzed. Morphology and size of nanoparticles were analyzed using a XL30 ESEM scanning electron microscope (FEI Company). The image-analysis application program ImageJ was used to measure particle diameters, to calculate average particle sizes, and to determine particle size distributions.2.3. Fabrication of nanoparticles from PDL-DES-MDEA terpolymers
Nanoparticles were prepared from three PDL-DES-MDEA terpolymers with 65%, 82% and 90% PDL using a single-emulsion solvent evaporation technique. Each polymer (50 mg) was dissolved in 2 mL of dichloromethane and the resultant polymer solution was added dropwise to 4 mL of 2.5% poly(vinyl alcohol) (PVA, molecular weight = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–70
000–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, 87–89% hydrolyzed) aqueous solution. The mixture was then sonicated three times (10 seconds each time) at 38% amplitude using a 600 Watts Ace Glass GEX 600-5 Ultrasonic Processor to yield an emulsion. This emulsion was quickly poured into 100 mL of aqueous solution containing 0.3% PVA and the whole mixture was stirred in an open beaker at room temperature for 4 h. This process allows gradual evaporation of the dichloromethane to form nanoparticles. Subsequently, the mixture containing nanoparticles was centrifuged at 3000 rpm for 15 min to remove the fraction of large particles. The remaining supernatant was subjected to a second centrifugation process at 11
000 Da, 87–89% hydrolyzed) aqueous solution. The mixture was then sonicated three times (10 seconds each time) at 38% amplitude using a 600 Watts Ace Glass GEX 600-5 Ultrasonic Processor to yield an emulsion. This emulsion was quickly poured into 100 mL of aqueous solution containing 0.3% PVA and the whole mixture was stirred in an open beaker at room temperature for 4 h. This process allows gradual evaporation of the dichloromethane to form nanoparticles. Subsequently, the mixture containing nanoparticles was centrifuged at 3000 rpm for 15 min to remove the fraction of large particles. The remaining supernatant was subjected to a second centrifugation process at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 hour to cause precipitation of the nanoparticles with desirable sizes. The precipitated particles were then re-suspended in 5 mL of water, frozen in liquid nitrogen and dried by lyophilization for 2 days.
000 rpm for 1 hour to cause precipitation of the nanoparticles with desirable sizes. The precipitated particles were then re-suspended in 5 mL of water, frozen in liquid nitrogen and dried by lyophilization for 2 days.
2.4. Preparation of DNA-loaded nanoparticles
Nanoparticles encapsulating DNA were fabricated by a double emulsion solvent evaporation method. Poly(amine-co-ester) terpolymer was dissolved in dichloromethane at 50 mg mL−1. Plasmid pGL4.13 encoding firefly luciferase (LucDNA, Promega) was dissolved in Tris-EDTA buffer and then added dropwise under votex to the polymer solution at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 polymer/DNA weight ratio. The resultant mixture was sonicated immediately with a probe sonicator (Ace Glass GEX 600-5 Ultrasonic Processor). After sonication, the formed emulsion was added dropwise to 5% (w/v) poly(vinyl alcohol) (PVA) aqueous solution under vortex and subjected to sonication again. Subsequently, the emulsion was poured into a beaker containing 0.3% (w/v) PVA aqueous solution and the whole mixture was stirred overnight to evaporate dichloromethane. Nanoparticles were collected by centrifugation at 13
1 polymer/DNA weight ratio. The resultant mixture was sonicated immediately with a probe sonicator (Ace Glass GEX 600-5 Ultrasonic Processor). After sonication, the formed emulsion was added dropwise to 5% (w/v) poly(vinyl alcohol) (PVA) aqueous solution under vortex and subjected to sonication again. Subsequently, the emulsion was poured into a beaker containing 0.3% (w/v) PVA aqueous solution and the whole mixture was stirred overnight to evaporate dichloromethane. Nanoparticles were collected by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and then washed with water three times. The obtained nanoparticles were resuspended in water and an equal mass of Trehalose was added. The particles were then frozen at −80 °C overnight and dried on a lyophilizer for 72 hours. Blank nanoparticles were fabricated without DNA following similar procedures.
