Au–ZnO bullet-like heterodimer nanoparticles: synthesis and use for enhanced nonenzymatic electrochemical determination of glucose†
Deivasigamani Ranjith Kumar,
Devaraj Manoj and
Jayadevan Santhanalakshmi*
Department of Physical Chemistry, University of Madras, Maraimalai Campus, Guindy, Chennai 600 025, India. E-mail: jslakshmi@yahoo.co.in; Tel: +91-44-2220 2819
First published on 3rd December 2013
Abstract
In this work, the gold nanoseed mediated growth of bullet-like gold–zinc oxide (Au–ZnO) heterodimer nanoparticles has been reported. A formation pathway for the bullet-like morphology has been proposed. The heterojunction effect of the Au–ZnO nanoparticles was studied using UV-vis spectroscopy, X-ray photoelectron spectroscopy (XPS), high resolution transmission electron microscopy (HRTEM) and electrochemical impedance spectroscopy (EIS). The bullet-like Au–ZnO heterodimer nanoparticles were effectively employed as a sensor for the nonenzymatic determination of glucose (GLU) in a Au–ZnO/MWCNTs/GC modified electrode. This showed good sensitivity and a wide linear range of 19–291 μM with a sensitivity of 0.0447 μA μM−1 and a limit of detection of 0.19 μM. The resulting sensor displayed excellent repeatability and long-term stability.
1. Introduction
Multicomponent heterodimer nanoparticles (HNPs) are composed of two or more particles bound as a single entity and possess great importance due to their unique electronic, optical, catalytic, optoelectronic, multimodal biological detection, solar energy conversion and electrochemical sensor applications.1–6 This tremendous amount of applications is mainly due to the special hetero metal–metal oxide junction effect in HNPs.7,8 Moreover, HNPs have a tunable morphology which is dependent on their composition and this can enhance their activity. Recently, bimetallic, core/shell, dumbbell like multifunctional nanomaterials and heterodimer nanoparticles have been used in technological and biological applications.9 In this work, the construction of Au–ZnO HNPs is considered. Recently, a seed mediated growth method has been widely used to construct heterodimer nanoparticles.10,11 Au nanoparticles are well known to the chemical community and have wide applications. Moreover, the construction of Au nanoseeds on a semiconducting material produces drastic changes in the electrical, catalytic and optical properties of the semiconductor.12,13 A number of metal–metal oxide HNPs are available with various synthesis methods, such as Au–Fe3O4, ZnO–Au, M–Pt–Fe3O4 (M = Au, Ag, Ni, Pd), Cu@Fe3O4, Pd–Fe3O4 and Au–ZnO.14–17Among the metal oxide nanoparticles, ZnO is significant because of its direct band gap (∼3 eV) with a large excitation binding energy (60 meV). Many reports on the catalytic, electrical and optical properties of ZnO nanoparticles have been published.18 Therefore, ZnO nanoparticle based devices have potential technological applications in solar cells, transparent conducting materials, ultraviolet-protection films, chemical sensors, transistors, light emitting or laser diodes, self-cleaning materials and so on.19 In this work, Au–ZnO HNPs are synthesized by a seed mediated growth method. Au–ZnO HNPs are biocompatible and have been used extensively for biological detection applications in biomedicine.20
Most diabetic patients who have complicated health disorders like cardiovascular diseases and other medical problems show serious health problems. Treatment courses require the control of hyperglycemia. For this, the detection of blood glucose from ≤5.55 mmol L−1 (100 mg dL−1) to >6.99 mmol L−1 (>126 mg dL−1) is essential.21 The proper treatment of complicated diseases in a diabetic patient requires the precise determination of blood glucose concentration. The enzymatic glucose sensors used for glucose determination are insufficient due to their long-term instability and unsatisfactory reproducibility originating from the nature and activity of immobilized enzymes.22 Also, the enzyme glucose oxidase is easily affected by experimental conditions such as pH, temperature, toxic chemicals, humidity and ionic detergents.23 Hence, the fabrication of highly sensitive and selective nonenzymatic glucose detectors in a cost effective manner is preferable.
Recently, continuous research effort has been directed at nonenzymatic glucose detection using electrodes with different nanostructural modifications. Noble metals (Au, Cu, Pt, Pd)24–28 and bimetals/alloys of Au–Ag,29 Pt–Au,30 Ni–Cu,31 and Pt–Pb,32 have been extensively used for nonenzymatic glucose detection. However, most of the above electrodes are adversely affected by other electroactive components such as ascorbic acid (AA) and uric acid (UA).33 Furthermore, noble metal electrode surfaces are easily poisoned by adsorbed intermediates and chloride, leading to a decrease in their sensitivity.34 Transition metals (Ni and Cu)35 have been investigated for nonenzymatic glucose detection, due to their lack of electrode poisoning on carbohydrate oxidation.33 However, the above transition metals are easily oxidized, in air and in solution, to their corresponding metal oxides, therefore their practical application is unfavorable. Hence, electrodes modified with the oxide forms of transition metals (CuO, NiO, and Co3O4)36–38 and hybrid type NiO–Au,39 CuNP/ZnO40 and Cu–CuO41 nanoparticles have been used for nonenzymatic glucose detection. In this paper, Au–ZnO bullet-like heterodimer nanoparticles designed by a seed mediated growth method are discussed. A Au–ZnO/MWCNTs/GC modified electrode for the determination of glucose (GLU) was optimized and tested. The Au–ZnO/MWCNTs/GC modified electrode exhibited an enhanced electron transfer rate, which significantly improved the sensitivity and selectivity of glucose determination.
