Synthesis and biological evaluation of oleanolic acid derivative–chalcone conjugates as α-glucosidase inhibitors†
Chu Tanga,
Linhui Zhua,
Yu Chenb,
Rui Qinc,
ZhiNan Meia,
Jing Xu*a and
Guangzhong Yang*a
aLaboratory for Natural Product Chemistry, College of Pharmacy, South Central University for Nationalities, 708 Minyuan Road, Wuhan 430074, P. R. China. E-mail: helloxujing6280@yahoo.com.cn; yanggz888@126.com
bCollege of Chemistry and Material Sciences, South Central University for Nationalities, 708 Minyuan Road, Wuhan 430074, P. R. China
cCollege of Life Sciences, South Central University for Nationalities, 708 Minyuan Road, Wuhan 430074, P. R. China
First published on 6th January 2014
Abstract
α-Glucosidase is a promising target for the treatment of obesity and diabetes mellitus. A series of oleanolic acid derivative–chalcone conjugates were designed and synthesized as α-glucosidase inhibitors. Their structures were determined by spectroscopic analysis and their α-glucosidase inhibitory activities were investigated in vitro. Most conjugates exhibited moderate inhibitory activity against α-glucosidase; among them, conjugate 1b (IC50 = 3.2 ± 0.2 μM) possessed the strongest α-glucosidase inhibitory activity, and the preliminary structure–activity relationship showed that the furan or thiophene rings in the chalcone units of the conjugates had a tendency to enhance the activity. Lineweaver–Burk plot analysis demonstrated competitive inhibition of α-glucosidase activity by 1b, 6b, 5c and 4d; their inhibition constant (Ki) values were 16.6, 29.3, 14.6 and 20.6 μM, respectively. The interaction forces between the conjugates and α-glucosidase were hydrogen bonding and van der Waals.
Introduction
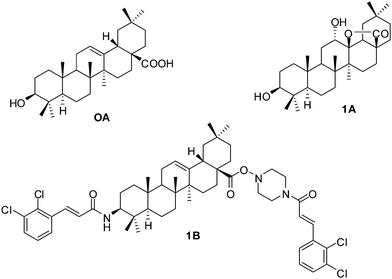
Diabetes mellitus (DM) is one of the most common and serious metabolic diseases characterized by high blood-glucose levels and alterations in carbohydrate, protein and lipid metabolism.1 Hyperglycemia and hyperlipidemia are involved in the development of microvascular and macrovascular complications of diabetes, which are the major causes of morbidity and mortality of diabetes sufferers.2 To date, therapy for type 2 DM is to suppress the postprandial hyperglycemia by reducing the absorption of gut glucose via inhibition of carbohydrate-hydrolyzing enzymes.3 α-Glucosidase, an enzyme catalyzing the cleavage of glycosidic bonds in oligosaccharides or glycoconjugates and for the final step in carbohydrate digestion. Therefore, the inhibition of α-glucosidase to control elevated glucose levels in blood is a popular choice.4Oleanolic acid (OA, Fig. 1), a natural pentacyclic triterpenoid, which has been used as an anti-hepatitis drug in China for over 20 years,5 exhibits various biological activities including anti-flammation, antitumor, anti-HIV, anti-oxidation activities.6–8 In previous reports, oleanolic acid and its derivatives have been designed and synthesized to suppress hyperglycemia as inhibitors of α-glucosidase,9,10 and some derivatives showed promising inhibitory activities (1A, 1B, Fig. 1).11,12 Although some other pentacyclic triterpenoid compounds like ursolic acid and lupeol have similar structures to OA, ursolic acid displayed weak activity against rat intestinal α-glucosidase,13 and lupeol derivatives also failed to inhibit α-glucosidase.14 Therefore, oleanolic acid was used as the lead compound. On the other hand, recent investigations have reported that some chalcones also possessed potential anti-diabetic activity.15,16 Therefore, on the basis of α-glucosidase inhibition activity of the aforementioned OA as well as chalcones, we carried on making further structural modifications to OA by incorporating different chalcone units that would allow us to find novel, more potent α-glucosidase inhibitors.
In this work, 26 analogues with an oleanolic acid core and different chalcone ligands were synthesized; the α-glucosidase inhibitory activities of these compounds were evaluated and their structure–activity relationships were also discussed.
Result and discussion
Chemistry
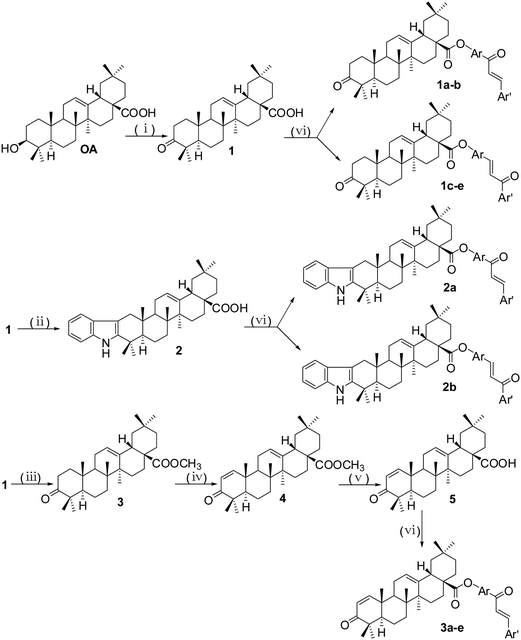
The conjugations (1a–e, 2a,b, 3a–e, 4a–f, 5a–c and 6a–e) of oleanolic acid derivatives with chalcones were achieved by a well-known esterification procedure using standard EDC/DMAP conditions (Schemes 1–3).17 In the initial step, chalcones (Cha1–Cha11) were synthesized by condensing the corresponding aldehyde with the corresponding acetophenone by Claisen–Schmidt condensation18,19 (Scheme 1). Compound 1, 3-keto OA, was prepared by Jones oxidation of oleanolic acid in 94.9% yield.20 Indole compound 2 was prepared by Fischer indolization of compound 1 with phenylhydrazine in the presence of acetic acid (Scheme 2).21 Refluxing compound 1 with CH3I in THF in the presence of KOH generated compound 3.19 Thereafter, compound 3 was further treated with phenylselenenyl chloride in the presence of H2O2 to yield methyl 3-oxo-olean-1,12-dien-28-oate 4,22 which subsequently was reacted with LiI in dry DMF to give the target acid 5.20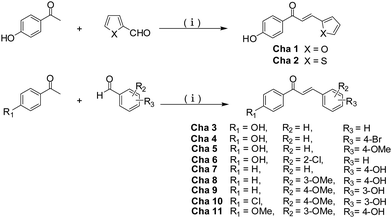 | ||
| Scheme 1 Synthesis of chalcones Cha1–Cha11. Reagents and conditions: (i) 5 eq. KOH, EtOH, r.t., 12 h. | ||
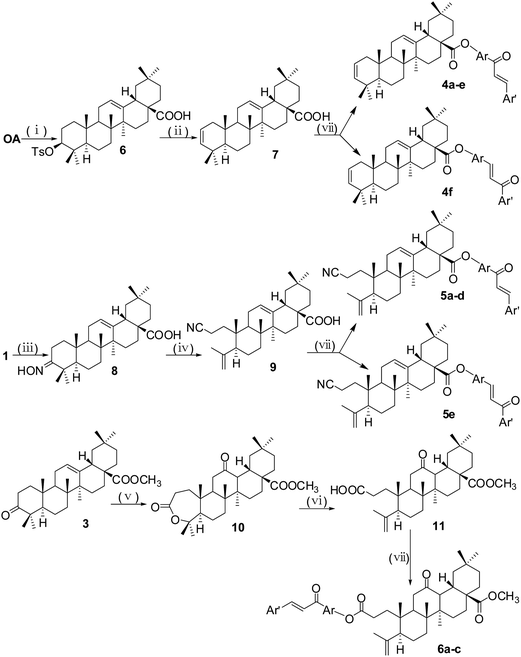
To prepare compound 7, we used oleanolic acid as the starting material, and it was deoxygenated via its 3-tosyl OA (Scheme 3). This 3-tosyl compound 6 was deoxygenated by treatment with sodium acetate in DMF at 120 °C for 24 h, giving compound 7, which had a double bond between the C-2 and C-3.23 3-Keto compound 1 reacted with hydroxylamine hydrochloride in pyridine to produce an oxime compound 8.21 Refluxing compound 8 with p-toluenesulfonyl chloride (p-TsCl) in dry pyridine in the presence of 4-N,N-dimethylamino-pyridine (DMAP) afforded the compound 9, which was the product of Beckmann fragmentation.24 The treatment of 28-methyl ester derivative 3 with m-chloroperbenzoic acid (m-CPBA) and NaHCO3 yielded lactone 10,25 and the lactone ring was cleaved by treatment of p-toluenesulfonic acid (p-TSA) in dichloromethane to give product 11.26
α-Glucosidase inhibitory activity
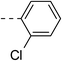
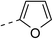
These twenty-six conjugates of oleanolic acid derivative–chalcone conjugates, together with oleanolic acid were evaluated by spectrophotometry for their inhibitory activities against α-glucosidase, and Acarbose was used as a reference. As shown in Table 1, most of the new conjugates (1a–d, 1f, 2a,b, 3b–e, 4a–f, 5a–c and 6a–c) exhibited stronger inhibitory activity against α-glucosidase than Acarbose, except for compounds 1d and 3a.| No. | Ar | Ar' | IC50a(μM) | No. | Ar | Ar' | IC50a(μM) |
|---|---|---|---|---|---|---|---|
| a Standard deviation (n = 3).b Not active, the IC50 is more than 1000 μM. | |||||||
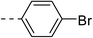
| 1a |  |
 |
159.3 ± 13.6 | 4a | 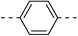 |
 |
30.8 ± 1.4 |
| 1b | 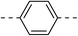 |
 |
3.2 ± 0.2 | 4b | 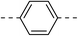 |
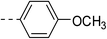 |
98.9 ± 4.0 |
| 1c | 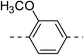 |
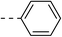 |
259.2 ± 14.1 | 4c | 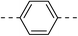 |
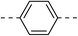 |
31.8 ± 2.2 |
| 1d | 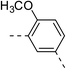 |
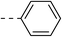 |
NAb | 4d | 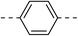 |
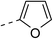 |
30.5 ± 1.4 |
| 1e | 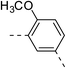 |
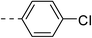 |
147.7 ± 4.9 | 4e | 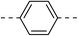 |
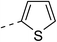 |
14.2 ± 0.7 |
| 2a | 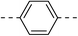 |
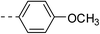 |
52.7 ± 6.8 | 4f | 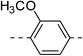 |
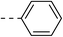 |
163.8 ± 8.0 |
| 2b | 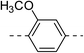 |
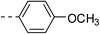 |
125.6 ± 10.7 | 5a | 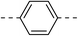 |
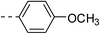 |
48.0 ± 3.7 |
| 3a | 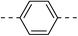 |
 |
NA | 5b | 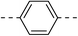 |
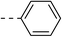 |
20.4 ± 0.8 |
| 3b | 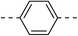 |
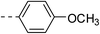 |
218.7 ± 1.5 | 5c | 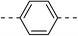 |
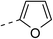 |
4.1 ± 0.2 |
| 3c | 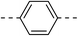 |
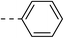 |
210.2 ± 1.7 | 5d | 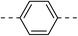 |
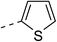 |
11.5 ± 1.0 |
| 3d | 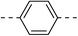 |
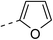 |
76.9 ± 4.7 | 5e | 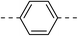 |
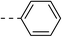 |
33.9 ± 0.4 |
| 3e | 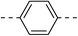 |
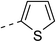 |
13.5 ± 1.5 | 6a | 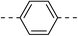 |
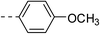 |
10.8 ± 0.9 |
| Oleanolic acid | 102.3 ± 2.4 | 6b | 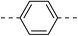 |
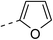 |
8.1 ± 0.7 | ||
| Acarbose | >300 | 6c |  |
 |
15.5 ± 1.0 | ||
| Cha 1 | 362.3 ± 7.6 | ||||||
Compared with currently referenced 1A (IC50 = 7.97 ± 0.21 μM), 1b and 5c displayed stronger inhibitory activity. Interestingly, different chalcone units obviously affected the inhibitory activities of the conjugates. Compared with oleanolic acid (IC50 = 102.3 ± 2.4 μM), the benzene ring in chalcone units (1a, 1c–e, 2b, 3a–c, 4b, 4f) did not improve the α-glucosidase inhibitory activity. The Br and Cl atom substation patterns on the chalcone portion (1a, 1e and 3a) reduced the activity. Among them, conjugate 1b (IC50 = 3.2 ± 0.2 μM) possessed the strongest α-glucosidase inhibitory activity, which approximately exhibited 34-fold enhanced activities compared with oleanolic acid (IC50 = 102.3 ± 2.4 μM), and the furan or thiophene rings in chalcone units of conjugates (1b, 3d, 3e, 4d, 4e) showed a tendency to enhance the activity. This result suggested that the furan chalcone unit might be required for strong activity, possibly related to protein binding. This exciting result prompted us to explore additional novel oleanolic acid derivative–chalcone analogs 5a–e and 6a–c. These eight conjugates were 3,4-seco-compounds, conjugates 6a–c bearing the oleanolic acid derivative ester on the 3-position. In this series, these eight conjugates dramatically enhanced α-glucosidase inhibitory activity compared to oleanolic acid. Among them, conjugate 5c (IC50 = 4.1 ± 0.2 μM) showed potent α-glucosidase inhibitory activity, being approximately 24-fold higher than oleanolic acid. These results suggest that the inhibitory activity was enhanced by the cleavage of the A ring on the oleanolic acid and that the C3 position of chalcone skeleton may be an important factor for the inhibitory activity.
