Off–on BODIPY-based chemosensors for selective detection of Al3+ and Cr3+ versus Fe3+ in aqueous media†
Andrea Barba-Bonac,
Laura Calabuigb,
Ana M. Costero*ab,
Salvador Gilab,
Ramón Martínez-Máñez*ac and
Félix Sancenónac
aCentro de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Unidad Mixta Universidad Politécnica de Valencia-Universidad de Valencia, Spain
bDepartamento de Química Orgánica, Universitat de València, Dr Moliner 50, 46100, Burjassot, Valencia, Spain. E-mail: ana.costero@uv.es
cDepartamento de Química, Universidad Politécnica de Valencia, Camino de Vera s/n, 46022, Valencia, Spain. E-mail: rmaez@qim.upv.es
First published on 20th January 2014
Abstract
Two new off–on BODIPY-based chemosensors that are highly sensitive for trivalent cations in aqueous solutions are described. Compound 2 exhibits selective sensing of Al3+ and Cr3+ versus Fe3+ through two different channels (UV-vis and fluorescence).
The design of new probes for transition and p-block metal cations is an important subject within the field of supramolecular chemistry because of their impact on the environment and human health. In this area, although a number of chemosensors for divalent transition metal cations have been described, there are a relatively small number of probes able to selectively respond to triple-charged metal cations1 and even less in aqueous environments.2 However, trivalent cations have important properties and play significant roles in different fields. For instance chromium is an essential element in human nutrition and has a huge impact on the metabolism of carbohydrates, fats, proteins and nucleic acids and has been reported to disturb glucose levels and lipid metabolism.3 On the other hand Fe3+ also plays a key role in many biochemical processes at the cellular level and it is indispensable for most organisms, and both its deficiency and overload can induce various disorders.4,5 Finally, it is well-known that Al3+ plays important roles in cells and environmental food chains and for instance was found to kill fishes in acidified water and cause damages to the central nerve system of human beings.6 Therefore, the design of new probes for the simple and easy detection of these metal cations in a number of different situation is of much interest.
Based in these concepts, and bearing in mind our interest in the design of chemosensors, we report herein two new probes (1 and 2, see Scheme 1) based in dipyrromethene boron difluoride (BODIPY) scaffold, for a simple optical detection of trivalent cations. BODIPY dyes are a class of well-known fluorophores with widespread applications as fluorescent probes due to their valuable characteristics, such as high molar absorption coefficients and high quantum yields leading to intense absorption and fluorescence bands.7 In this context, although a large number of BODIPY dyes have been designed and prepared for detecting metal cations,8 very few examples display sensing features in aqueous solutions.9
Probe 1 was synthesized through a bicondensation of N-methyl-N-(2-hydroxyethyl)-4-aminobenzaldehyde and 2,4-dimethylpyrrole in the presence of trifluoroacetic acid (TFA) as catalyst,10 followed by oxidation with p-chloranil. The boron difluoride bridge was introduced by treatment with boron trifluoride diethyl etherate (BF![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) Et2O) in the presence of triethylamine (TEA). For the synthesis of probe 2, the BODIPY derivative I was prepared from 2,4-dimethylpyrrole and benzaldehyde (see ESI†) following the same procedure as above.10 Condensation of I and N-methyl-N-(2-hydroxyethyl)-4-aminobenzaldehyde in benzene in presence of acetic acid and piperidine7b,11 yielded 2.
Et2O) in the presence of triethylamine (TEA). For the synthesis of probe 2, the BODIPY derivative I was prepared from 2,4-dimethylpyrrole and benzaldehyde (see ESI†) following the same procedure as above.10 Condensation of I and N-methyl-N-(2-hydroxyethyl)-4-aminobenzaldehyde in benzene in presence of acetic acid and piperidine7b,11 yielded 2.