2. Experimental
2.1. Chemicals and reagents
Zinc acetate dihydrate Zn(O2CCH3)2·2H2O, tetrachloroauric acid trihydrate (HAuCl4·3H2O), tert-butylamine borane complex, oleylamine, oleic acid, tetralin, dibenzyl ether, dodecanol multiwalled carbon nanotubes (MWCNTs), sodium hydroxide (NaOH) pellets and glucose were purchased from Sigma-Aldrich. All chemicals were used as received without further purification. All the solutions were prepared using triple distilled water.2.2. Instrumentation
UV-vis spectroscopic measurements were performed on a Techcomp 8500 double beam instrument. The optical length of the quartz cuvette is 1 cm. FT-IR spectra were recorded using a BRUKER TENSOR 27 in the region of 4000–400 cm−1 with a resolution of 2 cm−1. The morphology was studied using a TECNAI-G2 (model T-30) S-twin high resolution transmission electron microscope operated at an accelerating voltage of 300 kV, and X-ray diffraction (XRD) results were collected by using a BRUKER D8 advance X-ray diffractometer with monochromatic Cu Kα radiation (λ = 1.5418 Å). X-ray photoelectron spectroscopy (XPS) measurements were carried out in the ultra-high vacuum (UHV) chamber (evacuated to 3.5 × 10−10 mbar) of a photoelectron spectrometer (Omicron Nanotechnology GmbH, Germany) equipped with a monochromatic X-ray source (Al Kα, hυ = 1486.6 eV). The anode and filament were operated with 15 kV and 20 mA (300 W), respectively. The binding energy of the samples was calibrated by setting the C1s peak to 284.6 eV. The peaks were deconvoluted using standard Casa XPS software (v.2.3.13; product of Casa XPS Software Ltd., USA) to resolve the separate constituents after background subtraction.All the electrochemical experiments were carried out with a PGSTAT-12 electrochemical analyser (AUTOLAB BV, the Netherlands). A glassy carbon (GC) electrode with a geometric area of 0.0707 cm2 was used as the working electrode. It was used in the experiments after being polished down to a mirror polish with increasingly fine grade alumina powders (1, 0.3 and 0.05 μm), sonicated for about 15 min in triple distilled water and subsequently washed with a copious amount of triple distilled water. The reference electrode used was a saturated calomel electrode (ELICO, India). A Pt spiral wire with a high geometrical surface area (∼20 cm2) was used as the counter electrode.
2.3. Synthesis of Au nanoseeds
Au nanoseeds are prepared according to a literature method.42 The HAuCl4·3H2O (0.5 mmol) was dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction mixture of the capping agents oleylamine (3 mmol) and oleic acid (3 mmol), with the organic solvents tetralin (5 mL) and dibenzyl ether (5 mL) in a 50 mL three-neck round bottom flask equipped with a thermometer adapter and reflux condenser. The solution mixture was constantly stirred under N2 at 25 °C. Then, tert-butylamine borane complex as a reducing agent (1 mmol) dissolved in 1.5 mL of oleylamine and 1.5 mL of oleic acid was injected into the above solution. After addition of the reducing agent, the temperature was raised slowly to 120 °C and maintained for 10 min. The color of the reaction mixture changed from brownish yellow to black red. The color change of the reaction mixture confirmed the formation of Au nanoseeds. Au nanoseeds were precipitated by the addition of ethanol and collected by centrifugation at 6000 rpm. This procedure was repeated three times to get pure 5 nm Au nanoseeds.
1 reaction mixture of the capping agents oleylamine (3 mmol) and oleic acid (3 mmol), with the organic solvents tetralin (5 mL) and dibenzyl ether (5 mL) in a 50 mL three-neck round bottom flask equipped with a thermometer adapter and reflux condenser. The solution mixture was constantly stirred under N2 at 25 °C. Then, tert-butylamine borane complex as a reducing agent (1 mmol) dissolved in 1.5 mL of oleylamine and 1.5 mL of oleic acid was injected into the above solution. After addition of the reducing agent, the temperature was raised slowly to 120 °C and maintained for 10 min. The color of the reaction mixture changed from brownish yellow to black red. The color change of the reaction mixture confirmed the formation of Au nanoseeds. Au nanoseeds were precipitated by the addition of ethanol and collected by centrifugation at 6000 rpm. This procedure was repeated three times to get pure 5 nm Au nanoseeds.
2.4. Synthesis of Au–ZnO HNPs
Pre-synthesized 5 nm Au nanoseeds (25 mg) in hexane (1 mL) were added to a solution of zinc acetate dihydrate (0.5 mmol), oleylamine (6 mmol) and dodecanol (10 mmol).43 The reaction mixture was refluxed for 30 min at 120 °C in order to remove the water and hexane. The temperature of the reaction flask was increased to 180 °C and maintained for a few minutes. The residue was separated by three rounds of centrifugation at 6000 rpm and washed with ethanol several times. The same procedure was adopted for the synthesis of bundles of bullet-like ZnO without the addition of Au nanoseeds. The obtained Au–ZnO heterodimer nanoparticles and bundles of bullet-like ZnO were redispersed in hexane for further study.3. Result and discussion
HRTEM images of the monodispersed Au nanoseeds synthesized by using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar stoichiometric amounts of oleic acid and oleylamine are shown in Fig. 1A.44 The formation of bundles of bullet-like ZnO can be explained as follows. The oleylamine is likely to play a significant role in hindering the nucleation growth along certain crystal facets.43 Therefore, using the optimum amount of oleylamine during the synthesis enhances the growth of ZnO along the a and b axes but weakens the growth along the c axis,45 resulting in bundles of bullets merged together as shown in Fig. 1D (without the addition of Au nanoseeds). This is due to the homogenous nucleation growth that occurs. Homogenous growth commonly requires high temperatures to overcome the energy barrier of nucleation.46 The length and width of the bundles of bullets are 0.3–0.5 μm and 0.20–0.40 μm, respectively (Fig. 1F). When the Au nanoseeds are added to the reaction mixture, the formation of bundles of bullets is hindered and this results in the formation of individual Au–ZnO bullet-like HNPs (Fig. 1G). When heterogeneous nucleation occurs over a very short nucleation time it leads to a smaller particle size.47 The length and width of the bullets are in the ranges 220–280 nm and 100–180 nm, respectively (Fig. 1I). Moreover, we have observed that the formation of bundles of bullets occurred at a higher temperature (240 °C) in the absence of Au nanoseeds, whereas in the presence of Au nanoseeds the formation of individual bullets occurred at the lower temperature of 180 °C. It is clear that the formation of bullets can be achieved at lower temperatures by adding Au nanoseeds. The formation of bullets can be explained as follows: it is well known that the different crystal facets in the hexagonal wurtzite lattice have different energies. Based on this, they are anticipated to form a hexagonal prismatic shape in equilibrium. This equilibrium is similar to that found in hexagonal CdSe nanoparticles.48 Similarly, the growth of ZnO nanoparticles on (0001) facets (the c-direction) was controlled by the concentration of oleylamine.43 The nucleation growth in the direction of the (0001) facets has been reported to be twice as fast as in the direction of the (10