Kinetic analysis of α-glucosidase inhibition by compounds 1b, 6b, 5c and 4d
In order to gain further insight into how these conjugates interact with α-glucosidase, the inhibition mode of compounds 1b, 6b, 5c and 4d were chosen as typical examples, analyzed by Lineweaver–Burk plots using the data derived from enzyme assays containing various concentrations of p-nitrophenyl α-D-glucopyranoside (PNP-glycoside, 0.2–12 mM). Double-reciprocal plots of enzyme kinetics demonstrated competitive inhibition of α-glucosidase activity by 1b, 6b, 5c and 4d.27 Lineweaver–Burk plots of α-glucosidase kinetics are shown in Fig. 2. An increase of inhibitor concentration resulted in an increased gradient of the line, while their y-intercept was nearly the same. This indicated that the inhibitor could bind to the active sites of the enzyme. The Michaelis constant (Km) of PNP-glycoside for α-glucosidase was 2.14 mM and the Ki value of 1b, 6b, 5c and 4d were 16.6, 29.3, 14.6 and 20.6 μM, respectively. The differences of Ki values suggested that the α-glucosidase inhibitory activity of 5c was higher than that of 1b, 6b or 4d due to the differences in affinity to the enzyme inhibitor sites.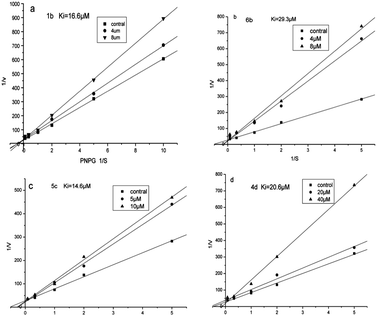 | ||
| Fig. 2 Lineweaver–Burk plots of the inhibition kinetics of yeast's α-glucosidase inhibitory effects by compounds 1b (a), 6b (b), 5c (c) and 4d (d). | ||
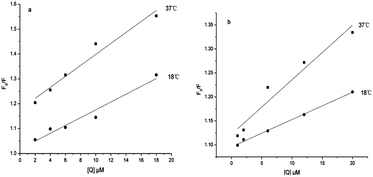
Fluorescence quenching spectra of α-glucosidase
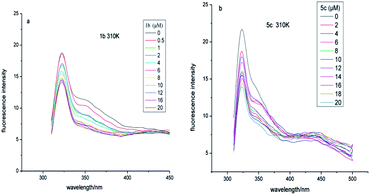
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka + n
Ka + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Q] (F0, fluorescence intensity in the absence of quencher; F, fluorescence intensity in the presence of quencher).30 From the plots obtained by log[(F0 − F)/F] vs. log[Q], the values of Ka could be calculated and the binding sites (n) are shown in Table 3. For conjugate 5c, the value of n exhibited a decrease with the increase of temperature, indicating that low temperatures were preferred for conjugate–α-glucosidase binding.
log[Q] (F0, fluorescence intensity in the absence of quencher; F, fluorescence intensity in the presence of quencher).30 From the plots obtained by log[(F0 − F)/F] vs. log[Q], the values of Ka could be calculated and the binding sites (n) are shown in Table 3. For conjugate 5c, the value of n exhibited a decrease with the increase of temperature, indicating that low temperatures were preferred for conjugate–α-glucosidase binding.
Types of interaction force between compound and α-glucosidase
There are four interaction forces between bio-molecules and small molecules, including electrostatic forces, hydrophobic interaction forces, hydrogen bonding and van der Waals forces. From the plots obtained by log[(F0 – F)/F] vs. log[Q], as shown in Fig. 5, the thermodynamic parameters were evaluated using the van't Hoff equation and the values are shown in Table 3. It has been reported that the types of interaction forces between bio-molecules and small molecules are associated with the signs of thermodynamic parameters.31 Only contributions to negative entropy and enthalpy changes arose from hydrogen bonding and van der Waals. As presented in Table 3, the negative ΔH value revealed that the reaction was an exothermic process, and low temperature was helpful for conjugate–α-glucosidase binding. At the same time, the negative value of ΔG demonstrated that the reaction process was spontaneous. In this circumstance, the interaction forces between the conjugate and α-glucosidase were hydrogen bonding and van der Waals.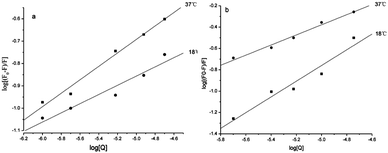 | ||
| Fig. 5 Modified Sten–Volmer plots for the fluorescence quenching of α-glucosidase by 1b (a) and 5c (b) at two different temperatures. | ||
Conclusions
In conclusion, a series of novel and potent α-glucosidase inhibitors were synthesized. Most of the conjugates exhibited moderate inhibitory activity against α-glucosidase. Among them, the conjugate 1b (IC50 = 3.2 ± 0.2 μM) possessed the strongest α-glucosidase inhibitory activity, the preliminary structure–activity relationships showed that the furan or thiophene rings in the chalcone units of the conjugates enhanced activities, and these conjugates exhibited inhibitory activities toward yeast α-glucosidase via a competitive mechanism. The inhibitor could bind to the active sites of the enzyme and the interaction process was spontaneous. The interaction forces between conjugates and α-glucosidases were hydrogen bonding and van der Waals. Thus, oleanolic acid derivative–chalcone conjugates as a promising new class of α-glucosidase inhibitor leads deserve further studies.Experimental section
General
Melting points were measured on an electro-thermal melting point apparatus and uncorrected. Infrared spectra were taken as KBr discs on a FTIR spectrometer. 1H NMR spectra were recorded in CDCl3 on a Bruker AVANCE-III-400 (or 500) spectrometer and resonances are in ppm relative to TMS. MS spectra were measured with a Finnigan MS spectrometer. All of the solvents and reagents were purified and dried by standard techniques. All compounds were routinely checked by thin-layer chromatography (TLC) on pre-coated silica gel GF254 plates (Qingdao Haiyang Chemical Co., Ltd., P. R. China). Column chromatography was performed using silica gel (200–300 mesh) from Qingdao Haiyang Chemical Group Co., China.General procedure for the synthesis of chalcones (Cha1–Cha11)
A mixture of the corresponding aldehyde (1.1 equiv.) and the corresponding acetophenone (1 equiv.) in anhydrous EtOH was stirred at room temperature for 15 min under a nitrogen atmosphere, and then KOH (5 equiv.) was added. The reaction mixture was stirred at room temperature overnight. After that, 10% HCl was added until pH 3, the aqueous layer was extracted with EtOAc (3 × 50 mL) and washed with water. The organic layer was dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, petroleum ether–acetone, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield the chalcone.
1) to yield the chalcone.
Synthesis of oleanolic acid derivatives (1–11)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to give 1 (9.31 g, 94.9%). 1H NMR (400 MHz, CDCl3): δ 5.31 (1H, s, H-12), 2.86 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 0.82 (s, CH3), 0.91 (s, CH3), 0.94 (s, CH3), 1.05 (s, CH3), 1.09 (s, CH3), 1.16 (s, CH3), 1.26 (s, CH3).
4) to give 1 (9.31 g, 94.9%). 1H NMR (400 MHz, CDCl3): δ 5.31 (1H, s, H-12), 2.86 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 0.82 (s, CH3), 0.91 (s, CH3), 0.94 (s, CH3), 1.05 (s, CH3), 1.09 (s, CH3), 1.16 (s, CH3), 1.26 (s, CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide indole derivative 2 (0.71 g, 60.1%). 1H NMR (400 MHz, CDCl3): δ 7.75 (1H, s, N–H), 7.45 (1H, d, J = 7.2 Hz, Ar-H), 7.33–7.28 (1H, m, Ar-H), 7.13–7.06 (2H, m, Ar-H), 5.42 (1H, s, H-12), 2.8 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 0.84 (s, CH3), 0.89 (s, CH3), 0.94 (s, CH3), 1.05 (s, CH3), 1.10 (s, CH3), 1.17 (s, CH3), 1.27 (s, CH3).
1) to provide indole derivative 2 (0.71 g, 60.1%). 1H NMR (400 MHz, CDCl3): δ 7.75 (1H, s, N–H), 7.45 (1H, d, J = 7.2 Hz, Ar-H), 7.33–7.28 (1H, m, Ar-H), 7.13–7.06 (2H, m, Ar-H), 5.42 (1H, s, H-12), 2.8 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 0.84 (s, CH3), 0.89 (s, CH3), 0.94 (s, CH3), 1.05 (s, CH3), 1.10 (s, CH3), 1.17 (s, CH3), 1.27 (s, CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford 3 (94% yield). 1H NMR (400 MHz, CDCl3): δ 5.31 (1H, s, H-12), 3.67 (3H, s, –COOCH3), 2.89 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 0.86 (s, CH3), 0.93 (s, CH3), 0.97 (s, CH3), 1.08 (s, CH3), 1.11 (s, CH3), 1.17 (s, CH3), 1.26 (s, CH3).
3) to afford 3 (94% yield). 1H NMR (400 MHz, CDCl3): δ 5.31 (1H, s, H-12), 3.67 (3H, s, –COOCH3), 2.89 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 0.86 (s, CH3), 0.93 (s, CH3), 0.97 (s, CH3), 1.08 (s, CH3), 1.11 (s, CH3), 1.17 (s, CH3), 1.26 (s, CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to yield the acid 5 as a white solid (0.13 g, 28.4%). 1H NMR (400 MHz, CDCl3): δ 7.06 (1H, d, J = 10.0 Hz, H-1), 5.84 (1H, d, J = 10.0 Hz, H-2), 5.36 (1H, s, H-12), 2.89 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 0.86 (s, CH3), 0.89 (s, CH3), 0.95 (s, CH3), 1.04 (s, CH3), 1.09 (s, CH3), 1.17 (s, CH3), 1.27(s, CH3).
4) to yield the acid 5 as a white solid (0.13 g, 28.4%). 1H NMR (400 MHz, CDCl3): δ 7.06 (1H, d, J = 10.0 Hz, H-1), 5.84 (1H, d, J = 10.0 Hz, H-2), 5.36 (1H, s, H-12), 2.89 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 0.86 (s, CH3), 0.89 (s, CH3), 0.95 (s, CH3), 1.04 (s, CH3), 1.09 (s, CH3), 1.17 (s, CH3), 1.27(s, CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 6 (1.875 g, 95%).
1) to provide 6 (1.875 g, 95%).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), yielding 7 (0.205 g, 42.1%). 1H NMR (400 MHz, CDCl3): δ 5.40 (3H, m, H-2, H-3, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.21 (s, CH3), 1.17 (s, CH3), 1.09 (s, CH3), 1.07 (s, CH3), 0.94 (s, CH3), 0.93 (s, CH3), 0.89 (s, CH3).
1), yielding 7 (0.205 g, 42.1%). 1H NMR (400 MHz, CDCl3): δ 5.40 (3H, m, H-2, H-3, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.21 (s, CH3), 1.17 (s, CH3), 1.09 (s, CH3), 1.07 (s, CH3), 0.94 (s, CH3), 0.93 (s, CH3), 0.89 (s, CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the oxime 8 was obtained as a white solid (2.208 g, 71.1%).
2), the oxime 8 was obtained as a white solid (2.208 g, 71.1%).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 9 (0.548 g, 42.4%). 1H NMR (400 MHz, CDCl3): δ 5.34 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.73 (3H, s, Me), 1.25 (6H, s, 2 × Me), 0.95 (3H, s, Me), 0.92 (3H, s, Me), 0.89 (3H, s, Me).
1) to give 9 (0.548 g, 42.4%). 1H NMR (400 MHz, CDCl3): δ 5.34 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.73 (3H, s, Me), 1.25 (6H, s, 2 × Me), 0.95 (3H, s, Me), 0.92 (3H, s, Me), 0.89 (3H, s, Me).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 10 (2.197 g, 48.8%).
1) to give 10 (2.197 g, 48.8%).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give 11 (1.758 g, 50.7%). 1H NMR (400 MHz, CDCl3): δ 4.87 (1H, s, H2-24), 4.67 (1H, s, H2-24), 3.67 (3H, s, –OMe), 2.80 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 2.63 (2H, m, H-2), 1.73 (3H, s, Me), 0.99 (3H, s, Me), 0.96 (6H, s, 2 × Me), 0.89 (3H, s, Me), 0.84 (3H, s, Me).