000 rpm for 15 min and then washed with water three times. The obtained nanoparticles were resuspended in water and an equal mass of Trehalose was added. The particles were then frozen at −80 °C overnight and dried on a lyophilizer for 72 hours. Blank nanoparticles were fabricated without DNA following similar procedures.
2.5. Analysis of DNA loading in the nanoparticles
DNA-loaded terpolymer nanoparticles were dissolved in dichloromethane for 2 hours. Subsequently, an equal volume of Tris-EDTA/heparin aqueous solution (approximately 50 units heparin per μg DNA) was added. The resultant mixture was shaked for 24 h to extract the DNA into the aqueous phase. The organic and aqueous phases were then separated by vortexing and centrifugation. This DNA extraction process was repeated and the aqueous fractions were combined. The amount of DNA loading was determined using Quant-it PicoGreen Kit (Invirogen) according to the manufacturer instructions.2.6. Quantification of in vitro luciferase transfection
HEK293 cells were seeded in a 24-well plate at a density of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in 500 μL of culture medium and were kept at 37 °C in 5% CO2 atmosphere over night. LucDNA-loaded nanoparticles suspended in the cell culture medium were added to each well at 0.5 μg DNA per well and were cultured with cells for 48 h. At the end of the incubation period, the cells were lysed with the reporter lysis buffer (Promega) and cell lysate was collected. Luciferase expression activity was measured as the fluorescence intensity (in terms of relative light units or RLU) using luciferase assay reagent (Promega) on a GloMax20/20 spectrophotometer according to the standard protocol. The protein content of the cell lysate was measured using BCA protein assay (Pierce) and gene transfection efficiency was calculated as relative light units (RLU) per mg of protein. Transfection by Lipofectamine 2000 was carried out according to the manufacturer's protocol.
000 cells per well in 500 μL of culture medium and were kept at 37 °C in 5% CO2 atmosphere over night. LucDNA-loaded nanoparticles suspended in the cell culture medium were added to each well at 0.5 μg DNA per well and were cultured with cells for 48 h. At the end of the incubation period, the cells were lysed with the reporter lysis buffer (Promega) and cell lysate was collected. Luciferase expression activity was measured as the fluorescence intensity (in terms of relative light units or RLU) using luciferase assay reagent (Promega) on a GloMax20/20 spectrophotometer according to the standard protocol. The protein content of the cell lysate was measured using BCA protein assay (Pierce) and gene transfection efficiency was calculated as relative light units (RLU) per mg of protein. Transfection by Lipofectamine 2000 was carried out according to the manufacturer's protocol.
3. Results and discussion
The poly(amine-co-ester) terpolymers were synthesized and purified according to the procedures reported in a previous publication.20 The synthesis of the copolymers was catalyzed by Candida antarctica lipase B (CALB) and was accomplished via ring-opening and polycondensation copolymerization of lactone with diethyl sebacate (DES) and N-methyldiethanolamine (MDEA) in diphenyl ether. All copolymerization reactions were performed in two stages: first stage oligomerization under 1 atm pressure of nitrogen gas followed by second stage polymerization under high vacuum (2.0 mmHg). In this work, four different lactones were employed to prepare the lactone-DES-MDEA terpolymers for measurements of the copolymer solid state properties, which include ε-caprolactone (CL), 12-dodecalactone (DDL), ω-pentadecalactone (PDL), and 16-hexadecalactone (HDL). Scheme 1 illustrates general reaction conditions to obtain these copolymers.All purified terpolymers were analyzed by GPC to measure their molecular weights, and by 1H and 13C NMR spectroscopy to determine their structure and composition. The characterization data on PDL-DES-MDEA (P), CL-DES-MDEA (C), DDL-DES-MDEA (D), and HDL-DES-MDEA (H) terpolymers are shown in Table 1. As reported previously,20 all terpolymers had random distributions of lactone, sebacate, MDEA repeat units in the copolymer chains.