1 molar stoichiometric amounts of oleic acid and oleylamine are shown in Fig. 1A.44 The formation of bundles of bullet-like ZnO can be explained as follows. The oleylamine is likely to play a significant role in hindering the nucleation growth along certain crystal facets.43 Therefore, using the optimum amount of oleylamine during the synthesis enhances the growth of ZnO along the a and b axes but weakens the growth along the c axis,45 resulting in bundles of bullets merged together as shown in Fig. 1D (without the addition of Au nanoseeds). This is due to the homogenous nucleation growth that occurs. Homogenous growth commonly requires high temperatures to overcome the energy barrier of nucleation.46 The length and width of the bundles of bullets are 0.3–0.5 μm and 0.20–0.40 μm, respectively (Fig. 1F). When the Au nanoseeds are added to the reaction mixture, the formation of bundles of bullets is hindered and this results in the formation of individual Au–ZnO bullet-like HNPs (Fig. 1G). When heterogeneous nucleation occurs over a very short nucleation time it leads to a smaller particle size.47 The length and width of the bullets are in the ranges 220–280 nm and 100–180 nm, respectively (Fig. 1I). Moreover, we have observed that the formation of bundles of bullets occurred at a higher temperature (240 °C) in the absence of Au nanoseeds, whereas in the presence of Au nanoseeds the formation of individual bullets occurred at the lower temperature of 180 °C. It is clear that the formation of bullets can be achieved at lower temperatures by adding Au nanoseeds. The formation of bullets can be explained as follows: it is well known that the different crystal facets in the hexagonal wurtzite lattice have different energies. Based on this, they are anticipated to form a hexagonal prismatic shape in equilibrium. This equilibrium is similar to that found in hexagonal CdSe nanoparticles.48 Similarly, the growth of ZnO nanoparticles on (0001) facets (the c-direction) was controlled by the concentration of oleylamine.43 The nucleation growth in the direction of the (0001) facets has been reported to be twice as fast as in the direction of the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (000
0) and (000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) facets.49 Owing to the intrinsic anisotropy of the ZnO crystal lattice together with the resulting significant differences in surface energies between the {10
) facets.49 Owing to the intrinsic anisotropy of the ZnO crystal lattice together with the resulting significant differences in surface energies between the {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} and the {0001} faces, anisotropic crystal growth along the c-axis is hindered by the addition of oleylamine and tends toward the formation of bullets rather than nanorods. Energy-dispersive X-ray spectroscopy (EDX) confirms the presence of Au, Zn, and O elements, as shown in Fig. S1,† in ratios that are qualitatively consistent with those expected for the Au–ZnO HNPs (Fig. 1G).
0} and the {0001} faces, anisotropic crystal growth along the c-axis is hindered by the addition of oleylamine and tends toward the formation of bullets rather than nanorods. Energy-dispersive X-ray spectroscopy (EDX) confirms the presence of Au, Zn, and O elements, as shown in Fig. S1,† in ratios that are qualitatively consistent with those expected for the Au–ZnO HNPs (Fig. 1G).
These results show the formation of micrometer sized oleylamine capped ZnO bundles of bullets (without Au nanoseeds). This can be explained via classical nucleation theory,50,51 where the homogeneous nucleation required to form nuclei in solution has a high energy barrier. High temperatures are needed to reach this high energy barrier and the capping ligand oleylamine hindered fast nucleation growth even at high temperatures, resulting in the formation of larger particles. In contrast, in seed mediated growth the kinetic barrier for heterogeneous nucleation is lower than for homogeneous nucleation. Moreover, the Au nanoseeds’ higher surface energy leads to fast nucleation and the HNPs reach a higher level of thermodynamic stability, because reducing the strain energy at the interface between the two different materials leads to a lower surface energy.51–53
The optical properties of Au and Au–ZnO nanoparticles were investigated in the UV-visible region. In Fig. 2A(a), the strong plasmon absorption peak observed at 521 nm indicates the formation of Au nanoparticles in the case of Au nanoseeds; while for Au–ZnO heterodimers, the surface plasmon resonance is shifted to 548 nm, showing it was significantly affected by the presence of the ZnO nanoparticles. The drastic change in peak shape and λmax is noteworthy. In comparison with pure Au nanoseeds, Au–ZnO nanoparticles show plasmon absorption at 548 nm (Fig. 2A(b)), a ∼27 nm higher wavelength than that of the pure Au particles, and also shows peak broadening. This noticeable change in the surface plasmon resonance of Au–ZnO heterodimer nanoparticles is due to the charge variation of the Au nanoparticles within the heterodimers, which arises because of the interfacial coupling between Au and ZnO. Also, Sun et al. explained that electron deficiencies in the Au nanoparticles cause the longer wavelength (red shift) of the plasmon absorption peak.2 The SPR peak shift owing to the interface communication between Au and ZnO nanoparticles, results in electron deficiencies in the Au nanoparticles.