2) to give 11 (1.758 g, 50.7%). 1H NMR (400 MHz, CDCl3): δ 4.87 (1H, s, H2-24), 4.67 (1H, s, H2-24), 3.67 (3H, s, –OMe), 2.80 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 2.63 (2H, m, H-2), 1.73 (3H, s, Me), 0.99 (3H, s, Me), 0.96 (6H, s, 2 × Me), 0.89 (3H, s, Me), 0.84 (3H, s, Me).General procedure for esterification (1a–e, 2a,b, 3a–e, 4a–f, 5a–c, 6a–e)
A DCM solution of the same molar ratio of the corresponding OA derivative and chalcone (Cha 1–11) with a two-fold mol ratio of 1-(3-dimethylamino-propyl)-3-ethylcarbodiimide hydrochloride (EDC) and 4-N,N-dimethylamino-pyridine (DMAP) was stirred at room temperature for 24 h under nitrogen atmosphere. The crude mixture was extracted with DCM, and the organic layer was washed with brine, dried (Na2SO4). Evaporation of the solvent gave a residue that was purified by column chromatography (silica gel, petroleum ether–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.75 (1H, d, J = 15.6 Hz, H-9′), 7.57 (2H, d, J = 8.0 Hz, Ar-H), 7.53 (2H, d, J = 8.0 Hz, Ar-H), 7.46 (1H, d, J = 16.0 Hz, H-8′), 7.16 (2H, d, J = 8.0 Hz, Ar-H), 5.38 (1H, s, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.24 (3H, s, Me), 1.14 (3H, s, Me), 1.08 (6H, s, 2 × Me), 1.04 (3H, s, Me), 0.97 (3H, s, Me), 0.93 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.7, 189.0, 175.7, 154.8, 143.5, 143.2, 135.3, 133.7, 132.2 (C × 2 in Ph), 130.0 (C × 2 in Ph), 129.8 (C × 2 in Ph), 124.9, 122.8, 122.3, 121.9 (C × 2 in Ph), 55.3, 47.4, 47.3, 46.8, 45.7, 41.9, 41.5, 39.5, 39.1, 36.7, 34.1, 33.8, 33.0, 32.3 (C × 2), 30.7, 27.8, 26.4, 25.7, 23.6 (C × 2), 23.0, 21.5, 19.5, 17.3, 15.0; ESI MS: m/z 739 ([M + H]+, 18.8); HRESIMS m/z 739.3349 [M + H]+ (calcd for C45H56O4Br, 739.3361).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.75 (1H, d, J = 15.6 Hz, H-9′), 7.57 (2H, d, J = 8.0 Hz, Ar-H), 7.53 (2H, d, J = 8.0 Hz, Ar-H), 7.46 (1H, d, J = 16.0 Hz, H-8′), 7.16 (2H, d, J = 8.0 Hz, Ar-H), 5.38 (1H, s, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.24 (3H, s, Me), 1.14 (3H, s, Me), 1.08 (6H, s, 2 × Me), 1.04 (3H, s, Me), 0.97 (3H, s, Me), 0.93 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.7, 189.0, 175.7, 154.8, 143.5, 143.2, 135.3, 133.7, 132.2 (C × 2 in Ph), 130.0 (C × 2 in Ph), 129.8 (C × 2 in Ph), 124.9, 122.8, 122.3, 121.9 (C × 2 in Ph), 55.3, 47.4, 47.3, 46.8, 45.7, 41.9, 41.5, 39.5, 39.1, 36.7, 34.1, 33.8, 33.0, 32.3 (C × 2), 30.7, 27.8, 26.4, 25.7, 23.6 (C × 2), 23.0, 21.5, 19.5, 17.3, 15.0; ESI MS: m/z 739 ([M + H]+, 18.8); HRESIMS m/z 739.3349 [M + H]+ (calcd for C45H56O4Br, 739.3361).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.05 (2H, d, J = 8.4 Hz, Ar-H), 7.60 (1H, d, J = 15.6 Hz, H-9′), 7.53 (1H, s, H-5′′), 7.40 (1H, d, J = 16.4 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 6.72 (1H, d, J = 3.2 Hz, H-3′′), 6.51 (1H, t, J = 1.6 Hz, H-4′′), 5.41 (1H, s, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.20 (3H, s, Me), 1.08 (3H, s, Me), 1.04 (6H, s, 2 × Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.7, 188.6, 175.7, 154.7, 151.6, 144.9, 143.2, 135.4, 130.8, 129.9 (C × 2 in Ph), 122.8, 121.8 (C × 2 in Ph), 118.9, 116.4, 112.7, 55.3, 47.4, 47.3, 46.8, 45.7, 41.9, 41.5, 39.5, 39.1, 36.7, 34.1, 33.8, 33.0, 32.3 (C × 2), 30.7, 27.8, 26.4, 25.7, 23.5 (C × 2), 23.0, 21.5, 19.5, 17.3, 15.0; ESI MS: m/z 651 ([M + H]+, 7.6); HRESIMS m/z 651.4054 [M + H]+ (calcd for C43H55O5, 651.4049).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.05 (2H, d, J = 8.4 Hz, Ar-H), 7.60 (1H, d, J = 15.6 Hz, H-9′), 7.53 (1H, s, H-5′′), 7.40 (1H, d, J = 16.4 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 6.72 (1H, d, J = 3.2 Hz, H-3′′), 6.51 (1H, t, J = 1.6 Hz, H-4′′), 5.41 (1H, s, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.20 (3H, s, Me), 1.08 (3H, s, Me), 1.04 (6H, s, 2 × Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.7, 188.6, 175.7, 154.7, 151.6, 144.9, 143.2, 135.4, 130.8, 129.9 (C × 2 in Ph), 122.8, 121.8 (C × 2 in Ph), 118.9, 116.4, 112.7, 55.3, 47.4, 47.3, 46.8, 45.7, 41.9, 41.5, 39.5, 39.1, 36.7, 34.1, 33.8, 33.0, 32.3 (C × 2), 30.7, 27.8, 26.4, 25.7, 23.5 (C × 2), 23.0, 21.5, 19.5, 17.3, 15.0; ESI MS: m/z 651 ([M + H]+, 7.6); HRESIMS m/z 651.4054 [M + H]+ (calcd for C43H55O5, 651.4049).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.4 Hz, Ar-H), 7.80 (1H, d, J = 15.6 Hz, H-9′), 7.61 (1H, m, H- 4′′), 7.53 (2H, d, J = 8.4 Hz, Ar-H), 7.48 (1H, d, J = 15.6 Hz, H-8′), 7.28 (1H, m, H-6′), 7.24 (1H, d, J = 2.4 Hz, H-2′), 7.00 (1H, d, J = 8.4 Hz, H-5′), 5.38 (1H, s, H-12), 3.89 (3H, s, –OMe), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.20 (3H, s, Me), 1.06 (3H, s, Me), 1.01 (6H, s, 2 × Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me); 13C NMR (125 M Hz, CDCl3): δ 217.8, 190.5, 175.5, 151.8, 144.4, 143.4, 142.1, 138.1, 133.5, 132.8, 128.6 (C × 2 in Ph), 128.5 (C × 2 in Ph), 123.4, 122.5, 122.0, 121.4, 111.7, 55.9, 55.3, 47.4, 47.3, 46.8, 45.7, 41.9, 41.5, 39.4, 39.1, 36.7, 34.1, 33.8, 33.1, 32.3 (C × 2), 30.7, 27.8, 26.4, 25.7, 23.6 (C × 2), 23.1, 21.5, 19.6, 17.2, 15.1; ESI MS: m/z 713.4 ([M + Na]+, 100); HRESIMS m/z 713.4204 [M + Na]+ (calcd for C46H58O5Na, 713.4182).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.4 Hz, Ar-H), 7.80 (1H, d, J = 15.6 Hz, H-9′), 7.61 (1H, m, H- 4′′), 7.53 (2H, d, J = 8.4 Hz, Ar-H), 7.48 (1H, d, J = 15.6 Hz, H-8′), 7.28 (1H, m, H-6′), 7.24 (1H, d, J = 2.4 Hz, H-2′), 7.00 (1H, d, J = 8.4 Hz, H-5′), 5.38 (1H, s, H-12), 3.89 (3H, s, –OMe), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.20 (3H, s, Me), 1.06 (3H, s, Me), 1.01 (6H, s, 2 × Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me); 13C NMR (125 M Hz, CDCl3): δ 217.8, 190.5, 175.5, 151.8, 144.4, 143.4, 142.1, 138.1, 133.5, 132.8, 128.6 (C × 2 in Ph), 128.5 (C × 2 in Ph), 123.4, 122.5, 122.0, 121.4, 111.7, 55.9, 55.3, 47.4, 47.3, 46.8, 45.7, 41.9, 41.5, 39.4, 39.1, 36.7, 34.1, 33.8, 33.1, 32.3 (C × 2), 30.7, 27.8, 26.4, 25.7, 23.6 (C × 2), 23.1, 21.5, 19.6, 17.2, 15.1; ESI MS: m/z 713.4 ([M + Na]+, 100); HRESIMS m/z 713.4204 [M + Na]+ (calcd for C46H58O5Na, 713.4182).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.02 (2H, d, J = 8.4 Hz, Ar-H), 7.78 (1H, d, J = 15.6 Hz, H-9′), 7.59 (1H, m, H-4′′), 7.53 (2H, d, J = 8.4 Hz, Ar-H), 7.49 (1H, m, H-6′), 7.39 (1H, d, J = 15.6 Hz, H-8′), 7.28 (1H, m, H-2′), 6.99 (1H, d, J = 8.4 Hz, H-5′), 5.39 (1H, s, H-12), 3.89 (3H, s, –OMe), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.23 (3H, s, Me), 1.12 (3H, s, Me), 1.07 (6H, s, 2 × Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.7, 190.3, 175.6, 153.5, 144.0, 140.4, 143.4, 138.4, 127.9, 132.6, 128.8, 128.5 (C × 2 in Ph), 128.4 (C × 2 in Ph), 122.5, 122.4, 121.4, 112.3, 55.9, 55.3, 47.4, 47.3, 46.8, 45.8, 41.9, 41.5, 39.5, 39.1, 36.7, 34.1, 33.9, 33.0, 32.3 (C × 2), 30.7, 27.7, 26.4, 25.7, 23.6 (C × 2), 23.1, 21.5, 19.6, 17.3, 15.1; ESI MS: m/z 691 ([M + H]+, 5.6); HRESIMS m/z 691.4356 [M + H]+ (calcd for C46H59O5, 691.4362).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.02 (2H, d, J = 8.4 Hz, Ar-H), 7.78 (1H, d, J = 15.6 Hz, H-9′), 7.59 (1H, m, H-4′′), 7.53 (2H, d, J = 8.4 Hz, Ar-H), 7.49 (1H, m, H-6′), 7.39 (1H, d, J = 15.6 Hz, H-8′), 7.28 (1H, m, H-2′), 6.99 (1H, d, J = 8.4 Hz, H-5′), 5.39 (1H, s, H-12), 3.89 (3H, s, –OMe), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.23 (3H, s, Me), 1.12 (3H, s, Me), 1.07 (6H, s, 2 × Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.7, 190.3, 175.6, 153.5, 144.0, 140.4, 143.4, 138.4, 127.9, 132.6, 128.8, 128.5 (C × 2 in Ph), 128.4 (C × 2 in Ph), 122.5, 122.4, 121.4, 112.3, 55.9, 55.3, 47.4, 47.3, 46.8, 45.8, 41.9, 41.5, 39.5, 39.1, 36.7, 34.1, 33.9, 33.0, 32.3 (C × 2), 30.7, 27.7, 26.4, 25.7, 23.6 (C × 2), 23.1, 21.5, 19.6, 17.3, 15.1; ESI MS: m/z 691 ([M + H]+, 5.6); HRESIMS m/z 691.4356 [M + H]+ (calcd for C46H59O5, 691.4362).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 7.79 (2H, d, J = 8.4 Hz, Ar-H), 7.75 (1H, d, J = 15.6 Hz, H-9′), 7.47 (1H, m, H-6′), 7.46 (2H, d, J = 8.4 Hz, Ar-H), 7.31 (1H, d, J = 15.6 Hz, H-8′), 7.27 (1H, d, J = 2.4 Hz, H-2′), 6.90 (1H, d, J = 8.8 Hz, H-4′), 5.36 (1H, s, H-12), 3.84 (3H, s, –OMe), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.24 (3H, s, Me), 1.11 (3H, s, Me), 1.07(3H, s, Me), 1.06 (3H, s, Me), 1.05 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.6, 188.9, 175.6, 153.7, 144.4, 143.4, 140.4, 139.0, 136.7, 128.0, 127.7, 129.8 (C × 2 in Ph), 128.9 (C × 2 in Ph), 122.5, 122.4, 119.9, 112.3, 55.9, 55.3, 47.4, 47.3, 46.8, 45.9, 42.0, 41.5, 39.5, 39.1, 36.7, 34.1, 33.9, 33.0, 32.3 (C × 2), 30.7, 27.7, 26.4, 25.6, 23.6 (C × 2), 23.2, 21.5, 19.6, 17.3, 15.1; ESI MS: m/z 747 ([M + Na]+, 3.7); HRESIMS m/z 725.3964 [M + H]+ (calcd for C46H58O5Cl, 725.3972).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 7.79 (2H, d, J = 8.4 Hz, Ar-H), 7.75 (1H, d, J = 15.6 Hz, H-9′), 7.47 (1H, m, H-6′), 7.46 (2H, d, J = 8.4 Hz, Ar-H), 7.31 (1H, d, J = 15.6 Hz, H-8′), 7.27 (1H, d, J = 2.4 Hz, H-2′), 6.90 (1H, d, J = 8.8 Hz, H-4′), 5.36 (1H, s, H-12), 3.84 (3H, s, –OMe), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.24 (3H, s, Me), 1.11 (3H, s, Me), 1.07(3H, s, Me), 1.06 (3H, s, Me), 1.05 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 217.6, 188.9, 175.6, 153.7, 144.4, 143.4, 140.4, 139.0, 136.7, 128.0, 127.7, 129.8 (C × 2 in Ph), 128.9 (C × 2 in Ph), 122.5, 122.4, 119.9, 112.3, 55.9, 55.3, 47.4, 47.3, 46.8, 45.9, 42.0, 41.5, 39.5, 39.1, 36.7, 34.1, 33.9, 33.0, 32.3 (C × 2), 30.7, 27.7, 26.4, 25.6, 23.6 (C × 2), 23.2, 21.5, 19.6, 17.3, 15.1; ESI MS: m/z 747 ([M + Na]+, 3.7); HRESIMS m/z 725.3964 [M + H]+ (calcd for C46H58O5Cl, 725.3972).