| Polymera | Lactone/DES/MDEA (feed molar ratio) | Lactone content (mol%)b | Mw | Mw/Mn |
|---|---|---|---|---|
| a Sample names P-x, C-x, D-x, and H-x represent PDL-DES-MDEA, CL-DES-MDEA, DDL-DES-MDEA, and HDL-DES-MDEA terpolymers with x mol% lactone units vs. (lactone + sebacate) units, respectively.b Molar percentage of lactone units vs. (lactone + sebacate) units in the polymer chains.c Homopolymer PPDL or poly(PDL) was prepared via ring-opening polymerization of PDL in diphenyl ether (200 wt% vs. monomer) at 80 °C for 24 h using 10 wt% Novozym 435 catalyst. The formed polymer was isolated via re-precipitation in CHCl3/CH3OH mixture. | ||||
| P-21 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
21 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.7 |
| P-35 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
35 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.8 |
| P-52 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
52 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.8 |
| P-65 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
65 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.0 |
| P-82 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
82 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.2 |
| P-90 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
90 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.1 |
| PPDLc | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.9 |
| C-80 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
80 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.0 |
| D-80 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
80 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.9 |
| H-80 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
80 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2.0 |
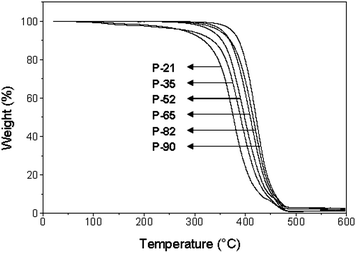
Fig. 1 shows the thermogravimetric curves of the PDL-DES-MDEA terpolymers with various PDL unit contents. It is observed that the thermal stability of the terpolymers increases with increasing PDL content in the polymer chains in agreement with the results obtained previously on other PDL-containing copolyesters.23,24 Unlike the PDL-rich copolymers that show minimal weight loss (≤0.2%) from room temperature to 200 °C, those with a low PDL content (e.g., P-21 and P-35) exhibit gradual weight loss over the whole T range up to the main degradation step, with Δm at 200 °C = 1.9–2.5%. Table 2 collects Tmax (temperature at the maximum weight loss rate) of the PDL-DES-MDEA samples. The Tmax increases by approximately 50 °C upon changing of the PDL content from 21 to 90 mol%. During the TGA measurements, the volatiles released from two representative terpolymers, P-21 and P-90, were analysed by Mass Spectrometry. No major differences were observed on the masses of the fragments from the two samples except a clear increment of masses 14 (N) and 18 (H2O) in the main degradation step of P-21 due to its high content of hydrophilic nitrogen-containing MDEA repeat units.
| Samplea | DSC | WAXS | TGA | ||||
|---|---|---|---|---|---|---|---|
| Tcb (°C) | ΔHcb (J g−1) | Tmc (°C) | ΔHmc (J g−1) | χc (%)d | Δm at 200 °C | Tmaxe (°C) | |
| a See Table 1 for the meaning of the abbreviated sample names.b Cooling run at 10 °C min−1.c Subsequent heating run at 20 °C min−1.d Crystallinity degree.e Temperature of the maximum weight loss rate.f Temperature of the higher-T peak in the multiple endotherm. | |||||||
| P-21 | 18 | 46 | 26f | 49 | 3 | 1.9 | 376 |
| P-35 | 30 | 64 | 36 | 65 | 18 | 2.5 | 379 |
| P-52 | 42 | 79 | 52 | 79 | 39 | 0.2 | 397 |
| P-65 | 54 | 99 | 63 | 99 | 49 | 0.2 | 410 |
| P-82 | 64 | 116 | 76 | 117 | 57 | 0.1 | 418 |
| P-90 | 73 | 134 | 85 | 134 | 63 | 0.1 | 422 |
| PPDL | 79 | 159 | 90 | 166 | 72 | 0.1 | 422 |
| C-80 | −8 | 55 | 18f | 55 | 4 | 2.4 | 398 |
| D-80 | 48 | 105 | 60 | 105 | 55 | 0.2 | 414 |
| H-80 | 63 | 120 | 74 | 120 | 58 | 0.1 | 420 |
The DDL-DES-MDEA terpolymer with 80% DDL (D-80) and HDL-DES-MDEA terpolymer with 80% HDL (H-80) displayed TGA curves (not shown) very similar to that of their PDL analogue P-82. On the other hand, CL-DES-MDEA terpolymer with 80% CL (C-80) exhibited a TGA curve (not shown) comparable to that of P-35 shown in Fig. 1. The main degradation step of C-80 occurs at a lower Tmax value compared to the values of the other terpolymers with analogous compositions (Table 2). Therefore, at a given lactone content, the terpolymers bearing CL units have lower thermal stability than the terpolymers with larger lactone units (i.e., DDL, PDL, and HDL). Nevertheless, the thermal stability of all terpolymers is well above that required to allow fabrication of medical devices from the polymers.