 | ||
| Fig. 2 (A) UV-vis absorption spectra of (a) Au nanoseeds and (b) Au–ZnO bullet-like HNPs. (B) FTIR spectra of (a) oleic acid, (b) oleylamine, (c) Au nanoseeds and (d) Au–ZnO bullet-like HNPs. | ||
Fig. 2B shows the FTIR spectra of (a) oleylamine, (b) oleic acid, (c) oleic acid and oleylamine capped Au nanoseeds and (d) heterodimer Au–ZnO nanoparticles. The spectrum of oleylamine (Fig. 2B(a)) has bands which are characteristic of alkyl chains and the band at 1622 cm−1 is assigned to the combined motions of NH2 scissoring and N–H bending.54 Fig. 2B(b) shows broad peaks at 3500 cm−1 and 2500 cm−1 which were due to the O–H stretching of the carboxylic acid. The bands at 2852 cm−1 and 2922 cm−1 are attributed to asymmetric and symmetric CH2 stretching vibrations in oleic acid. The band at 1710 cm−1 arises due to carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching in oleic acid.55 Fig. 2B(c) shows the FTIR spectrum of the oleic acid and oleylamine capped Au nanoseeds. The spectrum shows peaks resembling all of the pure oleic acid and oleylamine peak positions. However there is a drastic change in the oleic acid carbonyl peak position. The broadening and blue shift of the alkyl chain band indicates the close packing of oleic acid and oleylamine on the nanoparticles.54 The band at 580 cm−1 corresponds to the Zn–O bond vibration in the Au–ZnO heterodimer nanoparticles.56
O) stretching in oleic acid.55 Fig. 2B(c) shows the FTIR spectrum of the oleic acid and oleylamine capped Au nanoseeds. The spectrum shows peaks resembling all of the pure oleic acid and oleylamine peak positions. However there is a drastic change in the oleic acid carbonyl peak position. The broadening and blue shift of the alkyl chain band indicates the close packing of oleic acid and oleylamine on the nanoparticles.54 The band at 580 cm−1 corresponds to the Zn–O bond vibration in the Au–ZnO heterodimer nanoparticles.56
The representative XRD pattern of the as prepared Au nanoseeds (Fig. 3 inset) matches with the face-centered cubic (fcc) structure (JCPDS, file no. 04–0784), with the broad peaks at 38.35°, 44.85°, 64.5° and 77.48° corresponding to the (111), (200), (220) and (311) lattice planes respectively.57 The XRD pattern of the Au–ZnO HNPs shown in Fig. 3a indicates that the ZnO had a wurtzite hexagonal-structure (JCPDS no. 36–1451).58 The peaks at 2θ of 31.74°, 34.48°, 36.24°, 47.54°, 56.62°, 62.84°, 66.33°, 67.94° and 69.18° correspond to crystalline peaks that can be matched with the Bragg reflections of the (100), (002), (101), (102), (110), (103), (200), (112) and (201) planes, respectively. Moreover there is no peak shift or peak width change, thus further indicating that the crystal structures of the Au and ZnO nanoparticles are also preserved during the engraving processes. A similar XRD pattern was observed for the bundles of bullet-like ZnO (Fig. 3b).
 | ||
| Fig. 3 XRD patterns of (a) Au–ZnO bullet-like HNPs, (b) bundles of ZnO bullets, inset: Au nanoseeds. | ||
XPS was used to quantitatively analyse the surface chemical composition of the Au nanoseeds, the Au–ZnO and the ZnO nanoparticles. The binding energy for the C1s peak (284.6 eV) was considered for charge corrections. The as synthesized Au nanoseeds have characteristic peaks (Fig. S2A†) with binding energies of 83.6 and 87.3 eV, which are typical values of 4f for Au0. The Au–ZnO heterodimers show the binding energy of the Au4f electrons, and some of the peaks overlapped with the signal of Zn3p. In the deconvoluted spectra, the branches of the Au4f7/2 of the gold peak (Fig. 4A) in the Au–ZnO heterodimer were distinctive compared with the binding energy (83.0 eV) of metallic Au. The Au nanoseeds in this work show a negative shift of nearly ∼0.80 eV. This is due to the strong interaction between the Au nanoseeds and bullet-like ZnO nanoparticles; these results agree with those observed by Zhang et al.9 Furthermore, the Au4f range overlaps with the Zn3p1/2 and the Zn3p3/2 peak contributions, and these are deconvoluted into two (Zn3p) peaks: Zn3p1/2 located at 91.1 eV and Zn3p3/2 located at 88.62 eV.59 The Au–ZnO HNP spectrum has two distinct Zn2p peaks, matching the spin-orbit split between the Zn(2p3/2) and Zn(2p1/2) levels with binding energy peak positions at 1021.9 and 1044.8 eV, respectively. The spin-orbit splitting of 22.90 eV between the peaks is in good agreement with the value for Zn2+ (Fig. 4C).60 The peak at 529.7 eV in the O1s spectrum is the O2− ions in the wurtzite structure of the hexagonal Zn2+ ion array bound by zinc atoms with a full complement of nearest neighbor O2− ions. Fig. 4E and F show a high binding energy peak at 531.05 eV, which is attributed to O2− ions in the oxygen deficient regions within the ZnO matrix, and the binding energy centered at 532.35 eV is associated with the oleic acid capped Au nanoseeds O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH groups. The deconvoluted C(1s) XPS spectrum of the oleic acid capped Au nanoseed samples is shown in Fig. S2C.† The characteristic C1s binding energy at 284.6 eV is attributed to the C–C, C
C–OH groups. The deconvoluted C(1s) XPS spectrum of the oleic acid capped Au nanoseed samples is shown in Fig. S2C.† The characteristic C1s binding energy at 284.6 eV is attributed to the C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H bonds. The deconvoluted peaks centered at the binding energies of 285.7 and 287.3 eV are assigned to the C–OH and C
C and C–H bonds. The deconvoluted peaks centered at the binding energies of 285.7 and 287.3 eV are assigned to the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups, respectively.
