Determination of aromatic sulfur containing compounds in water samples by using TiO2 nanotube arrays as an adsorbent prior to high performance liquid chromatography with a fluorescence detector
Qingxiang Zhou* and
Zhi Fang
Beijing Key Laboratory of Oil and Gas Pollution Control, College of Geosciences, China University of Petroleum Beijing, Beijing 10224, PR China. E-mail: zhouqx@cup.edu.cn; Fax: +8610-89732300; Tel: +8610-89732300
First published on 9th January 2014
Abstract
A novel and simple analytical method was developed for the rapid determination of three aromatic sulfur containing compounds including dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT), 4,6-dimethyldibenzothiophene (4,6-DMDBT) in water samples. Highly ordered TiO2 nanotube arrays were used as the adsorbent in the extraction procedure. The possible factors that would affect the extraction efficiency have been investigated in detail. Under the optimal conditions, TiO2 nanotube arrays showed a good adsorptive property for the target analytes. The experimental results demonstrated that low limits of detection (LODs, S/N = 3) of 0.02, 0.037, 0.038 μg L−1 for DBT, 4-MDBT, 4,6-DMDBT were achieved. This proposed method was validated with four real environmental water samples, and the results indicated that good spiked recoveries were in the range of 79.3% to 106.3%. All these results indicated that it is an effective and alternative tool for monitoring trace pollutants.
1. Introduction
Sulfur is the most abundant heteroelement that exists in petroleum1 and is also commonly present in other types of fossil organic matter. Owing to different geological environments, some special prior compounds were formed during the process of generation, migration, and accumulation of petroleum. So aromatic sulfur containing compounds have been used for many years as maturity parameters to evaluate oil by petroleum geochemists, and it is found that immature oils are characterized with high amounts of thermally unstable non-thiophenic sulfur compounds, while high abundance of the more stable benzo- and dibenzothiophene2,3 exists in mature oil. Ratio of dibenzothiophene series, have also been used for oil fingerprint identification in environmental forensics to trace oil sources.4 However, these aromatic sulfur containing compounds enter the environment, which would throw put a severe threat on the environment and human health. The existed methods have been developed based on gas chromatography or high performance liquid chromatography such as gas chromatography with atomic emission detection, gas chromatography with flame photometric detection, gas chromatography-mass spectrometry, high performance liquid chromatography-tandem mass spectrometry, etc.5–9 As well known, most of them are expensive, and it is not available for a common lab. So it is of great value to establish rapid and sensitive method for their monitoring.In order to achieve sensitive results, a pretreatment step is often needed prior to gas chromatography or high performance liquid chromatography. Among the developed sample pretreatment techniques, solid phase extraction (SPE) is the popular one. In recent years, the research on the adsorbents for SPE has been put more attention. As a new nanomaterial, TiO2 nanotube powders have been proved to be an effective adsorbent in solid phase extraction in many reports. Niu et al. used surfactant modified TiO2 nanotube as adsorbent of solid phase extraction to enrich phthalate esters in natural water and a good result was obtained,10 and our research group have used TiO2 nanotube powders as adsorbents for the determination of trace organic pollutants such as nonylphenol,11 benzoylurea insecticides,12 paraquat and diquat13 and DDT and its metabolites14 in water samples. The results showed that TiO2 nanotube has a better enrich capability and proved the applicability of the TiO2 nanotube for the enrichment of trace pollutants. Nowadays, TiO2 nanotube array has been introduced as a new type of TiO2 nanotube material, in which the tubes grew vertically on the Ti substrate tightly.15 it will be expected to be an efficient adsorbent due to its large specific surface area.16 However, the studies on TiO2 nanotube arrays as adsorbent for trace analysis are very few.17,18
Polycyclic aromatic hydrocarbons (PAH) attract much attention as carcinogens to human beings, and many methods have been established for their determination. But there are very few methods on sulfur containing PAHs. Herein the goal is to develop a novel method with TiO2 nanotube arrays as a adsorbent to enrich target compounds (DBT, 4-MDBT, 4,6-DMDBT) in water samples. Some parameters affecting the procedure such as the surfactant concentration, pH value, desorption solvent, absorption time, desorption time are investigated.