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.8 Hz, Ar-H), 7.86 (1H, s, –NH), 7.77 (1H, d, J = 16.0 Hz, H-9′), 7.61 (2H, d, J = 8.4 Hz, Ar-H), 7.42 (1H, d, J = 7.2 Hz, Ar-H), 7.37 (1H, d, J = 15.6 Hz, H-8′), 7.29 (1H, d, J = 7.6 Hz, Ar-H), 7.19 (2H, d, J = 8.8 Hz, Ar-H), 7.12 (1H, m, Ar-H), 7.09 (1H, m, Ar-H), 6.95 (2H, d, J = 8.4 Hz, Ar-H), 5.50 (1H, s, H-12), 3.86 (3H, s, –OMe), 3.03 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 1.31 (3H, s, Me), 1.26 (6H, s, 2 × Me), 1.22(3H, s, Me), 1.02 (3H, s, Me), 0.97 (3H, s, Me), 0.96(3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 189.4, 175.8, 161.7, 154.6, 144.8, 142.9, 140.8, 136.1, 135.8, 130.2 (C × 2 in Ph), 129.9 (C × 2 in Ph), 128.2, 127.5, 123.3, 121.7 (C × 2 in Ph), 120.9, 119.5, 118.8, 117.9, 114.4 (C × 2 in Ph), 110.4, 106.8, 55.4, 53.2, 47.7, 46.3, 45.8, 42.0, 41.6, 39.6, 38.1, 36.8, 34.0, 33.9, 33.1, 32.3 (C × 2), 31.0, 30.7, 27.9, 25.7, 23.6, 23.5, 23.2, 23.1, 19.3, 17.3, 15.6; ESI MS: m/z 764 ([M + H]+, 4.2); HRESIMS m/z 764.4678 [M + H]+ (calcd for C52H62NO4, 764.4678).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.8 Hz, Ar-H), 7.86 (1H, s, –NH), 7.77 (1H, d, J = 16.0 Hz, H-9′), 7.61 (2H, d, J = 8.4 Hz, Ar-H), 7.42 (1H, d, J = 7.2 Hz, Ar-H), 7.37 (1H, d, J = 15.6 Hz, H-8′), 7.29 (1H, d, J = 7.6 Hz, Ar-H), 7.19 (2H, d, J = 8.8 Hz, Ar-H), 7.12 (1H, m, Ar-H), 7.09 (1H, m, Ar-H), 6.95 (2H, d, J = 8.4 Hz, Ar-H), 5.50 (1H, s, H-12), 3.86 (3H, s, –OMe), 3.03 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 1.31 (3H, s, Me), 1.26 (6H, s, 2 × Me), 1.22(3H, s, Me), 1.02 (3H, s, Me), 0.97 (3H, s, Me), 0.96(3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 189.4, 175.8, 161.7, 154.6, 144.8, 142.9, 140.8, 136.1, 135.8, 130.2 (C × 2 in Ph), 129.9 (C × 2 in Ph), 128.2, 127.5, 123.3, 121.7 (C × 2 in Ph), 120.9, 119.5, 118.8, 117.9, 114.4 (C × 2 in Ph), 110.4, 106.8, 55.4, 53.2, 47.7, 46.3, 45.8, 42.0, 41.6, 39.6, 38.1, 36.8, 34.0, 33.9, 33.1, 32.3 (C × 2), 31.0, 30.7, 27.9, 25.7, 23.6, 23.5, 23.2, 23.1, 19.3, 17.3, 15.6; ESI MS: m/z 764 ([M + H]+, 4.2); HRESIMS m/z 764.4678 [M + H]+ (calcd for C52H62NO4, 764.4678).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.87 (1H, s, –NH), 7.78 (1H, d, J = 15.6 Hz, H-9′), 7.48 (1H, d, J = 15.6 Hz, H-8′), 7.42 (1H, d, J = 7.6 Hz, Ar-H), 7.30 (1H, d, J = 7.6 Hz, Ar-H), 7.27 (1H, m, H-6′), 7.24 (1H, d, J = 2.4 Hz, H-2′), 7.12 (1H, m, Ar-H), 7.09 (1H, m, Ar-H), 7.03 (1H, d, J = 8.8 Hz, H-5′), 6.99 (2H, d, J = 8.0 Hz, Ar-H), 5.47 (1H, s, H-12), 3.89 (3H, s, –OMe), 3.88 (3H, s, –OMe), 3.04 (1H, dd, J1 = 4.0 Hz, J2 = 14.0 Hz, H-18), 1.33 (3H, s, Me), 1.27 (3H, s, Me), 1.26(3H, s, Me), 1.24 (3H, s, Me), 1.02 (3H, s, Me), 0.98(6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 188.7, 175.6, 163.4, 151.8, 143.5, 143.0, 142.0, 140.8, 136.1, 133.7, 131.0, 130.8 (C × 2 in Ph), 128.2, 123.4, 123.0, 121.8, 121.2, 120.9, 118.8, 117.9, 113.8 (C × 2 in Ph), 111.8, 110.3, 106.9, 55.9, 55.5, 53.2, 47.4, 46.3, 45.9, 42.1, 41.6, 39.6, 38.1, 36.8, 34.0, 34.0, 33.1, 32.4 (C × 2), 31.0, 30.7, 27.8, 25.6, 23.6, 23.5, 23.3, 23.1, 19.4, 17.2, 15.6; ESI MS: m/z 794 ([M + H]+, 6.7); HRESIMS m/z 794.4791 [M + H]+ (calcd for C53H64NO5, 794.4784).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.87 (1H, s, –NH), 7.78 (1H, d, J = 15.6 Hz, H-9′), 7.48 (1H, d, J = 15.6 Hz, H-8′), 7.42 (1H, d, J = 7.6 Hz, Ar-H), 7.30 (1H, d, J = 7.6 Hz, Ar-H), 7.27 (1H, m, H-6′), 7.24 (1H, d, J = 2.4 Hz, H-2′), 7.12 (1H, m, Ar-H), 7.09 (1H, m, Ar-H), 7.03 (1H, d, J = 8.8 Hz, H-5′), 6.99 (2H, d, J = 8.0 Hz, Ar-H), 5.47 (1H, s, H-12), 3.89 (3H, s, –OMe), 3.88 (3H, s, –OMe), 3.04 (1H, dd, J1 = 4.0 Hz, J2 = 14.0 Hz, H-18), 1.33 (3H, s, Me), 1.27 (3H, s, Me), 1.26(3H, s, Me), 1.24 (3H, s, Me), 1.02 (3H, s, Me), 0.98(6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 188.7, 175.6, 163.4, 151.8, 143.5, 143.0, 142.0, 140.8, 136.1, 133.7, 131.0, 130.8 (C × 2 in Ph), 128.2, 123.4, 123.0, 121.8, 121.2, 120.9, 118.8, 117.9, 113.8 (C × 2 in Ph), 111.8, 110.3, 106.9, 55.9, 55.5, 53.2, 47.4, 46.3, 45.9, 42.1, 41.6, 39.6, 38.1, 36.8, 34.0, 34.0, 33.1, 32.4 (C × 2), 31.0, 30.7, 27.8, 25.6, 23.6, 23.5, 23.3, 23.1, 19.4, 17.2, 15.6; ESI MS: m/z 794 ([M + H]+, 6.7); HRESIMS m/z 794.4791 [M + H]+ (calcd for C53H64NO5, 794.4784).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.75 (1H, d, J = 15.6 Hz, H-9′), 7.54 (2H, d, J = 8.0 Hz, Ar-H), 7.50 (2H, d, J = 8.0 Hz, Ar-H), 7.46 (1H, d, J = 16.0 Hz, H-8′), 7.17 (2H, d, J = 8.0 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 5.82 (1H, d, J = 10.0 Hz, H-2), 5.38 (1H, s, H-12), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 1.17 (3H, s, Me), 1.15 (3H, s, Me), 1.09 (3H, s, Me), 0.99 (3H, s, Me), 0.94 (3H, s, Me), 0.93 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.7, 189.4, 176.1, 159.0, 155.2, 143.9, 143.5, 135.8, 134.1, 132.2 (C × 2 in Ph), 130.1 (C × 2 in Ph), 129.8 (C × 2 in Ph), 125.5, 125.3, 122.3, 122.2, 121.8 (C × 2 in Ph), 53.8, 45.0, 47.8, 46.0, 42.6, 42.1 (C × 2), 40.7, 39.8, 34.2, 33.5, 33.0, 32.7, 31.2, 28.2 (C × 2), 25.7, 23.6, 23.4, 23.0, 22.1, 19.1, 18.7, 17.9; EI MS: m/z 738 ([M + 2]+, 4), 736 ([M]+, 3), 407 (100), 248 (31), 203 (70), 189 (38), 69 (39), 57 (54); HREIMS m/z 736.3125 (calcd for C45H53BrO4, 736.3105).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.75 (1H, d, J = 15.6 Hz, H-9′), 7.54 (2H, d, J = 8.0 Hz, Ar-H), 7.50 (2H, d, J = 8.0 Hz, Ar-H), 7.46 (1H, d, J = 16.0 Hz, H-8′), 7.17 (2H, d, J = 8.0 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 5.82 (1H, d, J = 10.0 Hz, H-2), 5.38 (1H, s, H-12), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 1.17 (3H, s, Me), 1.15 (3H, s, Me), 1.09 (3H, s, Me), 0.99 (3H, s, Me), 0.94 (3H, s, Me), 0.93 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.7, 189.4, 176.1, 159.0, 155.2, 143.9, 143.5, 135.8, 134.1, 132.2 (C × 2 in Ph), 130.1 (C × 2 in Ph), 129.8 (C × 2 in Ph), 125.5, 125.3, 122.3, 122.2, 121.8 (C × 2 in Ph), 53.8, 45.0, 47.8, 46.0, 42.6, 42.1 (C × 2), 40.7, 39.8, 34.2, 33.5, 33.0, 32.7, 31.2, 28.2 (C × 2), 25.7, 23.6, 23.4, 23.0, 22.1, 19.1, 18.7, 17.9; EI MS: m/z 738 ([M + 2]+, 4), 736 ([M]+, 3), 407 (100), 248 (31), 203 (70), 189 (38), 69 (39), 57 (54); HREIMS m/z 736.3125 (calcd for C45H53BrO4, 736.3105).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.79 (1H, d, J = 15.6 Hz, H-9′), 7.59 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 6.94 (2H, d, J = 8.0 Hz, Ar-H), 5.81 (1H, d, J = 10.0 Hz, H-2), 5.43 (1H, s, H-12), 3.84 (3H, s, –OMe), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.22 (3H, s, Me), 1.17 (3H, s, Me), 1.16 (3H, s, Me), 1.00 (3H, s, Me), 0.99 (3H, s, Me), 0.95 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.3, 189.3, 175.7, 161.7, 159.1, 154.5, 144.9, 143.6, 135.8, 130.8 (C × 2 in Ph), 129.9 (C × 2 in Ph), 127.5, 125.1, 122.3, 121.7 (C × 2 in Ph), 119.4, 114.4 (C × 2 in Ph), 55.4, 53.4, 44.5, 47.3, 45.5, 42.2, 41.7, 41.5, 40.2, 39.4, 33.8, 33.0, 32.2 (C × 2), 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 23.0, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 692 ([M + 4]+, 4), 688 ([M]+, 12), 484 (14), 407 (100), 217 (30), 203 (61), 189 (51), 105 (36), 69 (31); HREIMS m/z 688.4112 (calcd for C46H56O5, 688.4097).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.79 (1H, d, J = 15.6 Hz, H-9′), 7.59 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 6.94 (2H, d, J = 8.0 Hz, Ar-H), 5.81 (1H, d, J = 10.0 Hz, H-2), 5.43 (1H, s, H-12), 3.84 (3H, s, –OMe), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.22 (3H, s, Me), 1.17 (3H, s, Me), 1.16 (3H, s, Me), 1.00 (3H, s, Me), 0.99 (3H, s, Me), 0.95 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.3, 189.3, 175.7, 161.7, 159.1, 154.5, 144.9, 143.6, 135.8, 130.8 (C × 2 in Ph), 129.9 (C × 2 in Ph), 127.5, 125.1, 122.3, 121.7 (C × 2 in Ph), 119.4, 114.4 (C × 2 in Ph), 55.4, 53.4, 44.5, 47.3, 45.5, 42.2, 41.7, 41.5, 40.2, 39.4, 33.8, 33.0, 32.2 (C × 2), 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 23.0, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 692 ([M + 4]+, 4), 688 ([M]+, 12), 484 (14), 407 (100), 217 (30), 203 (61), 189 (51), 105 (36), 69 (31); HREIMS m/z 688.4112 (calcd for C46H56O5, 688.4097).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.82 (1H, d, J = 15.6 Hz, H-9′), 7.63 (2H, d, J = 8.4 Hz, Ar-H), 7.51 (1H, d, J = 15.6 Hz, H-8′), 7.47 (3H, m, Ar-H), 7.18 (2H, d, J = 8.0 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 5.81 (1H, d, J = 10.0 Hz, H-2), 5.47 (1H, s, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.22 (3H, s, Me), 1.17 (3H, s, Me), 1.15 (3H, s, Me), 1.10 (3H, s, Me), 0.99 (3H, s, Me), 0.96 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.3, 189.3, 175.7, 159.0, 154.7, 144.5, 143.6, 135.5, 134.7, 130.6, 130.1 (C × 2 in Ph), 128.9 (C × 2 in Ph), 128.4(C × 2 in Ph), 125.1, 122.3, 121.8 (C × 2 in Ph), 121.7, 53.4, 44.5, 47.3, 45.5, 42.2, 41.7, 41.4, 40.2, 39.4, 33.8, 33.0, 32.6, 32.2, 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 23.0, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 658 ([M]+, 7), 454 (13), 407 (100), 203 (38), 189 (34), 107 (18), 69 (11); HREIMS m/z 658.4028 (calcd for C45H54O4, 658.4034).