O functional groups, respectively.
 | ||
| Fig. 4 Deconvoluted XPS spectra corresponding to Au–ZnO bullet-like heterodimer nanoparticles Zn3p (A), Zn2p(C), O1s (E), and Zn3p (B), Zn2p (D) O1s (F) correspond to bundles of bullets. | ||
Electrochemical impedance spectroscopy (EIS) is a powerful technique for studying the surface of modified electrodes. The EIS spectra presented as Nyquist plots in the frequency range 0.1 Hz to 1000 kHz (Z′ versus -Z′′) of bare GC, MWCNTs/GC, Au/MWCNTs/GC and Au–ZnO/MWCNTs electrodes in 2 mM [Fe(CN)6]3−/4− in the presence of 0.1 M KCl solution are shown in Fig. 5A. In general, the EIS spectra have semicircular sections at higher frequencies corresponding to the electron transfer limiting process, and the linear sections at lower frequencies correspond to the diffusion limiting process. The impedance plot for the bare electrode is characterized by a semicircle at high frequency and a low frequency Warburg impedance (Rct 344 Ω). For MWCNTs/GC the plot is nearly a straight line (Fig. 5A inset), which represents Warburg resistance and the diffusion limiting step in the electrochemical process. Typically, an almost straight line in the plot implies low electron transfer resistance, indicating good conductivity of the redox probe. The Au/MWCNTs/GC electrode shows a drastic change in the Rct value (Rct 271 Ω), which indicates the good conductivity of the modified electrode when compared to that of the bare GC electrode. Furthermore, the Au–ZnO/MWCNTs/GC modified electrode shows a straight line due to the heterojunction effect and exhibits lower transfer resistance. These results showed that the Au–ZnO HNPs can act as a better electron transfer interface between the electrode surface and electrolyte solution.
The electrochemical properties of the bare GC, MWCNTs, Au/MWCNTs/GC and Au–ZnO/MWCNTs/GC modified electrodes were characterized by cyclic voltammetry (CV) in a K4[Fe(CN)6] system with a scan rate (υ) of 50 mV s−1 (Fig. 5B). The peak to peak separation (Epa − Epc = ΔEp) of the redox couples significantly changes in the modified electrodes and this is shown in Table S1.† In the Au–ZnO/MWCNTs/GC modified electrode, ΔEp appreciably decreased with increased redox peak current compared with the bare GC, MWCNTs/GC and Au/MWCNTs/GC modified electrodes. The very low ΔEp and high peak current observed for Au–ZnO HNPs is due to the interfacially accumulated electrons at the heterojunction (i.e., Au–ZnO) and the large active surface area of the nanoparticles which enhances the electron and mass transport properties of the electrode.61 Furthermore, the active surface area was determined using the Randles–Sevicik equation (eqn (1)) for the MWCNTs/GC, Au/MWCNTs/GC and Au–ZnO/MWCNTs/GC electrodes and the values are given in Table S1.† The heterogeneous electron transfer (ET) rate constants calculated according to the Nicholson method (eqn (2)) are also presented in Table S1.†62
 | (1) |
 | (2) |
The OH− concentration is very important to the current and potential response during glucose oxidation. Fig. 6A shows the cyclic voltammograms of 0.012, 0.025, 0.05, 0.1 and 0.25 M of NaOH medium in the presence of 6 mM GLU. The GLU oxidation peak current increases from 0.012 to 0.1 M NaOH (Fig. 6B). At higher concentrations (0.25 M) of NaOH, the oxidation peak current of GLU decreased, this may be due to the amphoteric oxide nature of the ZnO which is easily influenced by OH− ions.63 Hence, as the concentration of NaOH changes around the Au–ZnO/MWCNTs/GC modified electrode, it leads to a change in the Au–ZnO HNPs’ nature. Furthermore, the higher potential and pH values make more unrelated interferents unstable and reactive in the test solution and generate many intermediate reactants that would probably interact with the Au–ZnO/MWCNTs/GC modified electrode. Therefore, the optimum 0.1 M NaOH was selected for further studies of GLU detection.
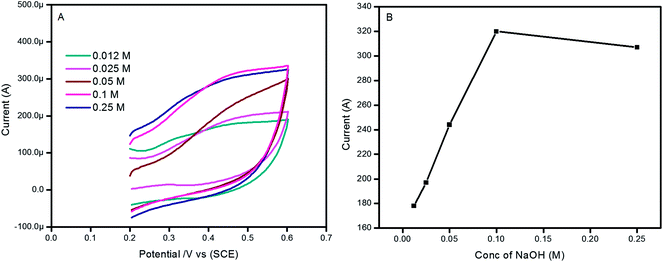 | ||
| Fig. 6 (A) Cyclic voltammograms of 6 mM glucose in different concentrations of NaOH at 50 mV s−1. (B) The effect of the concentration of NaOH vs. peak current response. | ||
The electrochemical oxidation of glucose was studied in a 0.1 M NaOH solution, at a scan rate of 50 mV s−1. As shown in Fig. 7A, the CV curves of the bare GC (a), MWCNTs/GC (b), Au/MWCNTs/GC (c) and Au–ZnO/MWCNTs/GC (d) modified electrodes in the absence of GLU showed no significant redox peaks and only a background current increase was observed. Upon addition of 5 mM glucose, CV shows that the bare GC electrode does not show any response, and the MWCNTs/GC electrode shows only a background current with a slight change in the shape of the curve compared to the absence of glucose. The Au/MWCNTs/GC and Au–ZnO/MWCNTs/GC modified electrodes exhibited significantly broad glucose oxidation peaks at potentials of +0.45 and +0.42 V, respectively. The Au/MWCNTs/GC and Au–ZnO/MWCNTs/GC electrodes exhibit glucose oxidation current increments due to the incorporation of Au and Au–ZnO nanoparticles that provide high surface area and greater electron transfer efficiency. The Au–ZnO nanoparticles modified electrode gave a high peak current at a lower operating peak potential, which is primarily due to the special heterodimer junction effect (Au–ZnO) which leads to the feasibility of a high conduction pathway of electrons from the analyte and the electrode. The CV response to 1–7 mM concentrations of glucose and the corresponding calibration plot of peak current versus glucose concentration are shown in Fig. 7B and C.