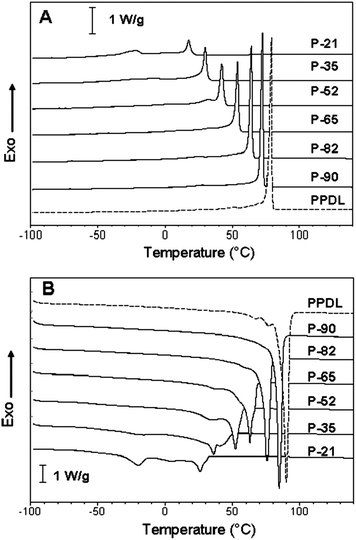
During the course of DSC analysis, PDL-DES-MDEA terpolymers were subjected to the same thermal treatment (heating to 150 °C followed by controlled cooling to −100 °C) to ensure that they possess the same thermal history prior to study of their calorimetric properties. The DSC curves of the copolymers obtained in a controlled cooling run at 10 °C min−1 from melt and in a subsequent heating scan at 20 °C min−1 are displayed in Fig. 2.
In the cooling runs (Fig. 2A), the terpolymers exhibit a composition-dependent exothermal phenomenon that starts with a very neat peak (Tc in Table 2), reminiscent of that of typical poly(PDL) (PPDL) homopolymer, followed by further exothermal events occurring at lower temperatures over a broad T-range. Upon re-heating (Fig. 2B), composition-dependent quite complex endothermal processes are observed in the DSC curves. The prominent peak of the endothermal phenomena (Tm in Table 2) gradually shifts to higher temperature with increasing PDL content in the terpolymer. Although the PDL-DES-MDEA terpolymers melt over a wide range of temperatures (26–85 °C), a majority of the copolymers (e.g., from P-52 to P-90) possess a sufficiently high Tm (≥52 °C) to remain as solids at the physiological temperature (37 °C). Table 2 reports the enthalpy values associated with the whole crystallization and melting processes observed in Fig. 2A and B. Both ΔHc and ΔHm values increase when the PDL content of PDL-DES-MDEA terpolymers increases.
In order to investigate if the crystal phase in the terpolymers is associated with PPDL-type crystals, WAXS analysis was performed and the results are shown in Fig. 3. The diffraction profile of all PDL-DES-MDEA terpolymers is characterized by two reflections centered at 2θ of approximately 21.2° and 23.7°, which are at the same positions of the (110) and (200) reflections of homopolymer PPDL.25 This is observable even for the poorly crystalline terpolymer P-21 with a very modest diffraction intensity. In the copolymers with PDL content ≥35 mol%, the other typical reflections of the PPDL lattice at higher 2θ values25 are also clearly detected. This result suggests that the crystal phase which is formed and melts during the DSC measurements (see Fig. 2) is composed of PPDL-type crystals. The high tendency of the terpolymers to form crystalline domains should enhance the stability of nanoparticles made from the polymers for effective gene or drug delivery. The trend of decreasing degree of crystallinity for the samples from P-90 to P-21 is shown by the enthalpy values in Table 2, and is confirmed by the WAXS diffractograms (see the values of crystallinity degree χc also in Table 2). In parallel, the amorphous phase scattering contribution to the X-ray profiles significantly increases with decreasing PDL content, being very relevant in P-35 and forming almost the whole scatter in P-21 (Fig. 3). For P-21, the apparent discrepancy between the near-zero χc value from WAXS and the significant crystallization and melting enthalpy values from DSC (Table 2) is ascribable to the fact that a large part of the melting endotherm of this terpolymer lays below ambient temperature (Fig. 2). Hence, X-ray diffractograms collected at room temperature cannot reveal such a crystal phase.
