Greener iodination of arenes using sulphated ceria–zirconia catalysts in polyethylene glycol†
Abstract
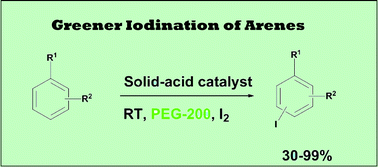
An environmentally benign method for the selective monoiodination of diverse aromatic compounds has been developed using reusable sulphated ceria–zirconia under mild conditions. The protocol provides moderate to good yields and selectively introduces iodine at the para/ortho position in monosubstituted arenes. SO42−/Ce0.07Zr0.93O2 was found to be the best choice for the synthesis of aryl iodides in high yield, presumably due to the maximum number of acid sites (4.23 mmol g−1) among the various compositions of the catalyst system.

 Please wait while we load your content...
Please wait while we load your content...