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.82 (1H, d, J = 15.6 Hz, H-9′), 7.63 (2H, d, J = 8.4 Hz, Ar-H), 7.51 (1H, d, J = 15.6 Hz, H-8′), 7.47 (3H, m, Ar-H), 7.18 (2H, d, J = 8.0 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 5.81 (1H, d, J = 10.0 Hz, H-2), 5.47 (1H, s, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.22 (3H, s, Me), 1.17 (3H, s, Me), 1.15 (3H, s, Me), 1.10 (3H, s, Me), 0.99 (3H, s, Me), 0.96 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.3, 189.3, 175.7, 159.0, 154.7, 144.5, 143.6, 135.5, 134.7, 130.6, 130.1 (C × 2 in Ph), 128.9 (C × 2 in Ph), 128.4(C × 2 in Ph), 125.1, 122.3, 121.8 (C × 2 in Ph), 121.7, 53.4, 44.5, 47.3, 45.5, 42.2, 41.7, 41.4, 40.2, 39.4, 33.8, 33.0, 32.6, 32.2, 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 23.0, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 658 ([M]+, 7), 454 (13), 407 (100), 203 (38), 189 (34), 107 (18), 69 (11); HREIMS m/z 658.4028 (calcd for C45H54O4, 658.4034).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.60 (1H, d, J = 15.6 Hz, H-9′), 7.52 (1H, s, H-5′′), 7.44 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 6.72 (1H, d, J = 3.2 Hz, H-3′′), 6.51 (1H, t, J = 1.6 Hz, H-4′′), 5.81 (1H, d, J = 10.0 Hz, H-2), 5.43 (1H, s, H-12), 3.02 (1H, dd, J1 = 4.0 Hz, J2 = 14.0 Hz, H-18), 1.15 (3H, s, Me), 1.09 (6H, s, 2 × Me), 1.04 (3H, s, Me), 0.97 (3H, s, Me), 0.94 (3H, s, Me), 0.92 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.3, 188.6, 175.7, 159.1, 154.6, 151.5, 145.0, 143.6, 135.4, 130.8, 129.9 (C × 2 in Ph), 125.0, 122.3, 121.7 (C × 2 in Ph), 118.9, 116.4, 112.7, 55.4, 44.5, 47.3, 45.5, 42.2, 41.6, 41.4, 40.2, 39.4, 33.8, 33.0, 32.2 (C × 2), 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 22.9, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 648 ([M]+, 10), 444 (14), 407 (100), 215 (24), 203 (44), 187 (32), 107 (16), 69 (29), 55 (15); HREIMS m/z 648.3792 (calcd for C43H52O5, 648.3770).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.60 (1H, d, J = 15.6 Hz, H-9′), 7.52 (1H, s, H-5′′), 7.44 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.04 (1H, d, J = 10.0 Hz, H-1), 6.72 (1H, d, J = 3.2 Hz, H-3′′), 6.51 (1H, t, J = 1.6 Hz, H-4′′), 5.81 (1H, d, J = 10.0 Hz, H-2), 5.43 (1H, s, H-12), 3.02 (1H, dd, J1 = 4.0 Hz, J2 = 14.0 Hz, H-18), 1.15 (3H, s, Me), 1.09 (6H, s, 2 × Me), 1.04 (3H, s, Me), 0.97 (3H, s, Me), 0.94 (3H, s, Me), 0.92 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.3, 188.6, 175.7, 159.1, 154.6, 151.5, 145.0, 143.6, 135.4, 130.8, 129.9 (C × 2 in Ph), 125.0, 122.3, 121.7 (C × 2 in Ph), 118.9, 116.4, 112.7, 55.4, 44.5, 47.3, 45.5, 42.2, 41.6, 41.4, 40.2, 39.4, 33.8, 33.0, 32.2 (C × 2), 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 22.9, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 648 ([M]+, 10), 444 (14), 407 (100), 215 (24), 203 (44), 187 (32), 107 (16), 69 (29), 55 (15); HREIMS m/z 648.3792 (calcd for C43H52O5, 648.3770).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.02 (2H, d, J = 8.0 Hz, Ar-H), 7.90 (1H, d, J = 15.6 Hz, H-9′), 7.41 (1H, d, J = 4.4 Hz, H-5′′), 7.34 (1H, d, J = 3.6 Hz, H-3′′), 7.30 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.06 (1H, t, J = 3.6 Hz, H-4′′), 7.03 (1H, d, J = 10.0 Hz, H-1), 5.80 (1H, d, J = 10.0 Hz, H-2), 5.42 (1H, s, H-12), 3.02 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.21 (3H, s, Me), 1.17 (3H, s, Me), 1.15 (3H, s, Me), 1.10 (3H, s, Me), 0.99 (3H, s, Me), 0.95 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.2, 188.6, 175.6, 159.0, 154.6, 143.6, 140.2, 137.4, 135.4, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 125.0, 122.3, 121.8 (C × 2 in Ph), 120.4, 53.4, 44.5, 47.3, 45.5, 42.1, 41.7, 41.5, 40.2, 39.4, 33.8, 33.0, 32.6, 32.2, 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 23.0, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 664 ([M]+, 6), 407 (76), 248 (44), 203 (100), 189 (41), 107 (30), 69 (26); HREIMS m/z 664.3594 (calcd for C43H52SO4, 664.3602).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.02 (2H, d, J = 8.0 Hz, Ar-H), 7.90 (1H, d, J = 15.6 Hz, H-9′), 7.41 (1H, d, J = 4.4 Hz, H-5′′), 7.34 (1H, d, J = 3.6 Hz, H-3′′), 7.30 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.06 (1H, t, J = 3.6 Hz, H-4′′), 7.03 (1H, d, J = 10.0 Hz, H-1), 5.80 (1H, d, J = 10.0 Hz, H-2), 5.42 (1H, s, H-12), 3.02 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.21 (3H, s, Me), 1.17 (3H, s, Me), 1.15 (3H, s, Me), 1.10 (3H, s, Me), 0.99 (3H, s, Me), 0.95 (3H, s, Me), 0.94 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 205.2, 188.6, 175.6, 159.0, 154.6, 143.6, 140.2, 137.4, 135.4, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 125.0, 122.3, 121.8 (C × 2 in Ph), 120.4, 53.4, 44.5, 47.3, 45.5, 42.1, 41.7, 41.5, 40.2, 39.4, 33.8, 33.0, 32.6, 32.2, 30.7, 27.8, 27.7, 25.7, 23.6, 23.3, 23.0, 21.6, 18.8, 18.7, 17.9; EI MS: m/z 664 ([M]+, 6), 407 (76), 248 (44), 203 (100), 189 (41), 107 (30), 69 (26); HREIMS m/z 664.3594 (calcd for C43H52SO4, 664.3602).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.20 (1H, d, J = 15.6 Hz, H-9′), 8.05 (2H, d, J = 8.0 Hz, Ar-H), 7.74 (1H, m, Ar-H), 7.48 (1H, d, J = 16.0 Hz, H-8′), 7.44 (1H, m, Ar-H), 7.19 (2H, d, J = 8.0 Hz, Ar-H), 7.32 (2H, m, Ar-H), 5.41 (3H, m, H-2, H-3, H-12), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.4 Hz, H-18), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.95 (3H, s, Me), 0.89 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 189.6, 176.28, 155.3, 143.4, 141.1, 138.3, 135.9 135.6, 133.6, 131.6, 130.7, 130.6 (C × 2 in Ph), 128.2, 127.5, 124.9, 123.3, 121.8, 122.3 (C × 2 in Ph), 52.4, 47.8, 46.5, 46.2, 42.4, 42.0, 41.2, 40.1, 36.6, 34.3, 33.5, 33.1, 32.7, 32.2, 31.8, 31.2, 27.8, 25.7, 24.0, 23.8, 23.6, 23.2, 19.9, 17.6, 16.0; EI MS: m/z 678 ([M]+, 8), 488 (24), 393 (68), 203 (100), 189 (54), 149 (91), 95 (63), 69 (74), 57 (94); HREIMS m/z 678.3838 (calcd for C45H55ClO3, 678.3826).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.20 (1H, d, J = 15.6 Hz, H-9′), 8.05 (2H, d, J = 8.0 Hz, Ar-H), 7.74 (1H, m, Ar-H), 7.48 (1H, d, J = 16.0 Hz, H-8′), 7.44 (1H, m, Ar-H), 7.19 (2H, d, J = 8.0 Hz, Ar-H), 7.32 (2H, m, Ar-H), 5.41 (3H, m, H-2, H-3, H-12), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.4 Hz, H-18), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.95 (3H, s, Me), 0.89 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 189.6, 176.28, 155.3, 143.4, 141.1, 138.3, 135.9 135.6, 133.6, 131.6, 130.7, 130.6 (C × 2 in Ph), 128.2, 127.5, 124.9, 123.3, 121.8, 122.3 (C × 2 in Ph), 52.4, 47.8, 46.5, 46.2, 42.4, 42.0, 41.2, 40.1, 36.6, 34.3, 33.5, 33.1, 32.7, 32.2, 31.8, 31.2, 27.8, 25.7, 24.0, 23.8, 23.6, 23.2, 19.9, 17.6, 16.0; EI MS: m/z 678 ([M]+, 8), 488 (24), 393 (68), 203 (100), 189 (54), 149 (91), 95 (63), 69 (74), 57 (94); HREIMS m/z 678.3838 (calcd for C45H55ClO3, 678.3826).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.79 (1H, d, J = 15.6 Hz, H-9′), 7.60 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, d, J = 15.6 Hz, H-8′), 7.17 (2H, d, J = 8.4 Hz, Ar-H), 6.94 (2H, d, J = 8.0 Hz, Ar-H), 5.40 (3H, m, H-2, H-3, H-12), 3.85 (3H, s, –OMe), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.91 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 189.4, 175.8, 161.7, 154.6, 144.8, 142.9, 137.9, 135.7, 130.2 (C × 2 in Ph), 129.9 (C × 2 in Ph), 127.5, 123.2, 121.3, 121.7 (C × 2 in Ph), 119.6, 114.4 (C × 2 in Ph), 55.4, 52.0, 47.3, 46.1, 45.7, 41.9, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.0, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.6, 23.6, 23.3, 23.1, 22.7, 19.5, 17.2, 15.6; EI MS: m/z 674 ([M]+, 4), 484 (10), 393 (42), 203 (100), 189 (44), 95 (28), 69 (22); HREIMS m/z 674.4338 (calcd for C46H58O4, 674.4340).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.0 Hz, Ar-H), 7.79 (1H, d, J = 15.6 Hz, H-9′), 7.60 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, d, J = 15.6 Hz, H-8′), 7.17 (2H, d, J = 8.4 Hz, Ar-H), 6.94 (2H, d, J = 8.0 Hz, Ar-H), 5.40 (3H, m, H-2, H-3, H-12), 3.85 (3H, s, –OMe), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.91 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 189.4, 175.8, 161.7, 154.6, 144.8, 142.9, 137.9, 135.7, 130.2 (C × 2 in Ph), 129.9 (C × 2 in Ph), 127.5, 123.2, 121.3, 121.7 (C × 2 in Ph), 119.6, 114.4 (C × 2 in Ph), 55.4, 52.0, 47.3, 46.1, 45.7, 41.9, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.0, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.6, 23.6, 23.3, 23.1, 22.7, 19.5, 17.2, 15.6; EI MS: m/z 674 ([M]+, 4), 484 (10), 393 (42), 203 (100), 189 (44), 95 (28), 69 (22); HREIMS m/z 674.4338 (calcd for C46H58O4, 674.4340).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.05 (2H, d, J = 8.0 Hz, Ar-H), 7.82 (1H, d, J = 15.6 Hz, H-9′), 7.64 (2H, d, J = 8.4 Hz, Ar-H), 7.52 (1H, d, J = 16.0 Hz, H-8′), 7.48 (3H, m, Ar-H), 7.18 (2H, d, J = 8.0 Hz, Ar-H), 5.40 (3H, m, H-2, H-3, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.20 (3H, s, Me), 0.99 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.95 (3H, s, Me), 0.90 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 189.4, 175.8, 154.7, 145.0, 142.9, 137.9, 135.4, 134.8, 130.6, 130.0 (C × 2 in Ph), 129.0 (C × 2 in Ph), 128.4(C × 2 in Ph), 121.4, 123.2, 121.8 (C × 2 in Ph), 121.7, 52.0, 47.4, 46.1, 45.7, 42.0, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.7, 23.6, 23.3, 23.1, 22.8, 19.5, 17.2, 15.6; EI MS: m/z 647 ([M + 3]+, 4), 644 ([M]+, 9), 454 (38), 393 (78), 203 (100), 189 (54), 107 (23), 81 (16); HREIMS m/z 644.4222 (calcd for C45H56O3, 644.4215).