 | ||
| Fig. 3 WAXS diffractograms of PDL-DES-MDEA terpolymers with various PDL unit contents. The insert shows a magnification of the low-2θ range. | ||
The insert in Fig. 3 shows a magnification of the low-angle part of the WAXS profiles. All PDL-DES-MDEA terpolymers except P-21 show the typical low-angle (001) reflection of PPDL, which is associated with periodicity along the chain axis.25 Earlier work showed that in random copolymers, the PDL monomer units have an exceptional ability to promote co-crystallization of a number of co-monomeric units in the PPDL crystal lattice. As the result, the degree of crystallinity in the copolymers was high over the whole copolymer composition range,23–28 and the chain periodicity responsible for the (001) reflection of the copolymers was altered. This alteration in the chain periodicity due to co-crystallization changed the location and intensity of the PPDL (001) reflection. In the present case, the invariance of the (001) reflection positions indicates that no foreign repeating units enter the crystal phase in PDL-DES-MDEA terpolymers.
The DSC curves of the lactone-DES-MDEA terpolymers containing approximately 80 mol% of different lactones (CL, DDL, PDL and HDL) are displayed in Fig. 4. The DSC analyses include both cooling runs from the melt at 10 °C min−1 (Fig. 4A) and subsequent heating scans at 20 °C min−1 (Fig. 4B). The crystallization and melting phenomena shown in Fig. 4A and B take place at temperatures that decrease with decreasing carbon numbers in the lactone units of the terpolymers (see Tc and Tm values in Table 2). The crystallization and melting of the PDL-DES-MDEA terpolymer occurs at slightly higher temperatures vs. those of the HDL-DES-MDEA terpolymer possibly due to the slightly higher lactone content in the former (82% in P-82 vs. 80% in H-80). The melting and crystallization enthalpies decrease with decreasing lactone size, showing a drop of crystallinity degree especially in C-80 (Table 2).
 | ||
| Fig. 4 DSC curves of lactone-DES-MDEA terpolymers with different lactone units: (A) cooling run at 10 °C min−1, (B) subsequent heating run at 20 °C min−1. | ||
Fig. 5 collects the WAXS diffractograms of terpolymers C-80, D-80, P-82 and H-80. The four terpolymers show a diffraction profile characterized by two major reflections at 2θ around 21° and 23.5°, although C-80 is mostly amorphous and shows very weak reflections at these 2θ values. For terpolymer C-80, a low crystallinity degree by WAXS measurements at ambient temperature is reasonably expected since DSC analysis reveals that the C-80 crystal phase melts around room temperature (Fig. 4).
 | ||
| Fig. 5 WAXS diffractograms of lactone-DES-MDEA terpolymers with different lactone units. The insert shows a magnification of the low-2θ range. | ||
The diffractograms of D-80 and H-80 resemble the already discussed P-82 profile and suggest that the crystal phase that develops is polyethylene-like, as earlier reported for the corresponding homopolymers including PCL.27,29,30 In addition to the major reflections, a small-angle reflection is also shown by terpolymers D-80, P-82, and H-80. The 2θ angle of this reflection, which appears at 5.6°, 4.8° and 4.5° for D-80, P-82 and H-80 respectively (see insert in Fig. 5), decreases with increasing chain length of the lactone repeating unit. This observation supports the earlier discussed attribution of the low-angle reflection in PDL-DES-MDEA terpolymers to the repeat length along the chain axis of PPDL-like crystals. With increasing lactone size in the terpolymers, the reflection is expected to shift to a lower 2θ angle. Such a reflection is not shown by C-80, in agreement with its absence in the X-ray profile of the poly(ε-caprolactone) homopolymer.
Visual inspection of the PDL-DES-MDEA terpolymers clearly evidences remarkable differences between the terpolymers with a high PDL content (≥52%) which are hard solids, and those with a low PDL content which are waxy (P-35) or gluey (P-21) at room temperature. The physical appearance of the other lactone-DES-MDEA terpolymers, all containing approximately 80% lactone, depends on the lactone size. The large lactone samples D-80 and H-80 are hard solids, but the small lactone sample C-80 is gluey.