Probe 1 shows in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (80
CH3CN (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) an intense absorption band at 490 nm (ε = 77600 cm−1 M−1) yet it is scarcely fluorescent (Φ = 0.002 using aqueous fluorescein as reference).12 This low emission is tentatively attributed to a photo-electron transfer (PET) from the lone pair of the amino group to the photo-excited BODIPY group.13 Fig. 1 shows the fluorescence spectrum (λexc = 480 nm) of 1 alone and in the presence of 1 equiv. of different metal cations. Addition of Fe2+, Cu2+, Zn2+, Cd2+, Co2+, Ni2+, Li+, Hg2+ and Ru3+ did not modify the emission of 1, whereas trivalent cations Al3+, Fe3+ and Cr3+ led to a very remarkable enhancement of the fluorescence emission (ΦAl = 0.29; ΦFe = 0.17; ΦCr = 0.24) at 515 nm.
20 v/v) an intense absorption band at 490 nm (ε = 77600 cm−1 M−1) yet it is scarcely fluorescent (Φ = 0.002 using aqueous fluorescein as reference).12 This low emission is tentatively attributed to a photo-electron transfer (PET) from the lone pair of the amino group to the photo-excited BODIPY group.13 Fig. 1 shows the fluorescence spectrum (λexc = 480 nm) of 1 alone and in the presence of 1 equiv. of different metal cations. Addition of Fe2+, Cu2+, Zn2+, Cd2+, Co2+, Ni2+, Li+, Hg2+ and Ru3+ did not modify the emission of 1, whereas trivalent cations Al3+, Fe3+ and Cr3+ led to a very remarkable enhancement of the fluorescence emission (ΦAl = 0.29; ΦFe = 0.17; ΦCr = 0.24) at 515 nm.
Moreover, no colour modulations in the presence of metal cations were found for 1. This was an expected result bearing in mind the presence of methyl groups in the pyrrole units that most likely impose a twist position of the phenyl ring that interrupts the conjugation between the N-methyl-N-(2-hydroxyethyl) coordination site and the signaling unit.14
In contrast, the signaling unit and binding site in probe 2 are electronically connected and therefore changes both in colour and emission were found (vide infra). In water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (40
CH3CN (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v), 2 exhibited a strong absorbance with a maximum at 603 nm (ε = 38400 M-1 cm−1). This band is bathochromically shifted by ca. 100 nm when compared with the parent BODIPY fluorophore due to the styryl extension at the α-position. Moreover probe 2 was poorly fluorescent (λexc = 530 nm, Φ = 0.007) most likely due to an efficient ICT quenching of the excited state of the BODIPY-chromophore from the electron-donating amino moiety.
60 v/v), 2 exhibited a strong absorbance with a maximum at 603 nm (ε = 38400 M-1 cm−1). This band is bathochromically shifted by ca. 100 nm when compared with the parent BODIPY fluorophore due to the styryl extension at the α-position. Moreover probe 2 was poorly fluorescent (λexc = 530 nm, Φ = 0.007) most likely due to an efficient ICT quenching of the excited state of the BODIPY-chromophore from the electron-donating amino moiety.
Addition of Fe2+, Zn2+, Cd2+, Co2+, Ni2+, Li+, Cu2+, Hg2+, Ru3+, or Fe3+ to solutions of 2 in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (40
CH3CN (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v) did not induce any change neither in the UV-Vis nor in the fluorescence spectra. By contrast, in the presence of the trivalent cations Cr3+ and Al3+ the colour of the solutions changed dramatically from blue to pink due to the appearance of a new band at 560 nm (see Fig. 2). Probe 2 also shows some colour change in the presence of Fe3+ but only when CH3CN alone or mixtures with a maximum of 8% water were used. Interestingly probe 2 also displays a remarkable strong fluorescence emission at 563 nm in water
60 v/v) did not induce any change neither in the UV-Vis nor in the fluorescence spectra. By contrast, in the presence of the trivalent cations Cr3+ and Al3+ the colour of the solutions changed dramatically from blue to pink due to the appearance of a new band at 560 nm (see Fig. 2). Probe 2 also shows some colour change in the presence of Fe3+ but only when CH3CN alone or mixtures with a maximum of 8% water were used. Interestingly probe 2 also displays a remarkable strong fluorescence emission at 563 nm in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (40
CH3CN (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v) upon addition of the metal cations Cr3+ and Al3+ (ΦAl = 0.33; ΦCr = 0.30).