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.05 (2H, d, J = 8.0 Hz, Ar-H), 7.82 (1H, d, J = 15.6 Hz, H-9′), 7.64 (2H, d, J = 8.4 Hz, Ar-H), 7.52 (1H, d, J = 16.0 Hz, H-8′), 7.48 (3H, m, Ar-H), 7.18 (2H, d, J = 8.0 Hz, Ar-H), 5.40 (3H, m, H-2, H-3, H-12), 3.00 (1H, dd, J1 = 4.0 Hz, J2 = 13.6 Hz, H-18), 1.20 (3H, s, Me), 0.99 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.95 (3H, s, Me), 0.90 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 189.4, 175.8, 154.7, 145.0, 142.9, 137.9, 135.4, 134.8, 130.6, 130.0 (C × 2 in Ph), 129.0 (C × 2 in Ph), 128.4(C × 2 in Ph), 121.4, 123.2, 121.8 (C × 2 in Ph), 121.7, 52.0, 47.4, 46.1, 45.7, 42.0, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.7, 23.6, 23.3, 23.1, 22.8, 19.5, 17.2, 15.6; EI MS: m/z 647 ([M + 3]+, 4), 644 ([M]+, 9), 454 (38), 393 (78), 203 (100), 189 (54), 107 (23), 81 (16); HREIMS m/z 644.4222 (calcd for C45H56O3, 644.4215).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.60 (1H, d, J = 15.6 Hz, H-9′), 7.52 (1H, s, H-5′′), 7.44 (1H, d, J = 15.6 Hz, H-8′), 7.17 (2H, d, J = 8.4 Hz, Ar-H), 6.71 (1H, d, J = 3.2 Hz, H-3′′), 6.50 (1H, t, J = 1.6 Hz, H-4′′), 5.39 (3H, m, H-2, H-3, H-12), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.91 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.8, 154.7, 151.6, 144.9, 142.9, 137.9, 135.4, 130.8, 129.9 (C × 2 in Ph), 123.2, 121.4, 121.8 (C × 2 in Ph), 119.0, 116.4, 112.7, 52.0, 47.4, 46.1, 45.7, 41.9, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.7, 23.6, 23.3, 23.1, 22.8, 19.5, 17.2, 15.6; EI MS: m/z 634 ([M]+, 8), 444 (28), 393 (67), 215 (34), 203 (98), 189 (50), 149 (100), 69 (17), 55 (14); HREIMS m/z 634.4037 (calcd for C43H54O4, 634.4052).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.60 (1H, d, J = 15.6 Hz, H-9′), 7.52 (1H, s, H-5′′), 7.44 (1H, d, J = 15.6 Hz, H-8′), 7.17 (2H, d, J = 8.4 Hz, Ar-H), 6.71 (1H, d, J = 3.2 Hz, H-3′′), 6.50 (1H, t, J = 1.6 Hz, H-4′′), 5.39 (3H, m, H-2, H-3, H-12), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.91 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.8, 154.7, 151.6, 144.9, 142.9, 137.9, 135.4, 130.8, 129.9 (C × 2 in Ph), 123.2, 121.4, 121.8 (C × 2 in Ph), 119.0, 116.4, 112.7, 52.0, 47.4, 46.1, 45.7, 41.9, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.7, 23.6, 23.3, 23.1, 22.8, 19.5, 17.2, 15.6; EI MS: m/z 634 ([M]+, 8), 444 (28), 393 (67), 215 (34), 203 (98), 189 (50), 149 (100), 69 (17), 55 (14); HREIMS m/z 634.4037 (calcd for C43H54O4, 634.4052).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.03 (2H, d, J = 8.0 Hz, Ar-H), 7.95 (1H, d, J = 15.6 Hz, H-9′), 7.41 (1H, d, J = 4.4 Hz, H-5′′), 7.35 (1H, d, J = 3.6 Hz, H-3′′), 7.31 (1H, d, J = 15.6 Hz, H-8′), 7.17 (2H, d, J = 8.4 Hz, Ar-H), 7.07 (1H, t, J = 3.6 Hz, H-4′′), 5.40 (3H, m, H-2, H-3, H-12), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.95 (3H, s, Me), 0.89 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.7, 154.7, 142.9, 140.3, 137.9, 137.3, 135.4, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 122.3, 121.8 (C × 2 in Ph), 121.4, 120.5, 52.0, 47.4, 46.1, 45.1, 42.0, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.8, 25.7, 23.6, 23.3, 23.1, 22.8, 19.5, 17.2, 15.6; EI MS: m/z 653 ([M + 3]+, 3), 650 ([M]+, 8), 460 (40), 393 (80), 203 (100), 189 (50), 95 (33), 69 (13); HREIMS m/z 650.3802 (calcd for C43H54SO3, 650.3810).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.03 (2H, d, J = 8.0 Hz, Ar-H), 7.95 (1H, d, J = 15.6 Hz, H-9′), 7.41 (1H, d, J = 4.4 Hz, H-5′′), 7.35 (1H, d, J = 3.6 Hz, H-3′′), 7.31 (1H, d, J = 15.6 Hz, H-8′), 7.17 (2H, d, J = 8.4 Hz, Ar-H), 7.07 (1H, t, J = 3.6 Hz, H-4′′), 5.40 (3H, m, H-2, H-3, H-12), 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.95 (3H, s, Me), 0.89 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.7, 154.7, 142.9, 140.3, 137.9, 137.3, 135.4, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 122.3, 121.8 (C × 2 in Ph), 121.4, 120.5, 52.0, 47.4, 46.1, 45.1, 42.0, 41.6, 40.7, 39.6, 36.2, 34.4, 33.8, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.8, 25.7, 23.6, 23.3, 23.1, 22.8, 19.5, 17.2, 15.6; EI MS: m/z 653 ([M + 3]+, 3), 650 ([M]+, 8), 460 (40), 393 (80), 203 (100), 189 (50), 95 (33), 69 (13); HREIMS m/z 650.3802 (calcd for C43H54SO3, 650.3810).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.4 Hz, Ar-H), 7.79 (1H, d, J = 15.6 Hz, H-9′), 7.61 (1H, m, H-4′′), 7.53 (2H, d, J = 8.0 Hz, Ar-H), 7.48 (H, d, J = 15.6 Hz, H-8′), 7.28 (1H, m, H-6′), 7.24 (1H, d, J = 2.4 Hz, H-2′), 7.01 (1H, d, J = 8.4 Hz, H-5′), 5.40 (3H, m, H-2, H-3, H-12), 3.89 (3H, s, –OMe), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.23 (3H, s, Me), 1.01 (3H, s, Me), 0.99 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.93 (3H, s, Me), 0.92 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 190.5, 175.5, 151.8, 144.4, 143.4, 142.0, 138.2, 137.9, 133.4, 132.7, 128.6 (C × 2 in Ph), 128.5 (C × 2 in Ph), 123.4, 123.0, 122.0, 121.3, 121.4, 111.8, 55.9, 52.0, 47.4, 46.1, 45.9, 42.0, 41.6, 40.7, 39.6, 36.2, 34.4, 33.9, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.6, 23.6, 23.3, 23.1, 22.8, 19.5, 17.1, 15.6; EI MS: m/z 674 ([M]+, 1), 407 (10), 393 (100), 203 (28), 189 (32), 95 (24), 69 (10); HREIMS m/z 674.4310 (calcd for C46H58O4, 674.4285).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.04 (2H, d, J = 8.4 Hz, Ar-H), 7.79 (1H, d, J = 15.6 Hz, H-9′), 7.61 (1H, m, H-4′′), 7.53 (2H, d, J = 8.0 Hz, Ar-H), 7.48 (H, d, J = 15.6 Hz, H-8′), 7.28 (1H, m, H-6′), 7.24 (1H, d, J = 2.4 Hz, H-2′), 7.01 (1H, d, J = 8.4 Hz, H-5′), 5.40 (3H, m, H-2, H-3, H-12), 3.89 (3H, s, –OMe), 3.00 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.23 (3H, s, Me), 1.01 (3H, s, Me), 0.99 (3H, s, Me), 0.97 (3H, s, Me), 0.96 (3H, s, Me), 0.93 (3H, s, Me), 0.92 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 190.5, 175.5, 151.8, 144.4, 143.4, 142.0, 138.2, 137.9, 133.4, 132.7, 128.6 (C × 2 in Ph), 128.5 (C × 2 in Ph), 123.4, 123.0, 122.0, 121.3, 121.4, 111.8, 55.9, 52.0, 47.4, 46.1, 45.9, 42.0, 41.6, 40.7, 39.6, 36.2, 34.4, 33.9, 33.1, 32.3 (C × 2), 31.8, 30.7, 27.7, 25.6, 23.6, 23.3, 23.1, 22.8, 19.5, 17.1, 15.6; EI MS: m/z 674 ([M]+, 1), 407 (10), 393 (100), 203 (28), 189 (32), 95 (24), 69 (10); HREIMS m/z 674.4310 (calcd for C46H58O4, 674.4285).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.03 (2H, d, J = 8.0 Hz, Ar-H), 7.79 (1H, d, J = 16.0 Hz, H-9′), 7.59 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, d, J = 16.0 Hz, H-8′), 7.15 (2H, d, J = 8.4 Hz, Ar-H), 6.94 (2H, d, J = 8.4 Hz, Ar-H), 5.39 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.85 (3H, s, –OMe) 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 1.74 (3H, s, Me), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 189.3, 175.7, 161.7, 154.6, 146.7, 144.9, 143.4, 135.8, 130.2 (C × 2 in Ph), 129.9 (C × 2 in Ph), 127.5, 122.3, 121.7 (C × 2 in Ph), 120.2, 119.5, 114.4 (C × 2 in Ph), 114.2, 55.4, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.3, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.5, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 688 ([M + 1]+, 4), 687 ([M]+, 5), 406 (82), 248 (58), 203 (100), 189 (85), 69 (48), 57 (55); HREIMS m/z 687.4271 (calcd for C46H57NO4, 687.4254).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.03 (2H, d, J = 8.0 Hz, Ar-H), 7.79 (1H, d, J = 16.0 Hz, H-9′), 7.59 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, d, J = 16.0 Hz, H-8′), 7.15 (2H, d, J = 8.4 Hz, Ar-H), 6.94 (2H, d, J = 8.4 Hz, Ar-H), 5.39 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.85 (3H, s, –OMe) 3.02 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 1.74 (3H, s, Me), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 189.3, 175.7, 161.7, 154.6, 146.7, 144.9, 143.4, 135.8, 130.2 (C × 2 in Ph), 129.9 (C × 2 in Ph), 127.5, 122.3, 121.7 (C × 2 in Ph), 120.2, 119.5, 114.4 (C × 2 in Ph), 114.2, 55.4, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.3, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.5, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 688 ([M + 1]+, 4), 687 ([M]+, 5), 406 (82), 248 (58), 203 (100), 189 (85), 69 (48), 57 (55); HREIMS m/z 687.4271 (calcd for C46H57NO4, 687.4254).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.82 (1H, d, J = 15.6 Hz, H-9′), 7.62 (2H, d, J = 8.4 Hz, Ar-H), 7.52 (1H, d, J = 15.6 Hz, H-8′), 7.42 (3H, m, Ar-H), 7.17 (2H, d, J = 8.0 Hz, Ar-H), 5.38 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.74 (3H, s, Me), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 189.7, 176.1, 155.1, 147.1, 145.4, 143.8, 135.9, 135.2, 131.1, 130.5 (C × 2 in Ph), 129.4 (C × 2 in Ph), 128.9 (C × 2 in Ph), 122.8, 122.2 (C × 2 in Ph), 121.7, 120.6, 114.6, 51.1, 47.2, 46.0, 42.7, 41.9, 39.9, 39.7, 38.2, 34.7, 34.2, 33.5, 32.7, 31.8, 31.2, 30.1, 27.7, 26.1, 24.5, 24.1, 24.0, 23.4, 19.5, 17.9, 12.0; EI MS: m/z 657 ([M]+, 3), 406 (63), 248 (78), 203 (100), 189 (21), 69 (54), 57 (44); HREIMS m/z 657.4190 (calcd for C45H55NO3, 657.4198).