For those lactone-DES-MDEA terpolymers that can be transformed via hot pressing into suitable films for tensile stress–strain tests, their mechanical behavior was investigated. The stress–strain curves of the copolymer samples P-65, P-82, D-80, and H-80 are shown in ESI section (Fig. 1S†). The average values of tensile modulus (E), stress at break (σ) and strain at break (ε) measured for these samples are reported in Table 3. The results show that the terpolymers, all of them with a crystallinity degree over 50%, possess a very modest elongation at break. They can be classified as rather fragile materials. The tensile modulus is in the range of 300–500 MPa. The softer terpolymers P-52, P-35, P-21 and C-80 are not suitable for tensile stress–strain tests. It needs to be noted that high mechanical strength is not a requisite for polymeric nanoparticles to serve as gene or drug carriers.
| Sample | E (MPa) | σ (MPa) | ε (%) |
|---|---|---|---|
| P-65 | 277 (±27) | 2.99 (±0.66) | 1.89 (±0.53) |
| P-82 | 488 (±68) | 3.00 (±0.84) | 1.07 (±0.55) |
| D-80 | 295 (±29) | 3.40 (±0.43) | 2.19 (±0.40) |
| H-80 | 345 (±51) | 5.91 (±0.85) | 4.24 (±0.72) |
As reported previously, the gene transfection efficiency of lactone-DES-MDEA terpolymers was substantially dependent on lactone content in the polymers. For example, PDL-DES-MDEA copolymer with 20% PDL was 6 times more efficient than the copolymer with 10% PDL in transfecting A549 cells.20 This enhanced gene transfection efficiency is assumably attributed to the higher hydrophobicity of the former polymer, which increases the stability of its polyplex with DNA in aqueous medium and facilitates the cellular uptake of the polyplex nanoparticles. Thus, it is important to quantitatively measure the degree of hydrophobicity of lactone-DES-MDEA terpolymers in order to better understand the influence of their structure on gene transfection efficacy. To determine the effects of structure and composition on hydrophobicity of the copolymers, water contact angle measurements were carried out on different lactone-DES-MDEA terpolymers spin-coated on glass slides. Fig. 6 shows the evolution of the water drop contact angle (θ) on terpolymer coatings over a period of 60 seconds. The θ values measured 30 seconds after contact of the water drop with surface of the investigated terpolymers are shown in ESI section (Table 1S†).
The water contact angle vs. time behavior of PDL-DES-MDEA terpolymers (Fig. 6a) remarkably changes with composition. It is almost constant when lactone content is ≥52 mol%, while a clear decrease of contact angle appears in MDEA-sebacate rich terpolymers (P-35 and P-21). Fig. 6a shows three representative drops 30 seconds after contact with the surface of P-90, P-52 and P-21. With increasing amount of the hydrophilic MDEA-sebacate moiety, the contact angle significantly decreases. Water contact angle results on lactone-DES-MDEA terpolymer with a high content (ca. 80 mol%) of different lactones are compared in Fig. 6b. Terpolymers with large lactones (H-80 and D-80) behave similarly as the already discussed P-82. The contact angle value increases in the order D-80 < H-80 < P-82, reflecting the effects of both lactone size and content on the overall hydrophobicity.
A case on its own is represented by C-80 whose water contact angle abruptly decreases during the initial 10–15 seconds after contact between drop and surface, indicating a strong water–polymer interaction notwithstanding the high lactone content. This behavior confirms the strong effect of lactone size on the overall hydrophilicity of the terpolymers, a small lactone (i.e. CL) contributing much less to enhance hydrophobicity than large ones (i.e. D, P, H).
The results of the water contact angle analysis confirm the earlier attribution of the TGA weight losses between RT and 200 °C (Table 2) to loss of moisture absorbed by the most hydrophilic terpolymers investigated (highest weight loss for P-21, P-35 and C-80).