2. Experimental
2.1 Reagents and materials
Dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT), 4,6-dimethyldibenzothiophene (4,6-DMDBT) were purchased from J&K Scientific (Beijing, China), HPLC grade methanol and acetonitrile were obtained from Thermofisher (New Jersey, USA) and MREDA (Dallas Alfa, USA), respectively. The ultra pure water used during our experiments was from Wahaha Company (Hangzhou, China). All the other solvents were analytical reagent grade unless otherwise stated.Standard stock solutions of 1 mg L−1 were prepared by dissolving each analyte with HPLC grade methanol and the standard solutions were stored at 4 °C in the refrigerator and protected from light.
Titanium sheets (99.6% purity) purchased from Beijing Hengli Taiye Co., Ltd. (Beijing, China), Pt electrode was obtained from Shanghai Ruosull Technology Co. Ltd (shanghai, China). 30 V potentiostat was from The Fourth Radio Factory in Shijiazhuang (JWY-30G, Shijiazhuang, China).
An Agilent 1260 high performance liquid chromatography system was used for the analysis of the analytes, which consists of quaternary pump, a column (Eclipse XDB-C18, 4.6 × 250 mm 5 μm), and fluorescence detector. The column temperature was controlled at 20 °C. The excitation wavelength and emission wavelength were set at 238 nm and 341 nm, respectively. Mobile phase consisted of methanol and water at a ratio of 90/10 (v/v) and the flow rate was set at 1 mL min−1.
2.2 Preparation of TiO2 nanotube arrays
A size of 20 × 20 mm with 0.2 mm thickness Ti sheet was polished by abrasive paper, and then immersed in acetone, isopropanol and methanol by sonicating in 10 min, respectively. The sheet was air-dried after rinsing with ultrapure water. The anodic oxidation by using titanium sheet as anode and platinum as cathode, and the distance between two electrodes was 3 cm in the experiment. 0.14 M NaF and 0.5 M H3PO4 were used as electrolyte. The anodic oxidation was carried out at 20 V for 1 h. After electrolysis, titanium sheet was rinsed with ultrapure water and then air-dried.2.3 Extraction procedure
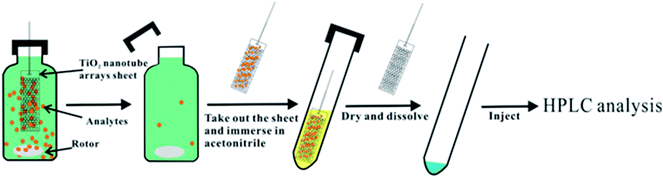
The micro solid phase equilibrium extraction (μ-SPEE) (Fig. 1) was carried out by immersing TiO2 nanotube arrays directly in solution with a certain depth. The extraction conditions during experiments were as optimized. After the equilibrium between adsorption and desorption, the TiO2 nanotube arrays sheet is taken out, and then rinsed with ultrapure water in order to remove co-adsorbed matrix and air-dried, TiO2 nanotube arrays sheet then immersed directly in a small amount of acetonitrile in order to desorb the target analytes. After 5 min, the TiO2 nanotube arrays sheet was taken out, and the acetonitrile solution was dried with nitrogen stream. Then the residues were dissolved in 200 μL methanol. Finally, 50 μL of the solution was injected for HPLC analysis.2.4 Water samples
Two reservoir samples and two river samples were collected from Ming Tombs Reservoir, Dingjiawa Reservoir, Yongding River and Shahe River in Beijing, respectively. All the collected water samples were filtered through 0.22 μm micropore membranes after sampling and were maintained in glass containers, then stored at a temperature of 4 °C and protected from light.3. Results and discussion
The μ-SPEE procedure is very like a SPE procedure, so many influencing factors such as surfactants concentration, eluting solvent, sample pH, absorption time and desorption time will affect the enrichment performance, herein in order to gain the optimal conditions, these parameters were investigated in detail.3.1 The effect of CTAB concentration