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.06 (2H, d, J = 8.0 Hz, Ar-H), 7.82 (1H, d, J = 15.6 Hz, H-9′), 7.62 (2H, d, J = 8.4 Hz, Ar-H), 7.52 (1H, d, J = 15.6 Hz, H-8′), 7.42 (3H, m, Ar-H), 7.17 (2H, d, J = 8.0 Hz, Ar-H), 5.38 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.74 (3H, s, Me), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 189.7, 176.1, 155.1, 147.1, 145.4, 143.8, 135.9, 135.2, 131.1, 130.5 (C × 2 in Ph), 129.4 (C × 2 in Ph), 128.9 (C × 2 in Ph), 122.8, 122.2 (C × 2 in Ph), 121.7, 120.6, 114.6, 51.1, 47.2, 46.0, 42.7, 41.9, 39.9, 39.7, 38.2, 34.7, 34.2, 33.5, 32.7, 31.8, 31.2, 30.1, 27.7, 26.1, 24.5, 24.1, 24.0, 23.4, 19.5, 17.9, 12.0; EI MS: m/z 657 ([M]+, 3), 406 (63), 248 (78), 203 (100), 189 (21), 69 (54), 57 (44); HREIMS m/z 657.4190 (calcd for C45H55NO3, 657.4198).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.07 (2H, d, J = 8.0 Hz, Ar-H), 7.61 (1H, d, J = 15.6 Hz, H-9′), 7.53 (1H, s, H-5′′), 7.44 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 6.73 (1H, d, J = 4.0 Hz, H-3′′), 6.52 (1H, t, J = 1.6 Hz, H-4′′), 5.38 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 4.0 Hz, J2 = 14.0 Hz, H-18), 1.73 (3H, s, Me), 1.20 (3H, s, Me), 0.98 (3H, s, Me), 0.94 (3H, s, Me), 0.93 (3H, s, Me), 0.89 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.7, 154.7, 151.5, 146.7, 145.0, 143.4, 135.4, 130.8, 129.9 (C × 2 in Ph), 122.3, 121.8 (C × 2 in Ph), 120.2, 118.9, 116.5, 114.2, 112.7, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.2, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.5, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 647 ([M]+, 3), 406 (100), 248 (16), 203 (38), 189 (13), 69 (11), 55 (9); HREIMS m/z 647.3946 (calcd for C43H53NO4, 647.3917).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.07 (2H, d, J = 8.0 Hz, Ar-H), 7.61 (1H, d, J = 15.6 Hz, H-9′), 7.53 (1H, s, H-5′′), 7.44 (1H, d, J = 15.6 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 6.73 (1H, d, J = 4.0 Hz, H-3′′), 6.52 (1H, t, J = 1.6 Hz, H-4′′), 5.38 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 4.0 Hz, J2 = 14.0 Hz, H-18), 1.73 (3H, s, Me), 1.20 (3H, s, Me), 0.98 (3H, s, Me), 0.94 (3H, s, Me), 0.93 (3H, s, Me), 0.89 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.7, 154.7, 151.5, 146.7, 145.0, 143.4, 135.4, 130.8, 129.9 (C × 2 in Ph), 122.3, 121.8 (C × 2 in Ph), 120.2, 118.9, 116.5, 114.2, 112.7, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.2, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.5, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 647 ([M]+, 3), 406 (100), 248 (16), 203 (38), 189 (13), 69 (11), 55 (9); HREIMS m/z 647.3946 (calcd for C43H53NO4, 647.3917).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.03 (2H, d, J = 8.4 Hz, Ar-H), 7.95 (1H, d, J = 16.0 Hz, H-9′), 7.43 (1H, s, H-5′′), 7.36 (1H, d, J = 3.6 Hz, H-3′′), 7.31 (1H, d, J = 16.4 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.08 (1H, t, J = 3.6 Hz, H-4′′), 5.38 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 1.73 (3H, s, Me), 1.20 (3H, s, Me), 0.98 (3H, s, Me), 0.94 (3H, s, Me), 0.93 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.7, 154.6, 146.7, 143.4, 140.3, 137.4, 135.4, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 122.3, 121.8 (C × 2 in Ph), 120.5, 120.2, 114.2, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.4, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.5, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 663 ([M]+, 8), 406 (100), 248 (34), 203 (43), 189 (11), 69 (9), 55 (7); HREIMS m/z 663.3757 (calcd for C43H53NSO3, 663.3768).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.03 (2H, d, J = 8.4 Hz, Ar-H), 7.95 (1H, d, J = 16.0 Hz, H-9′), 7.43 (1H, s, H-5′′), 7.36 (1H, d, J = 3.6 Hz, H-3′′), 7.31 (1H, d, J = 16.4 Hz, H-8′), 7.16 (2H, d, J = 8.4 Hz, Ar-H), 7.08 (1H, t, J = 3.6 Hz, H-4′′), 5.38 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 13.6 Hz, H-18), 1.73 (3H, s, Me), 1.20 (3H, s, Me), 0.98 (3H, s, Me), 0.94 (3H, s, Me), 0.93 (3H, s, Me), 0.90 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 188.6, 175.7, 154.6, 146.7, 143.4, 140.3, 137.4, 135.4, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 122.3, 121.8 (C × 2 in Ph), 120.5, 120.2, 114.2, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.4, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.5, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 663 ([M]+, 8), 406 (100), 248 (34), 203 (43), 189 (11), 69 (9), 55 (7); HREIMS m/z 663.3757 (calcd for C43H53NSO3, 663.3768).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.01 (2H, d, J = 8.0 Hz, Ar-H), 7.80 (1H, d, J = 16.0 Hz, H-9′), 7.65 (2H, d, J = 8.0 Hz, Ar-H), 7.57 (1H, d, J = 15.6 Hz, H-8′), 7.48 (3H, m, Ar-H), 7.09 (2H, d, J = 8.0 Hz, Ar-H), 5.37 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.74 (3H, s, Me), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.91 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 190.3, 175.9, 152.8, 146.7, 143.8, 143.4, 138.1, 132.3, 132.8, 139.5 (C × 2 in Ph), 128.6 (C × 2 in Ph), 128.5 (C × 2 in Ph), 122.3, 122.2 (C × 2 in Ph), 121.9, 120.2, 114.2, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.3, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.6, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 657 ([M]+, 1), 406 (100), 248 (8), 203 (17), 189 (8), 69 (7), 55 (6); HREIMS m/z 657.4175 (calcd for C45H55NO3, 657.4168).
O) cm−1; 1H NMR (400 MHz, CDCl3): δ 8.01 (2H, d, J = 8.0 Hz, Ar-H), 7.80 (1H, d, J = 16.0 Hz, H-9′), 7.65 (2H, d, J = 8.0 Hz, Ar-H), 7.57 (1H, d, J = 15.6 Hz, H-8′), 7.48 (3H, m, Ar-H), 7.09 (2H, d, J = 8.0 Hz, Ar-H), 5.37 (1H, s, H-12), 4.90 (1H, s, H2-24), 4.66 (1H, s, H2-24), 3.01 (1H, dd, J1 = 3.6 Hz, J2 = 14.0 Hz, H-18), 1.74 (3H, s, Me), 1.21 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.91 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 190.3, 175.9, 152.8, 146.7, 143.8, 143.4, 138.1, 132.3, 132.8, 139.5 (C × 2 in Ph), 128.6 (C × 2 in Ph), 128.5 (C × 2 in Ph), 122.3, 122.2 (C × 2 in Ph), 121.9, 120.2, 114.2, 50.6, 47.2, 45.6, 42.3, 41.5, 39.4, 39.2, 37.8, 34.3, 33.8, 33.0, 32.3, 31.4, 30.7, 29.7, 27.7, 25.6, 24.1, 23.7, 23.6, 23.0, 19.1, 17.5, 11.5; EI MS: m/z 657 ([M]+, 1), 406 (100), 248 (8), 203 (17), 189 (8), 69 (7), 55 (6); HREIMS m/z 657.4175 (calcd for C45H55NO3, 657.4168).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (500 MHz, CDCl3): δ 8.00 (2H, d, J = 8.5 Hz, Ar-H), 7.74 (1H, d, J = 15.5 Hz, H-9′), 7.55 (2H, d, J = 8.5 Hz, Ar-H), 7.36 (1H, d, J = 16.0 Hz, H-8′), 7.17 (2H, d, J = 8.5 Hz, Ar-H), 6.88 (2H, d, J = 8.5 Hz, Ar-H), 4.87 (1H, s, H2-24), 4.69 (1H, s, H2-24), 3.77 (3H, s, –OMe), 3.64 (3H, s, –OMe), 2.76 (1H, dd, J1 = 4.0 Hz, J2 = 13.5 Hz, H-18), 2.63 (2H, m, H-2), 1.72 (3H, s, Me), 0.97 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.86 (3H, s, Me), 0.84 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 210.8, 188.8, 178.0, 171.1, 161.5, 153.7, 146.3, 135.7, 127.2, 144.6, 130.1 (C × 2 in Ph), 129.7 (C × 2 in Ph), 121.4 (C × 2 in Ph), 124.2 (C × 2 in Ph), 119.1, 113.9, 55.2, 51.6, 51.3, 49.8, 47.0, 42.2, 40.8, 40.5, 38.7, 38.5, 36.0, 34.1, 33.2, 32.9, 32.6, 31.7, 30.4, 30.1, 28.1, 27.3, 24.1, 23.0 (C × 2), 22.5, 20.2, 18.8, 15.6; EI MS: m/z 736 ([M]+, 1), 482 (29), 467 (69), 407 (65), 278 (40), 254 (100), 218 (38), 65 (8); HREIMS m/z 736.4358 (calcd for C47H60O7, 736.4377).
O) cm−1; 1H NMR (500 MHz, CDCl3): δ 8.00 (2H, d, J = 8.5 Hz, Ar-H), 7.74 (1H, d, J = 15.5 Hz, H-9′), 7.55 (2H, d, J = 8.5 Hz, Ar-H), 7.36 (1H, d, J = 16.0 Hz, H-8′), 7.17 (2H, d, J = 8.5 Hz, Ar-H), 6.88 (2H, d, J = 8.5 Hz, Ar-H), 4.87 (1H, s, H2-24), 4.69 (1H, s, H2-24), 3.77 (3H, s, –OMe), 3.64 (3H, s, –OMe), 2.76 (1H, dd, J1 = 4.0 Hz, J2 = 13.5 Hz, H-18), 2.63 (2H, m, H-2), 1.72 (3H, s, Me), 0.97 (3H, s, Me), 0.95 (3H, s, Me), 0.93 (3H, s, Me), 0.86 (3H, s, Me), 0.84 (3H, s, Me); 13C NMR (125 MHz, CDCl3): δ 210.8, 188.8, 178.0, 171.1, 161.5, 153.7, 146.3, 135.7, 127.2, 144.6, 130.1 (C × 2 in Ph), 129.7 (C × 2 in Ph), 121.4 (C × 2 in Ph), 124.2 (C × 2 in Ph), 119.1, 113.9, 55.2, 51.6, 51.3, 49.8, 47.0, 42.2, 40.8, 40.5, 38.7, 38.5, 36.0, 34.1, 33.2, 32.9, 32.6, 31.7, 30.4, 30.1, 28.1, 27.3, 24.1, 23.0 (C × 2), 22.5, 20.2, 18.8, 15.6; EI MS: m/z 736 ([M]+, 1), 482 (29), 467 (69), 407 (65), 278 (40), 254 (100), 218 (38), 65 (8); HREIMS m/z 736.4358 (calcd for C47H60O7, 736.4377).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (500 MHz, CDCl3): δ 8.04 (2H, d, J = 8.5 Hz, Ar-H), 7.57 (1H, d, J = 15.5 Hz, H-9′), 7.50 (1H, s, H-5′′), 7.42 (1H, d, J = 15.5 Hz, H-8′), 7.19 (2H, d, J = 8.0 Hz, Ar-H), 6.70 (1H, d, J = 3.0 Hz, H-3′′), 6.48 (1H, t, J = 1.5 Hz, H-4′′), 4.89 (1H, s, H2-24), 4.71 (1H, s, H2-24), 3.67 (3H, s, –OMe), 2.80 (1H, dd, J1 = 3.5 Hz, J2 = 14.0 Hz, H-18), 2.64 (2H, m, H-2), 1.74 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.87 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 211.0, 188.4, 178.2, 171.3, 154.0, 151.4, 146.4, 144.9, 135.5, 130.7, 129.9 (C × 2 in Ph), 121.6 (C × 2 in Ph), 118.8, 116.3, 114.0, 112.6, 51.7 (C × 2), 50.0, 47.1, 42.4, 40.9, 40.7, 38.6, 38.5, 36.1, 34.3, 33.3, 33.0, 32.8, 31.8, 30.5, 30.2, 28.2, 27.5, 24.1, 23.1, 23.0, 22.6, 20.3, 19.0, 15.8; EI MS: m/z 696 ([M]+, 12), 485 (24), 407 (28), 214 (55), 149 (100), 69 (13), 57 (19); HREIMS m/z 696.4030 (calcd for C44H56O7, 696.4034).