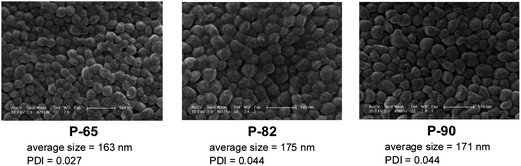
To demonstrate the feasibility of using solid terpolymers as non-viral vectors for gene delivery, PDL-DES-MDEA terpolymers with 65%, 82%, and 90% PDL were transformed into nanoparticles. SEM images of all three samples showed the particles being nearly spherical in shape with average particle size in the range between 160 nm and 180 nm and narrow particle size distribution (Fig. 7). P-65 nanoparticle sample was also loaded with LucDNA, which contained 1.47 μg DNA per mg of sample. The LucDNA-loaded P-65 nanoparticles exhibited luciferase transfection level of 2.3 × 107 RLU per mg-protein in HEK293 cells. For comparison, control experiments using blank P-65 nanoparticles under similar conditions yielded minimal transfection signal (<3 × 104 RLU per mg-protein) and LucDNA-loaded Lipofectamine 2000 (commercial) produced luciferase transfection level of 8.2 × 1010 RLU per mg-protein.
The possibility to fabricate nanoparticles from the hydrophobic terpolymers and use solid terpolymer nanoparticles for DNA delivery to transfect living cells is therefore demonstrated. This result is of interest because, in contrast to soft DNA/poly(amine-co-ester) polyplex particles,20 the solid nanoparticles are advantageous in that their surfaces can be readily modified to enhance the in vivo gene transfection efficacy of the particles (e.g., by incorporating targeting peptides for enhanced cellular uptake and/or by incorporating poly(ethylene glycol) chains for prolonged in vivo circulation).
4. Conclusions
The solid-state properties of lactone-DES-MDEA terpolymers are substantially dependent on lactone size and content. Thermal stability of the PDL-DES-MDEA terpolymers increases with increasing lactone content as the main degradation temperature shifts from a minimum of 376 °C in P-21 to a maximum of 422 °C in P-90. All terpolymers investigated develop a crystal phase upon cooling from the melt. Terpolymers with large lactones and a high lactone content develop crystals that melt well above room temperature whereas terpolymers with small lactones (e.g. CL) or with a low lactone content (e.g. P-21) possess a crystal phase that melts below/around RT. Consequentially, the physical aspect of lactone-DES-MDEA terpolymers at room temperature varies from hard solids to waxy/gluey materials. In all cases, the crystallization processes primarily involve the lactone units to form the lattice of homopolylactone. Terpolymers capable of forming suitable films for mechanical testing behave as rather fragile materials under tensile deformation with strain at break of a few percent and rigidity dependent on polymer composition and crystallinity degree. The ratio of sebacate-MDEA to lactone units strongly influences the hydrophilic/hydrophobic balance in the polymers. In terpolymers with large lactones such as PDL-DES-MDEA copolymers, the hydrophobic character prevails when lactone is the major component (>50 mol%). Conversely, terpolymers with small lactones are hydrophilic even at lactone content as high as 80 mol% (e.g. C-80). To evaluate the feasibility of using solid terpolymers as nanocarriers for DNA delivery, free-standing nanoparticles were fabricated from PDL-DES-MDEA copolymers with 65–90% PDL. Gene transfection of living cells was realized using LucDNA-loaded P-65 nanoparticles. The current results are expected to facilitate our understanding on how the solid state properties of these terpolymers influence their gene transfection efficiency.Acknowledgements
The authors wish to thank Yale University, the National Institutes of Health (R01 CA149128) and MIUR (Italian Ministry for University and Research) for the financial support of this work.References
- H. D. Martimprey, C. Vauthier, C. Malvy and P. Couvreur, Eur. J. Pharm. Biopharm., 2009, 71, 490 CrossRef PubMed.
- D. W. Pack, A. S. Hoffman, S. Pun and P. S. Stayton, Nat. Rev. Drug Discovery, 2005, 4, 581 CrossRef CAS PubMed.
- M. S. Al-Dosari and X. Gao, AAPS J., 2009, 11, 671 CrossRef CAS PubMed.
- C. Tros de Ilarduya, Y. Sun and N. Duzgunes, Eur. J. Pharm. Sci., 2010, 40, 159 CrossRef CAS PubMed.
- H. Lv, S. Zhang, B. Wang, S. Cui and J. Yan, J. Controlled Release, 2006, 114, 100 CrossRef CAS PubMed.
- W. T. Gogbey, K. K. Wu and A. G. Mikos, J. Controlled Release, 1999, 60, 149 CrossRef.