Many studies have shown that the surface of TiO2 nanotube arrays prepared through anodic oxidation is usually hydrophilic,19 which is unfavorable for the absorption of some hydrophobic compounds. Surfactants compose of hydrophilic and hydrophobic part, when it exists in solution, the hydrophilic part of surfactant will bond with the surface of TiO2 nanotube arrays and the hydrophobic part will be left directly to solution environment. The hydrophobic part will enhance the extraction of the target analytes and our previous experiment has proved this phenomenon.17 Cetyltrimethylammonium bromide (CTAB) was added into the sample solution to change the surface properties of TiO2 nanotube arrays. The results were shown in Fig. 3, the peak area of three target compounds increased while CTAB was added in solution. When the concentration of CTAB reached 120 mg L−1, the biggest peak area was obtained. However the peak area would decrease when the concentration was higher than 120 mg L−1. The reason may be that the more micelle contained analytes in the solution could not be absorbed onto the surface of the array, which would decrease the amount of enriched analytes. So 120 mg L−1 was adopted in this procedure.3.2 The effect of organic solvents for desorption
In the enrichment procedure, adsorption and desorption are the two key steps. Except adsorption, desorption is very important for an enrichment method. From the former experiments, we can see that the adsorption was not chemical adsorption, but van der Waals' force or electrostatic interaction. So the desorption could be realized by organic solvent which has high solubility for the target analytes, that is to say, the analytes are very easy to distribute in to this solvent. In this experiment, acetonitrile, methanol, ethanol, acetone, dichloromethane and so on are often used for desorption, in this experiment, these solvents were investigated and the results demonstrated that there were very little difference in desorption for all these five solvents, but among them acetonitrile was the best one for desorption due to the fact that acetonitrile has good elution ability and the analytes have high distribution coefficients than that in other solvents. So acetonitrile was adopted in following experiments.3.3 The effect of sample pH
Sample pH has an important role in this μ-SPEE procedure, because compounds will be present at different existing forms in solution at different pH values. Some the existing forms are not suitable for extraction, so sample pH will affects extraction efficiency. In this experiment, the role of different pH was investigated in the range of pH 3–12. The experimental results were shown in Fig. 2, and lower extraction efficiencies were observed when the solution in acid or alkaline conditions. When the solution is neutral, the highest extraction efficiency was obtained. The reason is that the analytes are easy to form association compounds at neutral conditions with CTAB, and further good sensitivity is achieved. Hence sample pH was adjusted to pH 7 in the following experiments.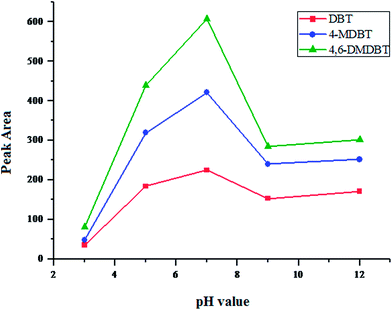 | ||
| Fig. 2 Effect of pH value. CTAB concentration, 120 mg L−1; eluent, acetonitrile; pH = 7; equilibrium time, 60 min; desorption time, 10 min; spiked concentration, 20 μg L−1 for three DBTs. | ||
3.4 The effect of absorption time
As the μ-SPEE procedure is concerned, equilibrium extraction time is important. If the time is too short, the equilibrium does not reach and the expected reliably high sensitivity is not obtained. However, if the absorption time is longer than that needed for equilibrium, it is also not economical for the whole analysis. Hence, extraction time was investigated in the range of 20–100 min. The results indicated that the equilibrium would reach in about 60 min. If the time is shorter than 60 min, it is not enough for absorption. In the meantime, the peak area will not increase continually when the time longer than 60 min. So the optimal extraction time was set as 60 min in the following experiments.3.5 The effect of desorption time