60 v/v) upon addition of the metal cations Cr3+ and Al3+ (ΦAl = 0.33; ΦCr = 0.30).
Furthermore in competitive experiments it was found that probes 1 and 2 respond to the presence of Al3 +, Cr3+ and Fe3+ (for 1) and to Al3+and Cr3+ (for 2) in the presence of Hg2+, Li+, Na+, K+, Ag+, Ca2+, Mg2+, Ni2+, Zn2+, Cd2+, Fe2+, Co2+ and Cu2+ cations. In addition, limits of detection (LOD) were determined from the equation LOD = K × Sb1/S, where K = 3, Sb1 is the standard deviation of the blank solution and S is the slope of the calibration curve.15 The obtained results were 0.14, 019 and 0.10 μM for Al3+, Cr3+ and Fe3+ respectively with ligand 1 and 0.08 and 0.18 μM for Al3+ and Cr3+ with ligand 2 using in both cases emission measurements. Moreover LOD of 0.18 and 0.52 μM were calculated for Al3+ and Cr3+ using 2 from UV-vis titrations.
Titration experiments of 1 and Fe3+, Cr3+ and Al3+ in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (80
CH3CN (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) (by fluorescence spectroscopy) and of 2 with Cr3+ and Al3+ in water
20 v/v) (by fluorescence spectroscopy) and of 2 with Cr3+ and Al3+ in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (40
CH3CN (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v) (by either UV-Vis or fluorescence) were carried out in order to determine the complexation constants by using the software Specfit program.16 As an example Fig. 3 shows the fluorescence titration of 1 with Al 3+.
60 v/v) (by either UV-Vis or fluorescence) were carried out in order to determine the complexation constants by using the software Specfit program.16 As an example Fig. 3 shows the fluorescence titration of 1 with Al 3+.
 | ||
Fig. 3 Fluorescence response (λexc. = 480 nm) for 1 (10−5 M) to increasing amounts of Al3+ in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (80 CH3CN (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) The inset shows the corresponding Job plot. 20 v/v) The inset shows the corresponding Job plot. | ||
A stepwise addition of Al3+ led to an enhancement of the band at 515 nm, which is saturated upon the addition of 1 equiv. of Al3+, strongly, suggesting the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal complexes. This was also demonstrated via the corresponding Job's plot and MS. The same stoichiometry was observed for Cr3+ and Fe3+ with 1.
1 ligand-to-metal complexes. This was also demonstrated via the corresponding Job's plot and MS. The same stoichiometry was observed for Cr3+ and Fe3+ with 1.
Moreover, similar fluorescence titration studies with 2 also indicated the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal complexes with the trivalent metal cations Cr3+ and Al3+. Moreover UV-vis spectroscopy titrations with 2 also resulted in similar results (see Table 1).
1 ligand-to-metal complexes with the trivalent metal cations Cr3+ and Al3+. Moreover UV-vis spectroscopy titrations with 2 also resulted in similar results (see Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal complexes
1 ligand-to-metal complexes
All these results are consistent with a sensing mechanism in which the metal cations form complexes with 1 and 2 via coordination with the N-methyl-N-(2-hydroxyethyl) moiety. The interaction of the cation with the lone pair on the nitrogen atom in 1 results in an inhibition of the PET process giving rise to the observed enhancement of the fluorescence emission. On the other hand, the binding of the cation with the lone pair on the nitrogen atom in 2 results in a reduction of the electron-donating ability of the nitrogen atom of N-methyl-N-(2-hydroxyethyl)-styryl group which is in conjugation to the BODIPY core, thus suppressing the ICT process causing the blue shift of the absorption spectrum band and an enhancement of the fluorescence.