The effect of scan rate on the oxidative peak current of glucose (2 mM) at the surface of the Au–ZnO/MWCNTs/GC modified electrode in a 0.1 M NaOH solution was studied. Fig. S3† shows the CV curves of glucose obtained in the range of 20–170 mV s−1 in order to investigate whether the oxidation behavior of glucose was due to diffusion, a surface confined process, or adsorption on the Au–ZnO/MWCNTs/GC modified electrode. The scan rate increases linearly with peak current (Ipa). The linear regression equation for the Au–ZnO/MWCNTs/GC electrode is: Ipa = 0.857 + 60.134 υ, R = 0.994; this indicates the diffusion of glucose molecules on the modified-electrode surface. This is further confirmed by the double logarithmic plot of scan rate vs. peak current.
Constant potential amperometry was performed to optimize the current response by varying the potential around the anodic peak potential to achieve a better current response. The effect of applied potentials (+0.35 V, +0.40 V, +0.42 V, +0.48 V and +0.52 V) on the amperometric response of the Au–ZnO/MWCNTs/GC modified electrode to 10 μM glucose was investigated. Fig. 8A shows that only +0.42 V caused a good steady state current response. The other operating potentials, even though they exhibit high current responses, lose their steady state current response when the concentration increases. Therefore, +0.42 V is chosen as the optimum working potential for GLU determination.
The typical time–current response at the optimized applied potential of 0.42 V is consistent upon the addition of 20 μM glucose, as shown in Fig. 8B. Glucose oxidation at the Au–ZnO/MWCNTs/GC modified electrode was studied using an amperometric technique in stirred solutions using an applied potential of 0.42 V. The Au–ZnO/MWCNTs/GC electrode yielded a current response on each successive addition of 20 μM glucose. This high current response observed for the Au–ZnO/MWCNTs/GC electrode, may be due to the special effect of the heterojunction of Au nanoseeds with ZnO nanobullets. The corresponding calibration plot results show that the Au–ZnO/MWCNTs/GC modified electrode possesses a high sensitivity of 0.0447 μA μM−1 with a detection limit of 0.19 μM (Fig. 8C). The analytical performance parameters of the Au and ZnO nanoparticle modified electrode compared with the values for Au–ZnO/MWCNTs/GC electrodes reported in the literature concerning nonenzymatic glucose sensors are presented in Table S2.† The results demonstrate that the Au–ZnO modified electrode in the present work has a wider linear range and better limit of detection for glucose detection.
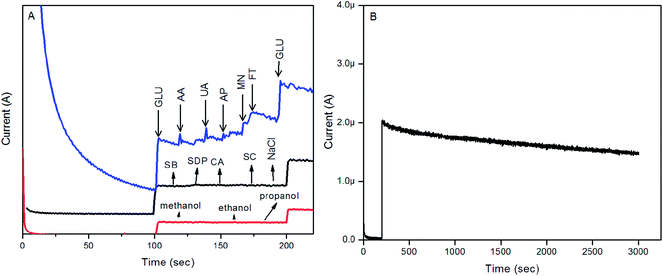
Selectivity is one of the very important characteristics for high-performance nonenzymatic glucose determination. In order to understand the factors that can affect the analytical estimation of GLU by the Au–ZnO/MWCNTs/GC modified electrode, we have studied the effect of potential interferents such as ascorbic acid (AA), uric acid (UA), acetaminophen (AP), mannose (MN), fructose (FT), dopamine (DA), citric acid (CA), sodium citrate (SC), potassium dihydrogen phosphate (SDP), sodium benzoate (SB), sodium chloride (NaCl), methanol, ethanol and propanol, which are the main interfering species in the direct electrochemical oxidation of glucose. Fig. 9A shows the typical amperometric responses of AA, UA, AP, MN and FT at 0.42 V in a 0.1 M NaOH solution with stirring. The glucose level in physiological samples is 3 to 8 mM as compared to other oxidizable interferents such as AA, UA, AP, MN and FT, which are present at levels as low as 0.1 mM. According to the results presented in Fig. 9A, adding 0.05 mM AA, DA, UA MN and FT only induced a current change compared to the oxidation current from 1 mM glucose. Chloride ions are one of the more challenging aspects in metal–metal oxide modified electrochemical nonenzymatic glucose determination. Here, chloride ions and also methanol, ethanol and propanol induced no current response change.
The reproducibility and stability of the Au–ZnO/MWCNTs/GC modified electrode was evaluated. The current responses of six Au–ZnO/MWCNTs/GC modified electrodes to 1 mM of GLU were studied. The relative standard deviation (RSD) was 2.06%, this result confirmed the Au–ZnO/MWCNTs/GC modified electrode’s reproducibility. The current signal response for 0.1 mM of GLU of the Au–ZnO/MWCNTs/GC modified electrode over a long operational potential period of 3000 seconds shown in Fig. 9B is acceptable. The stability of the Au–ZnO/MWCNTs/GC modified electrode was evaluated by measuring its sensitivity to 10 μM GLU within a 25 day period. The modified electrode was stored in air under ambient conditions and its sensitivity was tested once every 5 days (Fig. S4†).
In order to attempt to assess the feasibility of the Au–ZnO/MWCNTs/GC modified electrode for practical applications, the modified electrode was used to determine glucose in human blood serum samples. At an applied potential of +0.42 V, 40 μL of the serum sample was added to 10 mL of 0.1 M NaOH solution and the current response was recorded. The resulting GLU concentration was in good agreement with the value obtained by a hospital used glucose meter. The obtained GLU detection values are given in Table 1.