- Y.-B. Lim, Y. H. Choi and J.-S. Park, J. Am. Chem. Soc., 1999, 121, 5633 CrossRef CAS.
- D. Putnam and R. Langer, Macromolecules, 1999, 32, 3658 CrossRef CAS.
- Y.-B. Lim, C.-H. Kim, K. Kim, S. W. Kim and J.-S. Park, J. Am. Chem. Soc., 2000, 122, 6524 CrossRef CAS.
- Y.-B. Lim, S. O. Han, H. U. Kong, Y. Lee, J.-S. Park, B. Jeong and S. W. Kim, Pharm. Res., 2000, 17, 811 CrossRef CAS.
- D. M. Lynn and R. Langer, J. Am. Chem. Soc., 2000, 122, 10761 CrossRef CAS.
- D. M. Lynn, D. G. Anderson, D. Putnam and R. Langer, J. Am. Chem. Soc., 2001, 123, 8155 CrossRef CAS.
- Z. Jiang, Biomacromolecules, 2010, 11, 1089 CrossRef CAS PubMed.
- J. P. Leonetti, B. Raynerb, M. Lemaitre, C. Gagnor, P. G. Milhaud, J. L. Imbach and B. Lebleu, Gene, 1988, 72(1–2), 323 CrossRef CAS.
- M. Koping-Hoggard, I. Tubulekas, H. Guan, K. Edwards, M. Nilsson, K. M. Varum and P. Artursson, Gene Ther., 2001, 8, 1108 CrossRef CAS PubMed.
- Y. K. Valeeva, et al., J. Drug Delivery Sci. Technol., 2006, 16(4), 245 CAS.
- M. L. Read, P. R. Dash, A. Clark, K. A. Howard, D. Oupicky, V. Toncheva, H. O. Alpar, E. H. Schacht, K. Ulbrich and L. W. Seymour, Eur. J. Pharm. Sci., 2000, 10, 169 CrossRef CAS.
- J. J. Green, R. Langer and D. G. Anderson, Acc. Chem. Res., 2008, 41, 749 CrossRef CAS PubMed.
- J. Liu, Z. Jiang, J. Zhou, S. Zhang and W. M. Saltzman, J. Biomed. Mater. Res., Part A, 2011, 96, 456 Search PubMed.
- J. Zhou, J. Liu, C. J. Cheng, T. R. Patel, C. E. Weller, J. M. Piepmeier, Z. Jiang and W. M. Saltzman, Nat. Mater., 2012, 11, 82 CrossRef CAS PubMed.
- Z. Jiang, Biomacromolecules, 2008, 9, 3246 CrossRef CAS PubMed.
- Z. Liu, Z. Zhang, C. Zhou and Y. Jiao, Prog. Polym. Sci., 2010, 35, 1144 CrossRef CAS PubMed.
- L. Mazzocchetti, M. Scandola and Z. Jiang, Macromolecules, 2009, 42, 7811 CrossRef CAS.
- Z. Jiang, H. Azim, R. A. Gross, M. L. Focarete and M. Scandola, Biomacromolecules, 2007, 8, 2262 CrossRef CAS PubMed.
- M. Gazzano, V. Malta, M. L. Focarete, M. Scandola and R. A. Gross, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 1009 CrossRef CAS.
- L. Mazzocchetti, M. Scandola and Z. Jiang, Eur. Polym. J., 2011, 47, 942 CrossRef CAS PubMed.
- G. Ceccorulli, M. Scandola, A. Kumar, B. Kalra and R. A. Gross, Biomacromolecules, 2005, 6, 902 CrossRef CAS PubMed.
- M. L. Focarete, M. Gazzano, M. Scandola, A. Kumar and R. A. Gross, Macromolecules, 2002, 35, 8066 CrossRef CAS.
- H. Bittiger, R. H. Marchessault and W. D. Niegisch, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 1923 CrossRef CAS.
- C. Liu, F. Liu, J. Cai, W. Xie, T. E. Long, S. R. Turner, A. Lyons and R. A. Gross, Biomacromolecules, 2011, 12, 3291 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46918b |
| This journal is © The Royal Society of Chemistry 2014 |