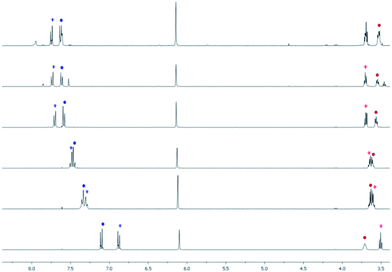
To confirm the propose sensing mechanism, 1H NMR experiments were carried out. Thus, 1H NMR spectra of ligand 1 free and the presence of different amounts of Al3+ were recorded in CD3CN (Fig. 4). The most important modifications of the signals of 1 after complexation were observed in the phenyl moiety (7.10 and 6.84 ppm) which underwent a significant downfield shift upon the addition of Al3+ (0.52 and 0.90 ppm respectively). The change is especially important in the ortho-protons to the amino group. On the other hand the methylene group that in the free ligand appears at 3.44 ppm show a downfield shift of 0.20 ppm and the signal corresponding to the hydroxymethylene protons, at 3.58 ppm, displayed a different behavior with an upper field shift of 0.18 ppm. Finally, there were no changes in the protons of the pyrrole units (at 6.15 ppm). These data strongly suggest the direct involvement of the amino group in the Al3+ coordination.
A similar behavior was observed with ligand 2 (see ESI†) upon addition of Al3+. Remarkable changes were observed in the N-methyl-N-(2-hydroxyethyl)styryl group, especially in the ortho-protons to the amino group. On the other hand, changes in the protons of the pyrrole units and phenyl moiety in the 8 position of the BODIPY were negligible.
Conclusions
In summary, we have successfully synthesized and characterized two BODIPY-based probes which show a “turn-on” response with trivalent cations of interest whereas the probe remained silent in the presence of competitive cations (Hg2+, Li+, Na+, K+, Ag+, Ca2+, Mg2+, Ni2+, Zn2+, Cd2+, Fe2+, Co2+, Ru3+, Fe2+, and Cu2+). This sensing behavior is highly selective and it is observed in mixed aqueous solutions. Compound 1 can be only used in fluorescence studies whereas compound 2 gives sensing response through two different channels, UV-vis and fluorescence. The observed colour change or enhanced fluorescence emission can be attributed to the binding of M3+ to the 2-aminoethanol moiety which reduces the electron-donating ability of the nitrogen atom conjugated to the BODIPY core. Finally, selective sensing of Al3+ and Cr3+ versus Fe3+ was observed with ligand 2.Acknowledgements
Financial support from the Spanish Government (Project MAT2012-38429-C04) and the Generalitat Valencia (Project PROMETEO/2009/016) is gratefully acknowledged. A.B.B. thanks for a pre-doctoral FPI fellowship.Notes and references
- (a) R. Kramer, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef CAS; (b) L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 1995, 24, 197–202 RSC; (c) Y. Wu, X. Peng, B. Guo, J. Fan, Z. Zhang, J. Wang, A. Cui and Y. Gao, Org. Biomol. Chem., 2005, 3, 1387–1392 RSC; (d) X. Peng, J. Du, J. Fan, J. Wang, Y. Wu, J. Zhao, S. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500–1501 CrossRef CAS PubMed; (e) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC; (f) A. Barba-Bon, A. M. Costero, S. Gil, M. Parra, J. Soto, R. Martínez-Máñez and F. Sncenón, Chem. Commun., 2012, 48, 3000–3002 RSC.
- (a) C.-H. Chen, D.-J. Liao, C.-F. Wan and A.-T. Wu, Analyst, 2013, 138, 2527–2530 RSC; (b) S.-D. Liu, L.-W. Zhang and X. Liu, New J. Chem., 2013, 37, 821–826 RSC; (c) S.-R. Liu and S.-P. Wu, Sens. Actuators, B, 2012, 171, 1110–1116 CrossRef PubMed.