| Blood sample | Commercial glucose meter measured (mM) | Au–ZnO/MWCNTs/GC electrode measured (mM) | Added (mM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 4.6 | 4.4 | 0.1 | 102% |
| 2 | 4.0 | 4.1 | 0.1 | 97% |
| 3 | 4.8 | 4.9 | 0.1 | 96% |
4. Conclusion
We have synthesized Au–ZnO heterodimer bullet-like nanoparticles through the reaction of zinc acetate, oleylamine and dodecanol by seed-mediated growth. The change in the electronic properties caused by the heterojunction between the Au nanoseeds and the ZnO bullet-like nanoparticles is explained based on UV-vis spectroscopy, XPS and EIS measurements. The Au–ZnO/MWCNTs/GC modified electrode was utilised to construct a novel nonenzymatic sensor for glucose determination, and the results shows that the Au–ZnO/MWCNTs/GC modified electrode has better electrocatalytic activity. This better electrocatalytic activity was mainly due to the heterojunction effect (i.e. Au–ZnO), high electron transfer rate, large electroactive surface area and the synergistic electrocatalytic activity resulting from the combination of Au–ZnO nanoparticles and MWCNTs. The Au–ZnO/MWCNTs/GC electrode can also be used in the presence of common biological interferants with negligible influence on GLU determination. These experimental results indicate that the Au–ZnO/MWCNTs/GC nanocomposite electrode has good prospects for the effective nonenzymatic determination of glucose at low concentrations with high sensitivity.Acknowledgements
Financial support by the National Centre for Nanoscience and Nanotechnology (NCNSNT) University of Madras, Chennai is gratefully acknowledged by the authors. We would like to thank NCNSNT for XPS and HRTEM characterization.References
- C. Xu, J. Xie, D. Ho, C. Wang, N. Kohler, G. E. Walsh, J. R. Morgan, Y. E. Chin and S. Sun, Angew. Chem., Int. Ed., 2008, 47, 173 CrossRef CAS PubMed.
- H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. Sun, Nano Lett., 2005, 5, 379 CrossRef CAS PubMed.
- F. Lin and R. Doong, J. Phys. Chem. C, 2011, 115, 6591 CAS.
- Y. Jang, J. Chung, S. Kim, S. W. Jun, B. H. Kim, D. W. Lee, B. M. Kim and T. Hyeon, Phys. Chem. Chem. Phys., 2011, 13, 2512 RSC.
- X. Sun, S. Guo, Y. Liu and S. Sun, Nano Lett., 2012, 12, 4859 CrossRef CAS PubMed.
- L. Wang, J. Wang, S. Zhang, Y. Sun, X. Zhu, Y. Cao, X. Wang, H. Zhang and D. Song, Anal. Chim. Acta, 2009, 653, 109 CrossRef CAS PubMed.
- C. Wang, Y. Wei, H. Jiang and S. Sun, Nano Lett., 2009, 9, 4544 CrossRef CAS PubMed.
- C. Wang, H. Yin, S. Dai and S. Sun, Chem. Mater., 2010, 22, 3277 CrossRef CAS.
- L. Zhang, H. Jing, G. Boisvert, J. Z. He and H. Wang, ACS Nano, 2012, 6, 3514 CrossRef CAS PubMed.
- X. Liu, C. Lee, W. C. Law, D. Zhu, M. Liu, M. Jeon, J. Kim, P. N. Prasad, C. Kim and M. T. Swihart, Nano Lett., 2013, 13, 4333 CrossRef CAS PubMed.
- M. R. Buck, J. F. Bondi and R. E. Schaak, Nat. Chem., 2012, 4, 37 CrossRef CAS PubMed.
- C. Wang, C. Xu, H. Zeng and S. Sun, Adv. Mater., 2009, 21, 3045 CrossRef CAS PubMed.
- X. Wang, X. Kong, Y. Yu and H. Zhang, J. Phys. Chem. C, 2007, 111, 3836 CAS.
- B. Nakhjavan, M. N. Tahir, F. Natalio, H. Gao, K. Schneider, T. Schladt, I. Ament, R. Branscheid, S. Weber, U. Kolb, C. Sonnichsen, L. M. Schreiber and W. Tremel, J. Mater. Chem., 2011, 21, 8605 RSC.
- N. S. Phala, G. Klatt, E. Van Steen, S. A. French, A. A. Sokol and C. R. A. Catlow, Phys. Chem. Chem. Phys., 2005, 7, 2440 RSC.
- K. X. Yao, X. Liu, L. Zhao, H. C. Zeng and Y. Han, Nanoscale, 2011, 3, 4195 RSC.
- M. N. Tahir, F. Natalio, M. A. Cambaz, M. Panthofer, R. Branscheid, U. Kolb and W. Tremel, Nanoscale, 2013, 5, 9944 RSC.
- Z. Guo, S. Wei, B. Shedd, R. Scaffaro, T. Pereiraa and H. T. Hahn, J. Mater. Chem., 2007, 17, 806 RSC.
- R. Brayner, S. A. Dahoumane, C. Yepremian, C. Djediat, M. Meyer, A. Cout and F. Fievet, Langmuir, 2010, 26, 6522 CrossRef CAS PubMed.
- L. H. Zhao, R. Zhang, J. Zhang and S. Q. Sun, CrystEngComm, 2012, 14, 945 RSC.
- S. C. Port, N. G. Boyle, W. A. Hsueh, M. J. Quinones, R. I. Jennrich and M. O. Goodarz, Am. J. Epidemiol., 2006, 163, 342 CrossRef PubMed.
- J. Luo, S. Jiang, H. Zhang, J. Jiang and X. Liu, Anal. Chim. Acta, 2012, 709, 47 CrossRef CAS PubMed.
- K. M. E. Khatib and R. M. A. Hameed, Biosens. Bioelectron., 2011, 26, 3542 CrossRef PubMed.
- S. Cherevko and C. H. Chung, Sens. Actuators, B, 2009, 142, 216 CrossRef CAS PubMed.
- J. Zhao, L. Wei, C. Peng, Y. Su, Z. Yang, L. Zhang, H. Wei and Y. Zhang, Biosens. Bioelectron., 2013, 47, 86 CrossRef CAS PubMed.
- Y. Zhang, L. Su, D. Manuzzi, H. V. E. Monteros, W. Jia, D. Huo, C. Hou and Y. Lei, Biosens. Bioelectron., 2012, 31, 426 CrossRef CAS PubMed.
