Enantioselective synthesis of N-allylindoles via palladium-catalyzed allylic amination/oxidation of indolines†
Abstract
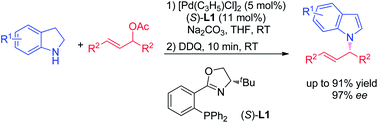
A highly efficient synthesis of N-allylindoles was realized via palladium-catalyzed asymmetric allylic amination/oxidation sequential reaction of indolines. The N-alkylated indole derivatives were obtained with up to 91% yield and 97% ee.

 Please wait while we load your content...
Please wait while we load your content...