Cotton fabrics coated with lignosulfonate-doped polypyrrole for flexible supercapacitor electrodes
Abstract
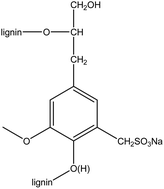
Polypyrrole/lignosulfonate (PPy/LGS) coated cotton fabrics have been prepared via in situ oxidation polymerization of pyrrole in the presence of lignosulfonate as both template and dopant. The mass loading on the fabric samples decreases dramatically with the increased LGS content. The electrical conductivity of the coated fabrics achieved 3.03 S cm−1 under the optimized conditions. The electrochemical properties of the coated fabrics were investigated using cyclic voltammetry and galvanostatic charge–discharge measurements. The specific capacitance of the coated fabrics can be as high as 304 F g−1 at a current density of 0.1 A g−1 in aqueous electrolyte. These novel fabrics are desirable for applications in wearable supercapacitors.

 Please wait while we load your content...
Please wait while we load your content...