- X. Zhou, X. Zheng, R. Lv, D. Kong and Q. L. Li, Electrochim. Acta, 2013, 107, 164 CrossRef CAS PubMed.
- X. Chen, Z. Lin, D. J. Chen, T. Jia, Z. Cai, X. Wang, X. Chen, G. Chen and M. Oyama, Biosens. Bioelectron., 2010, 25, 1803 CrossRef CAS PubMed.
- M. Tominaga, T. Shimazoe, M. Nagashima, H. Kusuda, A. Kubo, Y. Kuwahara and I. Taniguchi, J. Electroanal. Chem., 2006, 590, 37 CrossRef CAS PubMed.
- B. Singh, F. Laffir, T. McCormac and E. Dempsey, Sens. Actuators, B, 2010, 150, 80 CrossRef CAS PubMed.
- I. H. Yeo and D. C. Johnson, J. Electroanal. Chem., 2001, 495, 110 CrossRef CAS.
- J. Wang, D. F. Thomas and A. Chen, Anal. Chem., 2008, 80, 997 CrossRef CAS PubMed.
- W. Wang, L. Zhang, S. Tong, X. Li and W. Song, Biosens. Bioelectron., 2009, 25, 708 CrossRef CAS PubMed.
- J. Yang, L. C. Jiang, W. D. Zhang and S. Gunasekaran, Talanta, 2010, 82, 25 CrossRef CAS PubMed.
- K. C. Lin, Y. C. Lin and S. M. Chen, Electrochim. Acta, 2013, 96, 164 CrossRef CAS PubMed.
- F. Huang, Y. Zhong, J. Chen, S. Li, Y. Li, F. Wang and S. Feng, Anal. Methods, 2013, 5, 3050 RSC.
- S. Liu, B. Yu and T. Zhang, Electrochim. Acta, 2013, 102, 104 CrossRef CAS PubMed.
- C. Hou, Q. Xu, L. Yin and X. Hu, Analyst, 2012, 137, 5803 RSC.
- Y. Ding, Y. Liu, J. Parisi, L. Zhang and Y. Lei, Biosens. Bioelectron., 2011, 28, 393 CrossRef CAS PubMed.
- S. A. Kumar, H. W. Cheng, S. M. Chen and S. F. Wang, Mater. Sci. Eng., C, 2010, 30, 86 CrossRef CAS PubMed.
- G. Wang, Y. Wei, W. Zhang, X. Zhang, B. Fang and L. Wang, Microchim. Acta, 2010, 168, 87 CrossRef CAS.
- P. D. L. Presa, M. Multigner, J. D. L. Venta and M. A. Garcia, J. Appl. Phys., 2006, 100, 123915 CrossRef PubMed.
- Z. Zhang, M. Lu, H. Xu and W. S. Chin, Chem.–Eur. J., 2007, 13, 632 CrossRef CAS PubMed.
- W. Bu, Z. Chen, F. Chen and J. Shi, J. Phys. Chem. C, 2009, 113, 12176 CAS.
- R. A. Laudise and A. A. Ballman, J. Phys. Chem., 1960, 64, 688 CrossRef CAS.
- R. Costi, A. E. Saunders and U. Banin, Angew. Chem., Int. Ed., 2010, 49, 4878 CrossRef CAS PubMed.
- X. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120, 5343 CrossRef CAS.
- J. J. Shiang, A. V. Kadavanich, R. K. Grubbs and A. P. Alivisatos, J. Phys. Chem., 1995, 99, 17417 CrossRef CAS.
- B. Ludi and M. Niederberger, Dalton Trans., 2013, 42, 12554 RSC.
- J. W. Mullin, Crystallization. Butterworth-Heinemann, Oxford, 3rd edn, 1997 Search PubMed.
- I. V. Markov, Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth, and Epitaxy, World Scientific, Singapore, 2003 Search PubMed.
- V. Randle, The Role of the Coincidence Site Lattice in Grain Boundary Engineering, Woodhead Publishing, Cambridge, England, 1997 Search PubMed.
- A. Figuerola, A. Fiore, R. D. Corato, A. Falqui, C. Giannini, E. Micotti, A. Lascialfari, M. Corti, R. Cingolani, T. Pellegrino, P. D. Cozzoli and L. Manna, J. Am. Chem. Soc., 2008, 130, 1477 CrossRef CAS PubMed.
- M. Chen, Y. G. Feng, X. Wang, T. C. Li, J. Y. Zhang and D. Qian, Langmuir, 2007, 23, 5296 CrossRef CAS PubMed.
- K. Yang, H. Peng, Y. Wen and N. Li, Appl. Surf. Sci., 2010, 256, 3093 CrossRef CAS PubMed.
- S. G. S. Sindhu, K. A. Varman, P. C. Ramamurthy and G. Madras, RSC Adv., 2012, 2, 11536 RSC.
- Y. Chen, C. Somsen, S. Milenkovic and A. W. Hassel, J. Mater. Chem., 2009, 19, 924 RSC.
- M. S. Mo, S. H. Lim, Y. W. Mai, R. K. Zheng and S. P. Ringer, Adv. Mater., 2008, 20, 339 CrossRef CAS.
- S. B. Amor, M. Jacquet, P. Fioux and M. Nardin, Appl. Surf. Sci., 2009, 255, 5052 CrossRef PubMed.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, ULVAC-PHI, Inc., Japan, 1995 Search PubMed.
- Y. G. Zhou, F. W. Campbell, S. R. Belding and R. G. Compton, Chem. Phys. Lett., 2010, 497, 200 CrossRef CAS PubMed.
- J. P. Metters, S. M. Houssein, D. K. Kampouris and C. E. Banks, Anal. Methods, 2013, 5, 103 RSC.
- W. W. Zhan, Q. Kuang, J. Z. Zhou, X. J. Kong, Z. X. Xie and L. S. Zheng, J. Am. Chem. Soc., 2013, 135, 1926 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45269g |
| This journal is © The Royal Society of Chemistry 2014 |