In the μ-SPEE procedure, another important factor is desorption time. Desorption time will also affect the extraction performance. If the desorption time is too short, the adsorbed analytes will not be desorbed completely. Hence it is essential to optimize desorption time to elute target compound from adsorbent effectively and quickly. In this experiment, desorption time was investigated in the range of 3–12 min. The results showed the desorption performance increased over the range of 3–5 min then decreased slightly over the range of 5–12 min. Finally 5 min is selected for desorption of the targets.3.6 Analytical performance and real sample analysis
In this experiment, some important quantitative parameters of the proposed method such as linear range, correlation coefficients, limits of detection (LOD) and relative standard deviation (RSD) were evaluated by using 10 mL standard solutions. And the results were listed in Table 1. It was found that this method had excellent linearity between the peak area and concentration over the range of 0.1–100 μg L−1. The LODs of DBT, 4-MDBT, and 4,6-DMDBT were in the range of 0.02–0.038 μg L−1. The precisions of proposed method were investigated by using six replicate experiments, and the relative standard deviations (RSDs) were in the range of 3.82–5.85% (n = 6).| Compound | Linear range (μg L−1) | R2 | RSD (n = 6) | LOD (S/N = 3) |
|---|---|---|---|---|
| Dibenzothiophene | 0.1–100 | 0.998 | 4.95 | 0.037 |
| 4-Methyldibenzothiophene | 0.1–100 | 0.998 | 3.82 | 0.038 |
| 4,6-Dimethyldibenzothiophene | 0.1–100 | 0.998 | 5.85 | 0.02 |
3.7 Analysis of real water samples
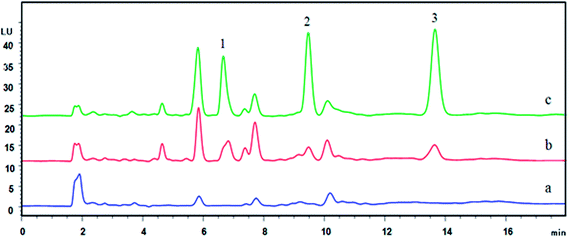
To investigate the practical applicability of the μ-SPEE procedure, four real water samples consist of two reservoir water and two river water samples were used. Real water samples were directly analyzed and analyzed after spiking two concentration levels of 5 and 20 μg L−1. As could be seen in Table 2, no target analytes were detected directly in four samples. The spiked recoveries were satisfied in the range of 79.3–106.3%. All these experimental data demonstrated that the proposed method was successfully used for the sensitive determination of three DBTs in complex sample matrices. The typical chromatogram of real water sample was demonstrated in Fig. 3.| Water samples | Added levels (μg L−1) | DBT | 4-MDBT | 4,6-DMDBT |
|---|---|---|---|---|
| a ND = not detected. | ||||
| Ming tombs reservoir | Blank | N.D.a | N.D. | N.D. |
| 5 | 96.9 ± 3.7 | 86.6 ± 1.0 | 79.3 ± 1.1 | |
| 20 | 99.7 ± 4.5 | 94.0 ± 4.2 | 92.2 ± 2.7 | |
| Yongding river | Blank | N.D. | N.D. | N.D. |
| 5 | 102.2 ± 3.8 | 87 ± 1.0 | 86.0 ± 1.5 | |
| 20 | 101.2 ± 5.1 | 91.4 ± 4 | 92.1 ± 3.9 | |
| Dingjiawa reservoir | Blank | N.D. | N.D. | N.D. |
| 5 | 94.3 ± 5.9 | 93.7 ± 5.2 | 86 ± 1.5 | |
| 20 | 100.1 ± 1.9 | 99.7 ± 0.2 | 98.9 ± 0.7 | |
| Shahe river | Blank | N.D. | N.D. | N.D. |
| 5 | 106.3 ± 1.1 | 87.0 ± 2.2 | 83.1 ± 4.5 | |
| 20 | 96.6 ± 3.9 | 97.8 ± 0.4 | 93.8 ± 2.4 | |
4. Conclusions
Present study investigated the applicability of TiO2 nanotube arrays for the enrichment of sulfur containing aromatic compounds. The results showed that the established micro-solid phase equilibrium extraction (μ-SPEE) was very effective for the extraction of three DBTs in environmental water samples. Good linear range, reproducibility and detection limits were obtained. Four real water samples were used to evaluate the analytical performance with two spiked concentration levels and satisfied spiked recoveries were achieved in the range of 79.3–106.3%. Based on these results, the proposed method was a simple and reliable method developed for the determination of DBTs in environmental water samples.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21377167), Program for New Century Excellent Talents in University (NCET-10-0813), Science Foundation of China University of Petroleum, Beijing (KYJJ2012-01-15).References
- P. Liu, C. Xu, Q. Shi, N. Pan, Y. Zhang, S. Zhao and K. H. Chung, Anal. Chem., 2010, 82, 6601–6606 CrossRef CAS PubMed.