- (a) H. Arakawa, R. Ahmad, M. Naoni and H.-A. Tajmir-Riahi, J. Biol. Chem., 2000, 275, 10150–10153 CrossRef PubMed; (b) H. Wu, P. Zhou, J. Wang, L. Zhao and C. Duan, New J. Chem., 2009, 33, 653–658 RSC.
- (a) P. Aisen, M. Wessling-Resnick and E. A. Leibold, Curr. Opin. Chem. Biol., 1999, 3, 200–206 CrossRef CAS; (b) R. S. Eisenstein, Annu. Rev. Nutr., 2000, 20, 627–662 CrossRef PubMed.
- (a) N. C. Andrews, N. Engl. J. Med., 1999, 341, 1986–2195 CrossRef CAS PubMed; (b) D. Touati, Arch. Biochem. Biophys., 2000, 373, 1–6 CrossRef CAS PubMed.
- S. Garcia-Medina, A. C. Razo-Estrada, L. M. Gomez-Olivan, A. Amaya-Chavez, E. Madrigal-Buijaidar and M. Galar-Martinez, Fish Physiol. Biochem., 2010, 36, 875–882 CrossRef CAS PubMed.
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (b) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- (a) D. Wang, Y. Shiraishi and T. Hiari, Tetrahedron Lett., 2010, 51, 2545–2549 CrossRef CAS PubMed; (b) X. Xie and Y. Qin, Sens. Actuators, B, 2011, 156, 213–217 CrossRef CAS PubMed; (c) D. Wang, Y. Shiraisi and T. Hirai, Chem. Commun., 2011, 47, 2673–2675 RSC; (d) H. B. Sun, S. J. Liu, T. C. Ma, N. N. Song, Q. Zhao and W. Huang, New J. Chem., 2011, 35, 1194–1197 RSC; (e) H. Son, J. H. Lee, Y. R. Kim, S. Han, X. Liu, J. Jaworski and H. Jung, Analyst, 2012, 137, 3914–3916 RSC.
- (a) M. Wang, J. Wang, W. Xue and A. Wu, Dyes Pigm., 2013, 97, 475–480 CrossRef CAS PubMed; (b) T. Hirata, T. Terai, T. Komatsu, K. Hanaoka and T. Nagano, Bioorg. Med. Chem. Lett., 2011, 21, 6090–6093 CrossRef CAS PubMed; (c) S. Zhu, J. Zhang, J. Janjanam, G. Vegesna, F.-T. Luo, A. Tiwari and H. Liu, J. Mater. Chem. B, 2013, 1, 1722–1728 RSC.
- (a) M. Baruah, W. Qin, N. Basarié, W. M. De Borggraeve and N. Boens, J. Org. Chem., 2005, 70, 4152–4157 CrossRef CAS PubMed; (b) Y. Ikawa, S. Moruyama and H. Furuta, Anal. Biochem., 2008, 378, 166–170 CrossRef CAS PubMed.
- N. Saki, T. Din and E. U. Akkaya, Tetrahedron, 2006, 62, 2721–2725 CrossRef CAS PubMed.
- J. Umberger and V. K. LaMer, J. Am. Chem. Soc., 1945, 67, 1099–1109 CrossRef CAS.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- M. Kollmannsberger, T. Gareis, S. Heinl, J. Breu and J. Daub, Angew. Chem., Int. Ed., 1997, 36, 1333–1335 CrossRef CAS.
- M. Zhu, M. Yuan, X. Liu, J. Xu, J. Lv, C. Huang, H. Liu, Y. Li, S. Wang and D. Zhu, Org. Lett., 2008, 10, 1481–1484 CrossRef CAS PubMed.
- SPECFIT/32TM GLOBAL ANALYSIS SYSTEM v.3.0, Spectrum Associates, Marlborough, MA, USA, http://www.bio-logic.info/rapidkinetics/specfit.html Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectroscopic data; NMR, fluorescence and UV titrations of 1 and 2 with Al3+, Cr3+ and Fe3+. See DOI: 10.1039/c3ra46845c |
| This journal is © The Royal Society of Chemistry 2014 |