O) cm−1; 1H NMR (500 MHz, CDCl3): δ 8.04 (2H, d, J = 8.5 Hz, Ar-H), 7.57 (1H, d, J = 15.5 Hz, H-9′), 7.50 (1H, s, H-5′′), 7.42 (1H, d, J = 15.5 Hz, H-8′), 7.19 (2H, d, J = 8.0 Hz, Ar-H), 6.70 (1H, d, J = 3.0 Hz, H-3′′), 6.48 (1H, t, J = 1.5 Hz, H-4′′), 4.89 (1H, s, H2-24), 4.71 (1H, s, H2-24), 3.67 (3H, s, –OMe), 2.80 (1H, dd, J1 = 3.5 Hz, J2 = 14.0 Hz, H-18), 2.64 (2H, m, H-2), 1.74 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.95 (3H, s, Me), 0.87 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 211.0, 188.4, 178.2, 171.3, 154.0, 151.4, 146.4, 144.9, 135.5, 130.7, 129.9 (C × 2 in Ph), 121.6 (C × 2 in Ph), 118.8, 116.3, 114.0, 112.6, 51.7 (C × 2), 50.0, 47.1, 42.4, 40.9, 40.7, 38.6, 38.5, 36.1, 34.3, 33.3, 33.0, 32.8, 31.8, 30.5, 30.2, 28.2, 27.5, 24.1, 23.1, 23.0, 22.6, 20.3, 19.0, 15.8; EI MS: m/z 696 ([M]+, 12), 485 (24), 407 (28), 214 (55), 149 (100), 69 (13), 57 (19); HREIMS m/z 696.4030 (calcd for C44H56O7, 696.4034).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1; 1H NMR (500 MHz, CDCl3): δ 8.04 (2H, d, J = 8.5 Hz, Ar-H), 7.92 (1H, d, J = 15.5 Hz, H-9′), 7.43 (1H, d, J = 5.0 Hz, H-5′′), 7.36 (1H, d, J = 3.5 Hz, H-3′′), 7.31 (1H, d, J = 15.5 Hz, H-8′), 7.22 (2H, d, J = 8.5 Hz, Ar-H), 7.08 (1H, t, J = 3.5 Hz, H-4′′), 4.91 (1H, s, H2-24), 4.73 (1H, s, H2-24), 3.68 (3H, s, –OMe), 2.82 (1H, dd, J1 = 3.5 Hz, J2 = 13.5 Hz, H-18), 2.66 (2H, m, H-2), 1.77 (3H, s, Me), 1.03 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.90 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 211.1, 188.6, 178.3, 171.4, 154.1, 146.5, 140.3, 137.4, 135.5, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 121.7 (C × 2 in Ph), 120.4, 114.1, 51.9, 51.8, 50.2, 47.3, 42.5, 41.0, 40.8, 38.9, 38.7, 36.2, 34.4, 33.4, 33.1, 32.9, 31.9, 30.6, 30.3, 28.3, 27.6, 24.4, 23.2, 23.1, 22.7, 20.4, 19.1, 15.9; EI MS: m/z 712 ([M]+, 13), 482 (32), 467 (65), 407 (70), 278 (47), 230 (100), 218 (48), 149 (41), 69 (12); HREIMS m/z 712.3808 (calcd for C44H56SO6, 712.3818).
O) cm−1; 1H NMR (500 MHz, CDCl3): δ 8.04 (2H, d, J = 8.5 Hz, Ar-H), 7.92 (1H, d, J = 15.5 Hz, H-9′), 7.43 (1H, d, J = 5.0 Hz, H-5′′), 7.36 (1H, d, J = 3.5 Hz, H-3′′), 7.31 (1H, d, J = 15.5 Hz, H-8′), 7.22 (2H, d, J = 8.5 Hz, Ar-H), 7.08 (1H, t, J = 3.5 Hz, H-4′′), 4.91 (1H, s, H2-24), 4.73 (1H, s, H2-24), 3.68 (3H, s, –OMe), 2.82 (1H, dd, J1 = 3.5 Hz, J2 = 13.5 Hz, H-18), 2.66 (2H, m, H-2), 1.77 (3H, s, Me), 1.03 (3H, s, Me), 1.00 (3H, s, Me), 0.98 (3H, s, Me), 0.90 (6H, s, 2 × Me); 13C NMR (125 MHz, CDCl3): δ 211.1, 188.6, 178.3, 171.4, 154.1, 146.5, 140.3, 137.4, 135.5, 132.2, 129.9 (C × 2 in Ph), 128.9, 128.4, 121.7 (C × 2 in Ph), 120.4, 114.1, 51.9, 51.8, 50.2, 47.3, 42.5, 41.0, 40.8, 38.9, 38.7, 36.2, 34.4, 33.4, 33.1, 32.9, 31.9, 30.6, 30.3, 28.3, 27.6, 24.4, 23.2, 23.1, 22.7, 20.4, 19.1, 15.9; EI MS: m/z 712 ([M]+, 13), 482 (32), 467 (65), 407 (70), 278 (47), 230 (100), 218 (48), 149 (41), 69 (12); HREIMS m/z 712.3808 (calcd for C44H56SO6, 712.3818).α-Glucosidase inhibitory activity determination
The α-glucosidase inhibitory activity of each compound was determined according to the chromogenic method described by Chapdelaine et al. with slight modifications.32 α-Glucosidase from Saccharomyces cerevisiae and substrate solution pNPG were prepared with 0.1 mol L−1 of Na-phosphate buffer (pH 6.8). The inhibitors were reconstituted in 80 μL phosphate buffer in a 96-well microplate and incubated with 30 μL α-glucosidase in 37 °C for 15 min, and then 30 μL substrate was added. After incubation with substrate for 5 min, release of p-nitrophenol was measured at 405 nm by spectrophotometry. Percentage of enzyme inhibition was calculated according to {1 − (Asample − Ablank)/Acontrol} × 100, where Asample represents absorbance of test samples, Acontrol represents absorbance of solution without sample, and Ablank represents absorbance in presence of solution without substrate.Kinetics of inhibition against α-glucosidase33
In order to evaluate the inhibition type of the conjugates against α-glucosidase activities, increasing concentrations of p-nitrophenyl α-D-glucopyranoside were used as substrates in the absence or presence of compounds at two different concentrations around the IC50 values. The inhibition types of 1b, 6b, 5c and 4d were determined by Lineweaver–Burk plots, using the methods reported in the literature. Inhibition types and Ki values of the inhibitors were determined by double-reciprocal plots.Fluorescence quenching measurements34
All fluorescence spectra were measured on a fluorescence spectrophotometer (Perkin-Elmer) equipped with a 10.0 mm quartz cell and a thermostat bath. In the fluorescence spectrophotometer, 30 μL of α-glucosidase solution (pH 6.8) with a concentration of 2 μM was added accurately to the quartz cell and then titrated by successive additions of inhibitor. The fluorescence emission spectra were measured at 18 and 37 °C. The excitation wavelength was 290 nm and the emission spectrum was recorded from 300 to 500 nm.Acknowledgements
The project was supported by National Key Technology R&D Program in the 12th Five Year Plan of China (2012BAI27B06).Notes and references
- S. Shobana, Y. N. Sreerama and N. G. Malleshi, Food Chem., 2009, 15, 1268–1273 CrossRef PubMed.
- A. M. Chang, M. J. Smith and C. J. Bloem, Am. J. Physiol.: Endocrinol. Metab., 2004, 287, E906–E911 CrossRef CAS PubMed.
- M. R. Bhandari, J. A. Nilubon, H. Gao and J. Kawabata, Food Chem., 2008, 106, 247–252 CrossRef CAS PubMed.
- W. Nie, J. G. Luo, X. B. Wang, H. Yin, H. B. Sun, H. Q. Yao and L. Y. Kong, Chem. Pharm. Bull., 2011, 59, 1051–1056 CrossRef CAS.
- J. Liu, Y. Liu, A. Parkinson and C. D. Klaassen, J. Pharmacol. Exp. Ther., 1995, 275, 768–774 CAS.
- E. M. Giner-Larza, S. Manez, M. C. Recio, R. M. Giner, J. M. Prieto, M. Cerda-Nicolas and J. L. Rios, Eur. J. Pharmacol., 2011, 428, 137–143 CrossRef.
- D. Deeb, X. Gao, S. A. Dulchavsky and S. C. Gautam, Anticancer Res., 2007, 27, 3035–3044 CAS.
- D. L. Yu, Y. Sakurai, C. H. Chen, F. R. Chang, L. Huang, Y. Kashiwada and K. H. Lee, J. Med. Chem., 2006, 49, 5462–5469 CrossRef CAS PubMed.
- J. G. Luo, L. Ma and L. Y. Kong, Bioorg. Med. Chem., 2008, 16, 2912–2920 CrossRef CAS PubMed.
- Y. Liu, L. Ma, W. H. Chen, B. Wang and Z. L. Xu, Bioorg. Med. Chem., 2007, 15, 2810–2814 CrossRef CAS PubMed.
- W. Nie, J. G. Luo, X. B. Wang, H. Yin, H. B. Sun, H. Q. Yao and L. Y. Kong, Chem. Pharm. Bull., 2011, 59, 1051–1056 CrossRef CAS.
- M. S. Ali, M. Jahangir, S. S. Hussan and M. I. Choudhary, Phytochemistry, 2002, 60, 295–299 CrossRef CAS.
- W. K. Kang, Y. L. Song and L. Zhang, Med. Chem. Res., 2013, 63, 162–169 Search PubMed.
- T. Narender, G. Madhur, N. Jaiswal, M. Agrawal, C. K. Maurya, N. Rahuja, A. K. Srivastava and A. K. Tamrakar, Eur. J. Med. Chem., 2008, 43, 1865–1877 CrossRef PubMed.
- W. D. Seo, J. H. Kim, J. E. Kang, H. W. Ryu, M. J. Curtis-Long, H. S. Lee, M. S. Yang and K. H. Park, Bioorg. Med. Chem. Lett., 2005, 15, 5514–5516 CrossRef CAS PubMed.
- S. K. Choi, Synthetic Multivalent Molecules, Wiley-Inter-science, New York, 2004, pp. 219–221 Search PubMed.
- K. W. Lin, A. M. Huang, T. C. Hour, S. C. Yang, Y. S. Pu and C. N. Lin, Bioorg. Med. Chem., 2011, 19, 4274–4285 CrossRef CAS PubMed.
- A. Modzelewska, C. Pettit, G. Achanta, N. E. Davaidson, P. Huang and S. R. Khan, Bioorg. Med. Chem., 2006, 14, 3491–3495 CrossRef CAS PubMed.
- M. Cabrera, M. Simoens, G. Falchi, M. L. Lavaggi, O. E. Piro, E. E. Castellano, A. Vidal, A. Azqueta, A. Monge, A. L. Cerain, G. Sagrera, H. Cerecetto and M. Gonzalez, Bioorg. Med. Chem., 2007, 15, 3356–3367 CrossRef CAS PubMed.
- D. Liu, D. D. Song, G. Guo, R. Wang, J. L. Lv, Y. K. Jing and L. X. Zhao, Bioorg. Med. Chem., 2007, 15, 5432–5439 CrossRef CAS PubMed.
- J. F. Li, Y. Zhao, M. M. Cai, X. F. Li and J. X. Li, Eur. J. Med. Chem., 2009, 44, 2796–2806 CrossRef CAS PubMed.
- Y. J. You, Y. Kim, N. H. Nam and B. Z. Ahn, Bioorg. Med. Chem. Lett., 2003, 13, 3137–3140 CrossRef CAS.
- A. Parra, P. E. Lopez and G. G. Andres, Nat. Prod. Res., 2010, 24, 177–196 CrossRef CAS PubMed.
- S. B. Gnoatto, A. Dassonville-Klimpt, S. D. Nascimento, P. Galera, K. Boumediene, G. Gosmann, P. Sonnet and S. Moslemi, Eur. J. Med. Chem., 2008, 43, 1865–1877 CrossRef CAS PubMed.
- S. Qian, H. Li, Y. Chen, W. Zhang, S. Yang and Y. Wu, J. Nat. Prod., 2010, 73, 1743–1750 CrossRef CAS PubMed.
- H. Y. Tu, A. M. Huang, B. L. Wei, K. H. Gan, T. C. Hour, S. C. Ynag, Y. S. Pu and C. N. Lin, Bioorg. Med. Chem., 2009, 17, 7265–7274 CrossRef CAS PubMed.
- B. K. Suresh, A. K. Tiwari, P. V. Srinivas, A. Z. Ali, B. Raju and J. M. Rao, Bioorg. Med. Chem. Lett., 2004, 14, 3841–3845 CrossRef PubMed.
- A. Papadopoulou, R. J. Green and R. A. Frazier, J. Agric. Food Chem., 2005, 53, 158–163 CrossRef CAS PubMed.
- J. R. Lakowicz and B. P. Maliwal, J. Biol. Chem., 1983, 258, 4794–4801 CAS.
- M. X. Xie, X. Y. Xu and Y. D. Wang, Biochim. Biophys. Acta, 2005, 1724, 215–224 CrossRef CAS PubMed.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096–3102 CrossRef CAS.
- P. Chapdelaine, R. R. Tremblay and J. Y. Dube, Clin. Chem., 1978, 24, 208–211 CAS.
- W. Hou, Y. Li, Q. Zhang, X. Wei, A. Peng, L. Chen and Y. Wei, Phytother. Res., 2009, 23, 614–618 CrossRef CAS PubMed.
- M. Aguilar-Moncayo, M. I. García-Moreno, A. E. Stutz, J. M. G. Fernandez, T. M. Wrodnigg and C. O. Mellet, Bioorg. Med. Chem., 2010, 18, 7439–7445 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46492j |
| This journal is © The Royal Society of Chemistry 2014 |