- T. Ho, M. Rogers, H. Drushel and C. Koons, AAPG Bull., 1974, 58, 2338–2348 Search PubMed.
- Z. Wang, M. Fingas and D. S. Page, J. Chromatogr. A, 1999, 43, 369–411 CrossRef.
- Z. Wang and M. Fingas, Environ. Sci. Technol., 1995, 29, 2842–2849 CrossRef CAS PubMed.
- H. Niu, Y. Cai, Y. Shi, F. Wei, S. Mou and G. Jiang, J. Chromatogr. A, 2007, 1172, 113–120 CrossRef CAS PubMed.
- G. P. Yang, X. L. Liu and J. W. Zhang, Environ. Pollut., 1998, 101, 405–414 CrossRef CAS.
- R. G. Christensen and E. White V, J. Chromatogr. A, 1985, 323, 33–36 CrossRef CAS.
- S. Chiaberge, T. Fiorani and P. Cesti, Fuel Process. Technol., 2011, 92, 2196–2201 CrossRef CAS PubMed.
- F. Liang, M. Lu, M. E. Birch, T. C. Keener and Z. Liu, J. Chromatogr. A, 2006, 1114, 145–153 CrossRef CAS PubMed.
- H. Yang, J. Chen, Y. Briker, R. Szynkarczuk and Z. Ring, Catal. Today, 2005, 109, 16–23 CrossRef CAS PubMed.
- X. F. Su, X. N. Zhao, G. H. Xie and Q. X. Zhou, Chin. Chem. Lett., 2012, 23, 969–972 CrossRef CAS PubMed.
- Y. Huang, Q. Zhou, G. Xie, H. Liu and H. Lin, Microchim. Acta, 2011, 172, 109–115 CrossRef CAS.
- Q. Zhou, J. Mao, J. Xiao and G. Xie, Anal. Methods, 2010, 2, 1063–1068 RSC.
- Q. Zhou, Y. Ding, J. Xiao, G. Liu and X. Guo, J. Chromatogr. A, 2007, 1147, 10–16 CrossRef CAS PubMed.
- D. Gong, C. Grimes, O. K. Varghese, W. Hu, R. Singh, Z. Chen and E. C. Dickey, J. Mater. Res., 2001, 16, 3331–3334 CrossRef CAS.
- M. Bonato, K. V. Ragnarsdottir and G. C. Allen, Water, Air, Soil Pollut., 2012, 223, 3845–3857 CrossRef CAS.
- Y. Huang, Q. Zhou and G. Xie, J. Hazard. Mater., 2011, 193, 82–89 CrossRef CAS PubMed.
- Q. Zhou, Y. Huang and G. Xie, J. Chromatogr. A, 2012, 1237, 24–29 CrossRef CAS PubMed.
- Y.-Y. Song, F. Schmidt-Stein, S. Bauer and P. Schmuki, J. Am. Chem. Soc., 2009, 131, 4230–4232 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |