Ionic liquid coated sulfonated carbon/silica composites: novel heterogeneous catalysts for organic syntheses in water†
Princy Guptaa,
Manmeet Koura,
Satya Paul*a and
James H. Clarkb
aDepartment of Chemistry, University of Jammu, Jammu-180 006, India. E-mail: paul7@rediffmail.com; Fax: +91-191-2451365; Tel: +91-191-2453841
bGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, Heslington, York, UK. E-mail: james.clark@york.ac.uk; Fax: +44 (0)1904 322705; Tel: +44 (0)1904 322567
First published on 21st November 2013
Abstract
Ionic liquid coated sulfonic acid functionalized amorphous carbon/silica composites derived from a starch–glucose mixture were developed and their catalytic activities were evaluated for Knoevenagel condensation, reductive amination of aldehydes and ketones, and for Michael addition of indole to α,β-unsaturated ketones in aqueous medium. The catalyst prepared from starch–glucose mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) [CSC-Star-Glu-IL2] showed the highest activity in water. The catalysts were characterized by FTIR, TGA, elemental analysis, and the most active was further characterized by XRD, SEM and TEM.
1) [CSC-Star-Glu-IL2] showed the highest activity in water. The catalysts were characterized by FTIR, TGA, elemental analysis, and the most active was further characterized by XRD, SEM and TEM.
Introduction
Reactions in water are now of great interest in organic syntheses.1 In addition to scientific interest in aqueous media, social and legislative pressures to find environmentally benign alternatives to current organic chemical processes have made these reactions attractive from industrial and ecological points of view.2 Many common organic solvents are likely to be banned or at least restricted in use as a consequence of REACH and similar multinational, national and regional regulations. These issues are beginning to present enormous challenges to the chemical, pharmaceutical and some other industries. Reactions in water offer significant environmental advantages and have attracted a great deal of interest since water is a desirable solvent due to its: low cost, safety, and environmentally benign nature. Although many reactions work well in water, some reactions proceed very slowly because the solubility of most organic molecules in pure water is limited. Because solubility is generally a great advantage for reactivity, a variety of strategies expanding the scope of water-based organic syntheses have been investigated. The concept of ionic liquid coating for solid acid catalysts was first reported by Valkenberg3a and co-workers. They have reported ionic liquid coated iron chloride catalyzed Friedel–Crafts acylation of aromatic compounds. Palladium acetate supported on amorphous silica with the aid of an ionic liquid, [bmim]PF6 was reported for Mizoroki–Heck reaction by Hagiwara3b and co-workers. Kobayashi and co-workers have developed silica supported metal catalysts with hydrophobic ionic liquids for organic reactions in water.3c,d The authors suggested that the ionic liquid creates a hydrophobic environment on the siliceous surface resulting in better diffusion of organic substrates to the catalytic sites. Gu and co-workers4a have reported that coating of silica supported sulfonic acid catalysts with hydrophobic ionic liquid leads to a significant improvement of catalyst selectivity in an aqueous medium. Hara4b and co-workers reported sulfonated carbon derived from sugar for the first time and used it for the production of biodiesel. Recently, we have reported amorphous carbon/silica composites bearing sulfonic acid as solid acid catalysts for the chemoselective protection of aldehydes as 1,1-diacetates and for N-, O- and S-acylations.5 As an extension of this work, herein, we prepared ionic liquid coated sulfonated carbon/silica composites as heterogeneous catalysts for Knoevenagel condensation and reductive amination of aldehydes and ketones, and for Michael addition of indole to α,β-unsaturated ketones in aqueous medium.In our previous study,5 it was found that starch and glucose are the best choice of biomaterials for preparing sulfonated carbon/silica composites. So, here we have a selected mixture of starch and glucose for preparing ionic liquid coated sulfonated carbon/silica composites. While ionic liquids themselves have had very limited success as alternative solvents (due to their high cost and difficulties in separations and reuse), their use in an immobilized state overcomes many of these difficulties while taking advantage of their useful properties (high solubilities of many compounds and high stabilization of polar reaction intermediates).
Due to the widespread synthetic utility of Knoevenagel condensation, a large number of methodologies have been developed for this reaction. In recent years, a wide range of catalysts, including Lewis acids,6 zeolites,7 solid bases,8 heterogeneous catalysts9 and amines immobilized on polymers,10a Yb(OTf)310b have been employed, each affording variable yields of products in solution or under solvent-free conditions. Al-Omran F. et al.10c have reported stereochemical Knoevenagel condensation of 2-(benzothiazol-2-ylthio) acetonitrile with furan-2-carbaldehyde and thiophene-2-carbaldehydes. Recently, a number of basic catalysts such as lithium hydroxide,11 silica supported ammonium acetate,12 L-proline,13 chitosan hydrogel,14 silica immobilized piperazine,15 titanium isopropoxide/pyridine,16 fly ash supported calcium oxide,17 amine functionalized mesoporous silica,18 thiourea,19 mesoporous Mg–Fe bimetal oxides,20 lipase,21 and tertiary amine functionalized polyacrylonitrile22 have been used to catalyze Knoevenagel condensation. However, basic catalysts cannot be applied to the substrates possessing base sensitive groups. To overcome these problems, number of acid catalyzed23–26a Knoevenagel condensation protocols have been developed. Moreover, many Knoevenagel condensations catalyzed by ionic liquids26b–f have also been reported. But, there is still scope for the development of new environment-friendly procedure using novel solid catalyst for Knoevenagel condensation. Herewith we have reported a novel ionic liquid coated sulfonated carbon/silica composites as a heterogeneous catalyst for the Knoevenagel condensation of aldehydes and ketones.
Reductive amination of aldehydes and ketones is a convenient method for the synthesis of functionalized amines which are important organic intermediates and have found wide applications in synthetic and combinatorial chemistry.27 A number of reagents such as NaBH4–silica phosphoric acid,28 zinc borohydride N-methyl pyrrolidine,29 NaBH4–silica gel supported sulfuric acid,30 NaBH4–Amberlyst-15,31 NaBH4–silica chloride,32 hydrido-iridium (III) complex,33 fluorous organocatalyst,34 NaBH4–cellulose sulfuric acid35 and NaBH4 using carbon based solid acid catalyst36 for reductive amination of aldehydes and ketones have been developed. However, most of the methods have limitations such as functional group tolerance, side reactions and harsh reaction conditions. So, there is a demand for an efficient and environment-friendly protocol for the reductive amination of aldehydes and ketones.
Michael reaction of indoles to α,β-unsaturated carbonyl compounds provides easy access to 3-substituted indoles, which are important building blocks for the synthesis of important biologically active compounds and natural products.37–39 Recently, catalysts such as polyvinyl sulfonic acid,40 metal halide hydrates,41 task-specific ionic liquids,42 bimetallic iron–palladium catalysts,43a and iron salts43b have been reported for the Michael addition of indole to α,β-unsaturated ketones. However, the acid-catalyzed conjugate addition of indoles requires careful control of acidity to prevent side reactions such as dimerization or polymerization. Further, many of these procedures involve strongly acidic conditions, expensive reagents, longer reaction time, give low yields of products due to the dimerization of indoles or polymerization of vinyl ketones and cumbersome product isolation. Keeping all these facts in view, we have developed a new environment-friendly procedure for Michael addition of indole to α,β-unsaturated ketones.
Results and discussion
Preparation and characterization of ionic liquid coated sulfonated carbon/silica composites
Firstly, sulfonated carbon/silica composites were prepared according to the method already reported,5 except that instead of using different biomaterials, a mixture of starch and glucose in three different ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 respectively was taken. The sulfonated carbon/silica composites (CSC-Star-Glu) prepared were then coated with ionic liquid, 1-butyl-3-methylimidazolium bromide, [Bmim][Br] to get CSC-Star-Glu-IL1 [(prepared from starch
3 respectively was taken. The sulfonated carbon/silica composites (CSC-Star-Glu) prepared were then coated with ionic liquid, 1-butyl-3-methylimidazolium bromide, [Bmim][Br] to get CSC-Star-Glu-IL1 [(prepared from starch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1
glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1))], CSC-Star-Glu-IL2 [(prepared from starch
1))], CSC-Star-Glu-IL2 [(prepared from starch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (3
glucose (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1))] and CSC-Star-Glu-IL3 [(prepared from starch
1))] and CSC-Star-Glu-IL3 [(prepared from starch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1
glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3))]. The preparation procedure is represented in Scheme 1. The characterization of the ionic liquid coated sulfonated carbon/silica composites CSC-Star-Glu-ILs viz. CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 was carried out with FTIR, TGA, and elemental analysis. The most active catalyst CSC-Star-Glu-IL2 was also characterized by XRD, SEM and TEM. The FTIR spectra of CSC-Star-Glu-ILs showed bands from 1625–1629 cm−1 which were assigned to aromatic C
3))]. The preparation procedure is represented in Scheme 1. The characterization of the ionic liquid coated sulfonated carbon/silica composites CSC-Star-Glu-ILs viz. CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 was carried out with FTIR, TGA, and elemental analysis. The most active catalyst CSC-Star-Glu-IL2 was also characterized by XRD, SEM and TEM. The FTIR spectra of CSC-Star-Glu-ILs showed bands from 1625–1629 cm−1 which were assigned to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes in polyaromatic rings. The bands from 2930–2934 cm−1 in CSC-Star-Glu-ILs were due to the phenolic –OH groups. CSC-Star-Glu-ILs also showed absorption bands from 1455–1466 cm−1 (due to asymmetric stretching of SO2) and from 1102–1110 cm−1 (due to symmetric stretching of SO2), which indicated the presence of –SO3H groups. CSC-Star-Glu-ILs showed bands from 3100–3110 and 3146–3152 cm−1 due to imidazolium ring –CH stretching. The bands from 2873–2875 and 2960–2962 cm−1 were due to aliphatic –CH stretching modes of methyl and butyl groups. The bands from 1570–1574 were assigned to stretching modes of imidazole ring and from 1160–1170 cm−1 were assigned to imidazole H–C–N bending modes. Further, the bands from 803–810, 750–753 and 651–655 cm−1 were due to in-plane imidazole ring bending, out of plane –CH bending and imidazole C2–N1–C5 bending modes respectively. The presence of these bands showed the coating of ionic liquid on the surface of the catalyst. The major absorption frequencies are presented in Table 1. The FTIR spectra of CSC-Star-Glu-IL2 is shown in Fig. S1 (see ESI†). The stability of CSC-Star-Glu-ILs was determined by thermogravimetric analysis. The TGA curves showed almost negligible weight losses upto 240 °C, and then significant weight losses are observed after 247 °C. Thus, the catalysts are stable upto 240 °C and hence it is safe to carry out the reaction at room temperature, 60 and 100 °C. The weight losses of CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 are represented in Table 2. The TGA of CSC-Star-Glu-IL2 is represented in Fig. S2 (see ESI†). The –SO3H loading in the CSC-Star-Glu-ILs was determined by elemental analysis. The CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 were found to contain 0.55, 0.60 and 0.52 mmol of SO3H per gram of catalysts respectively. The most active catalyst CSC-Star-Glu-IL2 was also characterized by XRD, SEM and TEM. XRD patterns of CSC-Star-Glu-IL2 (Fig. S3, see ESI†) showed a broad diffraction peak from 2θ = 15–30°, C(002), attributed to amorphous carbon sheets oriented in a considerable random fashion. SEM images (Fig. S4, see ESI†) showed the morphology of the catalyst and indicated that the catalyst possesses broad rod like shapes. TEM micrographs (Fig. S5, see ESI†) showed that the sulfonated carbon/silica composite is spherical in shape with ionic liquid coated on the surface of the catalyst (shown by dots on spheres in Fig. S5†).
C stretching modes in polyaromatic rings. The bands from 2930–2934 cm−1 in CSC-Star-Glu-ILs were due to the phenolic –OH groups. CSC-Star-Glu-ILs also showed absorption bands from 1455–1466 cm−1 (due to asymmetric stretching of SO2) and from 1102–1110 cm−1 (due to symmetric stretching of SO2), which indicated the presence of –SO3H groups. CSC-Star-Glu-ILs showed bands from 3100–3110 and 3146–3152 cm−1 due to imidazolium ring –CH stretching. The bands from 2873–2875 and 2960–2962 cm−1 were due to aliphatic –CH stretching modes of methyl and butyl groups. The bands from 1570–1574 were assigned to stretching modes of imidazole ring and from 1160–1170 cm−1 were assigned to imidazole H–C–N bending modes. Further, the bands from 803–810, 750–753 and 651–655 cm−1 were due to in-plane imidazole ring bending, out of plane –CH bending and imidazole C2–N1–C5 bending modes respectively. The presence of these bands showed the coating of ionic liquid on the surface of the catalyst. The major absorption frequencies are presented in Table 1. The FTIR spectra of CSC-Star-Glu-IL2 is shown in Fig. S1 (see ESI†). The stability of CSC-Star-Glu-ILs was determined by thermogravimetric analysis. The TGA curves showed almost negligible weight losses upto 240 °C, and then significant weight losses are observed after 247 °C. Thus, the catalysts are stable upto 240 °C and hence it is safe to carry out the reaction at room temperature, 60 and 100 °C. The weight losses of CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 are represented in Table 2. The TGA of CSC-Star-Glu-IL2 is represented in Fig. S2 (see ESI†). The –SO3H loading in the CSC-Star-Glu-ILs was determined by elemental analysis. The CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 were found to contain 0.55, 0.60 and 0.52 mmol of SO3H per gram of catalysts respectively. The most active catalyst CSC-Star-Glu-IL2 was also characterized by XRD, SEM and TEM. XRD patterns of CSC-Star-Glu-IL2 (Fig. S3, see ESI†) showed a broad diffraction peak from 2θ = 15–30°, C(002), attributed to amorphous carbon sheets oriented in a considerable random fashion. SEM images (Fig. S4, see ESI†) showed the morphology of the catalyst and indicated that the catalyst possesses broad rod like shapes. TEM micrographs (Fig. S5, see ESI†) showed that the sulfonated carbon/silica composite is spherical in shape with ionic liquid coated on the surface of the catalyst (shown by dots on spheres in Fig. S5†).
| Catalyst | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
Phenolic-OH | SO2 stretch | Imidazole ring | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asym. | Sym. | (C–H) stretch | H–C–N bending | C2–N1–C5 bending | In plane bending | Out of plane C–H bending | Aliphatic C–H stretch | |||
| a FTIR was recorded on Perkin-Elmer FTIR spectrophotometer using KBr discs. | ||||||||||
| CSC-Star-Glu-IL1 | 1628 | 2930 | 1455 | 1106 | 3146, 3100 | 1160 | 653 | 807 | 750 | 2872, 2960 |
| CSC-Star-Glu-IL2 | 1625 | 2934 | 1466 | 1102 | 3152, 3106 | 1166 | 651 | 803 | 753 | 2875, 2962 |
| CSC-Star-Glu-IL3 | 1629 | 2933 | 1460 | 1110 | 3150, 3110 | 1170 | 655 | 810 | 752 | 2873, 2960 |
| Entry | Catalyst | Loss of organic functionality (°C) |
|---|---|---|
| a Thermal analysis was carried out on DTG-60 Shimadzu make thermal analyzer at the rate of 10 °C min−1. | ||
| 1 | CSC-Star-Glu-IL1 | 245 |
| 2 | CSC-Star-Glu-IL2 | 247 |
| 3 | CSC-Star-Glu-IL3 | 240 |
In order to optimize the ionic liquid used for the preparation of catalysts, we firstly prepare CSC-Star-Glu [(prepared from starch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (3
glucose (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1))] and then coated it with different ionic liquids viz. 1-methyl-3-methylimidazolium bromide, [Mmim][Br], 1-ethyl-3-methylimidazolium bromide, [Emim][Br], 1-propyl-3-methylimidazolium bromide, [Pmim][Br], 1-butyl-3-methylimidazolium bromide, [Bmim][Br] and 1-pentyl-3-methylimidazolium bromide, [Pentmim][Br] to prepare different ionic liquid coated catalysts. Then we carried out Knoevenagel condensation taking 4-methoxybenzaldehyde and malononitrile as the test substrates and the results are presented in Table 3, which indicated that out of various ionic liquids, [Bmim][Br] exhibited highest activity. So, we chose [Bmim][Br] for coating sulfonated carbon/silica composites.
1))] and then coated it with different ionic liquids viz. 1-methyl-3-methylimidazolium bromide, [Mmim][Br], 1-ethyl-3-methylimidazolium bromide, [Emim][Br], 1-propyl-3-methylimidazolium bromide, [Pmim][Br], 1-butyl-3-methylimidazolium bromide, [Bmim][Br] and 1-pentyl-3-methylimidazolium bromide, [Pentmim][Br] to prepare different ionic liquid coated catalysts. Then we carried out Knoevenagel condensation taking 4-methoxybenzaldehyde and malononitrile as the test substrates and the results are presented in Table 3, which indicated that out of various ionic liquids, [Bmim][Br] exhibited highest activity. So, we chose [Bmim][Br] for coating sulfonated carbon/silica composites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on Knoevenagel condensationa in water
1) on Knoevenagel condensationa in water
Catalyst testing for Knoevenagel condensation of aldehydes and ketones
Knoevenagel condensation was carried out by stirring a mixture of aldehyde or ketone, active methylene compound in the presence of CSC-Star-Glu-IL in water at room temperature (for aldehydes) or 60 °C (for ketones) (Scheme 2). To select the appropriate CSC-Star-Glu-IL, 4-methoxybenzaldehyde and malononitrile were selected as the test substrates and the reaction was carried using CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 using water as the reaction medium. The results are presented in Table 4, which indicated that out of various catalysts, CSC-Star-Glu-IL2 exhibited highest activity.| Entry | Catalyst | Knoevenagel condensation | Reductive amination | Michael addition | |||
|---|---|---|---|---|---|---|---|
| Time (h) | Yielde (%) | Time (min) | Yieldf (%) | Time (h) | Yieldf (%) | ||
a CSC-Star-Glu-IL1: catalyst prepared using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of starch and glucose; CSC-Star-Glu-IL2: catalyst prepared using 3 1 ratio of starch and glucose; CSC-Star-Glu-IL2: catalyst prepared using 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of starch and glucose; CSC-Star-Glu-IL3: catalyst prepared using 1 1 ratio of starch and glucose; CSC-Star-Glu-IL3: catalyst prepared using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of starch and glucose.b Reaction conditions: 4-methoxybenzaldehyde (1 mmol), malononitrile (1 mmol) and CSC-Star-Glu-IL (0.2 g)at room temperature in water (5 mL) for 0.5 h.c Reaction conditions: aldehyde or ketone (1 mmol), primary amine (1 mmol), CSC-Star-Glu-IL (0.2 g) in water (5 mL) at 60 °C in microwave synthesizer.d Reaction conditions: α,β-unsaturated ketone (1 mmol), indole (1.2 mmol), CSC-Star-Glu-IL (0.2 g) at 100 °C in water (7 mL).e Isolated yields.f Column chromatographic yields. 3 ratio of starch and glucose.b Reaction conditions: 4-methoxybenzaldehyde (1 mmol), malononitrile (1 mmol) and CSC-Star-Glu-IL (0.2 g)at room temperature in water (5 mL) for 0.5 h.c Reaction conditions: aldehyde or ketone (1 mmol), primary amine (1 mmol), CSC-Star-Glu-IL (0.2 g) in water (5 mL) at 60 °C in microwave synthesizer.d Reaction conditions: α,β-unsaturated ketone (1 mmol), indole (1.2 mmol), CSC-Star-Glu-IL (0.2 g) at 100 °C in water (7 mL).e Isolated yields.f Column chromatographic yields. |
|||||||
| 1 | CSC-Star-Glu-IL1 | 0.5 | 90 | 10 | 85 | 13 | 78 |
| 2 | CSC-Star-Glu-IL2 | 0.5 | 95 | 10 | 90 | 13 | 85 |
| 3 | CSC-Star-Glu-IL3 | 0.5 | 88 | 10 | 80 | 13 | 75 |
This is because, out of these three catalysts, CSC-Star-Glu-IL2 contains 3 equivalent of starch and one equivalent of glucose. Since this catalyst contains maximum amount of amylopectin which will lead to the formation of more number of small polycyclic aromatic rings, providing more anchoring sites for SO3H groups.5,43c Hence, more the number of SO3H groups, more is the activity of the catalyst. The molar ratio of test substrates was also optimized after carrying out series of reactions. It was found that for 1 mmol of carbonyl compound, 1 mmol of active methylene compound was required. To select the appropriate amount of CSC-Star-Glu-IL2, the reaction with test substrates was carried using different amounts of the catalyst i.e. 0.050 g (3 mol% SO3H), 0.10 g (6 mol% SO3H), 0.15 g (9 mol% SO3H) and 0.2 g (12 mol% SO3H) and found that best results in terms of reaction time and yield were obtained with 0.2 g of CSC-Star-Glu-IL2. Further, to optimize the reaction temperature, we carried out the reaction with test substrates at room temperature, 60 and 80 °C and found that room temperature was the optimum reaction temperature for aldehydes and 60 °C for ketones (acetophenone as test substrate). Thus, the optimum conditions selected are: aldehyde or ketone (1 mmol), active methylene compound (1 mmol), CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H) and room temperature for aldehydes and 60 °C for ketones. The generality of the developed protocol was demonstrated by using various aldehydes and ketones possessing both electron-releasing and electon-withdrawing groups and excellent results were obtained (Table 5).
| Entry | Aldehyde/ketone | X | Time (h) | Yieldb (%) | m.p./lit. m.p. (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde or ketone (1 mmol), active methylene compound (1 mmol), CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H) in water (5 mL) at room temperature for aldehydes and at 60 °C for ketones in water (5 mL).b Isolated yields.c Boiling point. | |||||
| 1 |  |
CN | 2 | 94 | 82–84/82–84 (ref. 12) |
| 2 |  |
CN | 0.5 | 95 | 112–114/113–114 (ref. 11) |
| 3 | 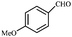 |
COOEt | 1 | 86 (E isomer) | 82–84/85 (ref. 11) |
| 4 |  |
CN | 1 | 94 | 188–190/187–188 (ref. 12) |
| 5 |  |
CN | 1.5 | 88 | 158–160/161–163 (ref. 12) |
| 6 | 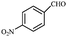 |
CN | 0.5 | 90 | 157–160/159–160 (ref. 12) |
| 7 | 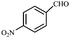 |
COOEt | 1 | 88 (E isomer) | 128–130/129–131 (ref. 12) |
| 8 |  |
CN | 1 | 93 | 124–126/126 (ref. 11) |
| 9 |  |
CN | 2 | 85 | 90–91/92 (ref. 12) |
| 10 |  |
COOEt | 5 | 84 (E isomer) | 83–85/84 (ref. 12) |
| 11 |  |
COOEt | 4 | 85 (E isomer) | 140–143/144 (ref. 12) |
| 12 |  |
COOEt | 2 | 88 (E isomer) | 135–138/138–140 (ref. 11) |
| 13 |  |
CN | 1.5 | 90 | 132–135/134–136 (ref. 11) |
| 14 | 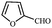 |
COOEt | 1 | 88 (E isomer) | 82–83/82–83 (ref. 22) |
| 15 | C3H7CHO | COOEt | 5 | 85 (E isomer) | 108-110c/109–111 (ref. 47) |
Catalyst testing for reductive amination of aldehydes and ketones
One-pot reductive amination of aldehydes and ketones was carried out by stirring a mixture of carbonyl compound, amine and NaBH4 in the presence of CSC-Star-Glu-IL in water using microwave heating (Scheme 2). To select the appropriate CSC-Star-Glu-IL for reductive amination, 4-methoxybenzaldehyde and aniline were selected as the test substrates and the reaction was carried using CSC-Star-Glu-IL1, CSC-Star-Glu-IL2 and CSC-Star-Glu-IL3 in water. The results are presented in Table 4, which indicated that out of various catalysts, CSC-Star-Glu-IL2 exhibited highest activity. The test reaction was carried out under different set of conditions to select the optimum reaction conditions. First of all, molar ratio of test substrates was optimized. It was found that for 1 mmol of each of 4-methoxybenzaldehyde and aniline, 1.5 mmol of NaBH4 was required. To select the optimum reaction temperature, we carried out the test reaction at different temperatures 60, 80 and 100 °C under stirring on a magnetic stirrer but the reaction did not go to completion even after 20 h at either of these temperatures. Then the reaction with test substrates was carried out in a microwave synthesizer at 60 °C and to our surprise, complete conversion of test substrates took place in just 10 min giving N-(4-methoxybenzyl)aniline in 90% isolated yield. To select the appropriate amount of catalyst, the reaction was carried out using different amounts of CSC-Star-Glu-IL2 i.e. 0.050 g (3 mol% SO3H), 0.10 g (6 mol% SO3H), 0.15 g (9 mol% SO3H) and 0.2 g (12 mol% SO3H) and found that best results in terms of reaction time and yield were obtained with 0.2 g of CSC-Star-Glu-IL2. Thus, the optimum conditions selected for the reductive amination are: aldehyde (1 mmol), amine (1 mmol), NaBH4 (1.5 mmol), CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H) in water (5 mL) under microwave heating at 60 °C. To demonstrate the generality of the developed protocol, various aldehydes and ketones possessing both electron-releasing and electron-withdrawing groups were chosen and excellent results were obtained (Table 6).| Entry | Carbonyl compound | Amine | Product | Yieldb (%) | m.p./lit. m.p. (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde or ketone (1 mmol), primary amine (1 mmol), CSC-Star-Glu-IL2 ( 0.2 g, 12 mol% SO3H) in water (5 mL) using MW heating at 60 °C for 10 min.b Column chromatographic yields.c Hydrochloride salt of amine. | |||||
| 1 |  |
 |
 |
96 | 36–37/35.5–37.8 (ref. 44) |
| 2 |  |
 |
 |
86 | 47–48/48.6–48.9 (ref. 34) |
| 3 |  |
 |
 |
90 | 47–48/48–49 (ref. 44) |
| 4 | 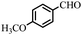 |
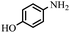 |
 |
88 | 100–101/102–103 (ref. 44) |
| 5 | 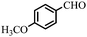 |
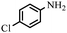 |
 |
90 | 76–77/78–81 (ref. 44) |
| 6 |  |
 |
 |
86 | 140–141/140–141 (ref. 34) |
| 7 |  |
 |
 |
93 | 209–211c/210–211 (ref. 44) |
| 8 |  |
 |
 |
95 | 66–67/67–68 (ref. 44) |
| 9 |  |
 |
 |
85 | 95–96/97.7–97.9 (ref. 34) |
| 10 |  |
 |
 |
90 | 176–177c/179–181 (ref. 44) |
| 11 |  |
 |
 |
85 | Oil/oil (ref. 48) |
| 12 |  |
 |
 |
90 | Oil/oil (ref. 49) |
| 13 | C3H7CHO | (C2H5)2NH | C3H7CH2N(C2H5)2 | 88 | Oil/oil (ref. 49) |
Catalyst testing for the Michael addition of indole to α,β-unsaturated ketones
Michael addition was carried out by stirring a mixture of α,β-unsaturated ketone and indole in the presence of CSC-Star-Glu-IL in water (Scheme 2). Again CSC-Star-Glu-IL2 was found to be the best catalyst for the Michael addition of indole (1.2 mmol) to 3-(4-methylphenyl)-1-phenylpropenone (1 mmol) (Table 4). Further, 0.2 g (12 mol% SO3H) of CSC-Star-Glu-IL2 gave the best results in terms of reaction time and yield. To optimize the reaction temperature, the Michael addition was carried at 50, 80 and 100 °C using water as the reaction medium and found that 100 °C was the optimum reaction temperature. Thus, the optimum conditions selected are: α,β-unsaturated ketone (1 mmol), indole (1.2 mmol), CSC-Star-Glu-IL 0.2 g (12 mol% SO3H), water (7 mL) and 100 °C was selected as the reaction temperature. To demonstrate the generality of the developed protocol, various α,β-unsaturated ketones possessing both electron-releasing and electron-withdrawing groups were chosen and excellent results were obtained (Table 7).| Entry | Ar | R2 | Time (h) | Yieldb (%) | m.p./lit. m.p. (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: α,β-unsaturated ketone (1 mmol), indole (1.2 mmol), CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H) at 100 °C in water (7 mL).b Column chromatographic yields. | |||||
| 1 | C6H5 | C6H5 | 12 | 83 | 126–127/127–128 (ref. 46) |
| 2 | 4-(OCH3)C6H4 | C6H5 | 12.5 | 80 | 122–123/124–125 (ref. 45) |
| 3 | 4-(CH3)C6H4 | C6H5 | 13 | 85 | 129–130/130–131 (ref. 46) |
| 4 | 4-(CH3)C6H4 | 4-ClC6H4 | 13 | 78 | 146–147/146–149 (ref. 46) |
| 5 | 4-ClC6H4 | C6H5 | 13.5 | 75 | 126–127/127–128 (ref. 46) |
Since the products are solids and insoluble in water, the effect of reaction time on yield of the products is required to be studied because the catalyst was also solid and exist with the product as the reaction progresses. To study this, the reaction mixture in case of entry 1 (Tables 5–7) was stirred under the reaction conditions for different periods of time i.e. upto 2 h for Knoevenagel condensation, 10 min for reductive amination and 16 h for Michael addition. In Knoevenagel condensation, initially, there was a sharp increase in yield up to 0.5 h i.e. 45%, then yield goes to 85% in 1.5 h until the reaction goes to completion in 2 h (Fig. 1a). In case of reductive amination, again sharp increase in yield was observed up to 2 min i.e. 40%, then up to 8 min yield goes to 88% till the reaction goes to completion in 10 min (Fig. 1b). In case of Michael addition, from 4 to 12 h (Fig. 1b) there is again sharp increase in yield and at 12 h, the yield of product reached its maximum level i.e. 83% and no further enhancement in yield was observed if the reaction was allowed to occur for longer reaction time.
 | ||
| Fig. 1 (a) Effect of reaction time on the yield of products for Knoevenagel condensation (entry 1, Table 4). (b) Effect of reaction time on the yield of products for reductive amination and Michael addition (entry 1, Table 5 and Table 6). | ||
Comparison of activity of CSC-Star-Glu-IL2 with CSC-Star-Glu (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1) in water
1) in water
We carried out Knoevenagel condensation (using 4-methoxybenzaldehyde and malononitrile as test substrates), reductive amination (4-methoxybenzaldehyde and aniline as test substrates) and Michael addition (indole and 3-(4-methylphenyl)-1-phenylpropenone as test substrates) with CSC-Star-Glu (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in different solvents and found that lower yields of products were obtained in case of water as a solvent. To our surprise, a great improvement on the yield of products were obtained when CSC-Star-Glu (3
1) in different solvents and found that lower yields of products were obtained in case of water as a solvent. To our surprise, a great improvement on the yield of products were obtained when CSC-Star-Glu (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) coated with ionic liquid i.e. CSC-Star-Glu-IL2 was used as catalyst to carry out the similar reactions in water. The results are presented in Fig. 2.
1) coated with ionic liquid i.e. CSC-Star-Glu-IL2 was used as catalyst to carry out the similar reactions in water. The results are presented in Fig. 2.
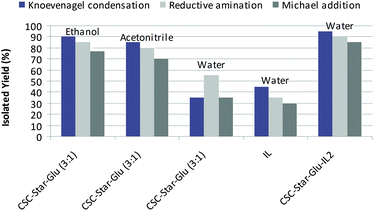 | ||
| Fig. 2 Comparison of activity of CSC-Star-Glu-IL2 with CSC-Star-Glu for Knoevenagel condensation (Table 4, entry 2), reductive amination of aldehydes and ketones (Table 5, entry 3) and for Michael addition of indole to α,β-unsaturated ketones (Table 6, entry 3) in different solvents. | ||
Finally, to examine the effect of ionic liquid loading on CSC-Star-Glu (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we prepared catalysts with different loadings of ionic liquid and found that excellent yields in case of test substrates for Knoevenagel condensation, reductive amination and Michael addition were obtained when 50 wt% of ionic liquid was loaded on CSC-Star-Glu (3
1), we prepared catalysts with different loadings of ionic liquid and found that excellent yields in case of test substrates for Knoevenagel condensation, reductive amination and Michael addition were obtained when 50 wt% of ionic liquid was loaded on CSC-Star-Glu (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The results are presented in Fig. 3.
1). The results are presented in Fig. 3.
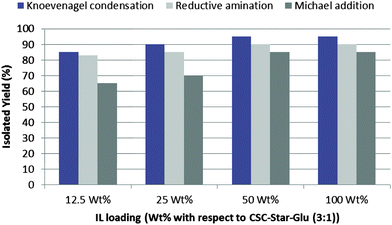 | ||
| Fig. 3 Effect of ionic liquid loading on the activity of CSC-Star-Glu-IL2 for Knoevenagel condensation (Table 4, entry 2), reductive amination of aldehydes and ketones (Table 5, entry 3) and Michael addition of indole to α,β-unsaturated ketones (Table 6, entry 3) in water. | ||
Recyclability
Recyclability of CSC-Star-Glu-IL2 was investigated in case of entry 2 (Table 5), entry 5 (Table 6) and entry 3 (Table 7). The catalyst was separated by filtration after completion of the reaction and again used for subsequent reactions after adding fresh substrates under similar conditions for five consecutive runs (Fig. 4). It was found that catalyst could be recycled for five consecutive runs without loss of significant activity. The elemental analysis and FTIR of the CSC-Star-Glu-IL2 after 5th run indicated negligible change in the sulfur content and coating of ionic liquid respectively. The slight loss of activity may be due to the microscopic changes on the surface of the catalyst. | ||
| Fig. 4 Recyclability of CSC-Star-Glu-IL2 for Knoevenagel condensation (Table 4, entry 2), reductive amination of aldehydes and ketones (Table 5, entry 3) and Michael addition of indole to α,β-unsaturated ketones (Table 6, entry 3) in water. | ||
Heterogeneity
In order to rule out the possibility of leaching of ionic liquid film from the surface of the catalyst, we carried out the hot filtration test. The reaction in case of entry 1 (Table 5) has been carried out in the presence of CSC-Star-Glu-IL2, until the conversion was 45% (0.5 h) and at that point, CSC-Star-Glu-IL2 was filtered off at the reaction temperature. The liquid phase was then transferred to another flask and allowed to react, but no further significant conversion was observed. Moreover, the elemental analysis and FTIR of the used CSC-Star-Glu-IL2 indicated negligible change in the sulfur content and coating of ionic liquid respectively. So, we conclude that there is no significant leaching of ionic liquid from the surface of the catalyst.Experimental section
General remarks
The chemicals used were purchased from Aldrich chemical company and Merck. The products were characterized by their spectral data and comparison of their physical data with those of known samples. The 1H NMR data were recorded in CDCl3 or CDCl3+DMSO-d6 on Bruker DPX 200 (200 MHz) spectrometer using TMS as an internal standard. The FTIR spectra were recorded on Perkin-Elmer FTIR spectrophotometer using KBr windows and mass spectral data were recorded on Bruker Esquires 3000 (ESI). XRD diffraction patterns were determined on Bruker AXSD8 X-ray diffraction spectrometer and SEM using Jeol make T-300 Scanning electron Microscope. Transmission Electron Micrographs (TEM) were recorded on H7500 Hitachi. The amount of sulfur in composites was determined by elemental analysis on Elementar Analysensyteme GmbH VarioEL. Thermal analysis was carried out on DTG-60 Shimadzu make thermal analyzer. Microwave synthesizer manufactured by CEM (DISCOVER SYSTEM) was used for carrying out the reductive amination of aldehydes and ketones.General procedure for the synthesis of CSC-Star-Glu-ILs
Sulfonated carbon/silica composites were prepared according to the method already reported,5 except that instead of taking natural organic compounds, a mixture of starch and glucose in three different ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 respectively was taken. In the second step, the CSC-Star-Glu composite (1 g) was added to a solution of 1-butyl-3-methyl imidazolium bromide (0.5 g) in acetonitrile (8 mL) in a round bottom flask (25 mL). The solution was then stirred at room temperature for 30 minutes followed by removal of acetonitrile in a rotary evaporator at 50 °C. Finally, the solid obtained was dried under vacuum for 30 minutes to get a free flowing white powder (50 wt% of IL was loaded on CSC-Star-Glu).
3 respectively was taken. In the second step, the CSC-Star-Glu composite (1 g) was added to a solution of 1-butyl-3-methyl imidazolium bromide (0.5 g) in acetonitrile (8 mL) in a round bottom flask (25 mL). The solution was then stirred at room temperature for 30 minutes followed by removal of acetonitrile in a rotary evaporator at 50 °C. Finally, the solid obtained was dried under vacuum for 30 minutes to get a free flowing white powder (50 wt% of IL was loaded on CSC-Star-Glu).
General procedure for Knoevenagel condensation of aldehydes and ketones
To a mixture of aldehyde or ketone (1 mmol), active methylene compound (1 mmol) in a round-bottom flask (25 mL), CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H) was added and the reaction mixture was stirred at room temperature (for aldehydes) or 60 °C (for ketones) in water (5 mL) for an appropriate time (Table 5). After completion of the reaction (monitored by TLC), the reaction mixture was extracted with hot EtOAc (2 × 5 mL). The product was obtained after removal of the solvent under reduced pressure.The geometry of Knoevenagel products may be E or Z. It is well known that the E and Z isomers can be distinguished by 1H NMR spectral characteristics. When ethyl cyanoacetate was used as the active methylene compound, the reaction is highly stereoselective with only formation of E-isomer (Table 5). The assignment of stereochemistry of the isomer formed was made on the basis of chemical shift of vinylic (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) protons which migrated to lower field (higher δ values) for the E isomers (when CH
C) protons which migrated to lower field (higher δ values) for the E isomers (when CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C protons are cis to the ester group).11,22,43d–43f Thus, from the 1H NMR data which is in coincidence with the data already reported,11,22,43d–43f it is confirmed that the products obtained are E-isomers, when ethyl cyanoacetate was used as the active methylene compound.
C protons are cis to the ester group).11,22,43d–43f Thus, from the 1H NMR data which is in coincidence with the data already reported,11,22,43d–43f it is confirmed that the products obtained are E-isomers, when ethyl cyanoacetate was used as the active methylene compound.
General procedure for reductive amination of aldehydes and ketones
To a mixture of aldehyde or ketone (1 mmol), amine (1 mmol), NaBH4 (1.5 mmol) and CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H) in a round-bottom flask (25 mL), water (5 mL) was added and reaction mixture was stirred at 60 °C in a microwave synthesizer for 10 min (Table 6). After completion of the reaction (monitored by TLC), the reaction mixture was extracted with hot ethyl acetate (2 × 5 mL). The organic layer was washed with water and dried over anhyd. Na2SO4. The product was obtained after removing the solvent under reduced pressure followed by passing through column of silica gel and elution with EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pet. ether (2
pet. ether (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
General procedure for Michael addition of indole to α,β-unsaturated ketone
To a mixture of indole (1.2 mmol), α,β-unsaturated ketone (1 mmol) in a round-bottom flask (25 mL) and CSC-Star-Glu-IL2 (0.2 g, 12 mol% SO3H), water (7 mL) was added and reaction mixture was stirred at 100 °C for an appropriate time (Table 7). After completion of the reaction (monitored by TLC), the reaction mixture was extracted with hot ethyl acetate (2 × 5 mL). The organic layer was washed with water and dried over anhyd. Na2SO4. Finally, the product was obtained after removal of the solvent under reduced pressure followed by passing through column of silica gel and elution with EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pet. ether (1
pet. ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6).
6).
The catalyst was dried in a vacuum desiccator for 1 h and reused in the next run.
The structures of the products were confirmed by IR, 1H NMR, mass spectral data and comparison with authentic samples available commercially or prepared according to the literature methods.
Spectroscopic data of some selected compounds
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str.) 1H NMR (CDCl3): δ 1.40 (t, J = 7 Hz, 3H, CH2CH3), 3.88 (s, 3H, OCH3), 4.23–4.47 (q, J = 7 Hz, 2H, CH2CH3), 7.05 (d, J = 7 Hz, 2H, Ar-H), 7.94 (d, J = 7 Hz, 2H, Ar-H), 8.31 (s, 1H, CH). MS (ESI): 243 (M+), 266 (M+ + 23).
C str.) 1H NMR (CDCl3): δ 1.40 (t, J = 7 Hz, 3H, CH2CH3), 3.88 (s, 3H, OCH3), 4.23–4.47 (q, J = 7 Hz, 2H, CH2CH3), 7.05 (d, J = 7 Hz, 2H, Ar-H), 7.94 (d, J = 7 Hz, 2H, Ar-H), 8.31 (s, 1H, CH). MS (ESI): 243 (M+), 266 (M+ + 23).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str.) 1H NMR (CDCl3): δ 1.35–1.39 (t, J = 8 Hz, 3H, CH2CH3), 3.91 (s, 3H, OCH3), 4.32–4.37 (q, J = 8 Hz, 2H, CH2CH3), 6.47 (s, 1H, Ar-H), 6.70 (d, J = 8 Hz, 2H, Ar-H), 8.43 (d, J = 8 Hz, 2H, Ar-H), 8.71 (s, 1H, CH). MS (ESI): 261.9 (M+).
C str.) 1H NMR (CDCl3): δ 1.35–1.39 (t, J = 8 Hz, 3H, CH2CH3), 3.91 (s, 3H, OCH3), 4.32–4.37 (q, J = 8 Hz, 2H, CH2CH3), 6.47 (s, 1H, Ar-H), 6.70 (d, J = 8 Hz, 2H, Ar-H), 8.43 (d, J = 8 Hz, 2H, Ar-H), 8.71 (s, 1H, CH). MS (ESI): 261.9 (M+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str.). 1H NMR (CDCl3): δ 3.82 (s, 3H, OCH3), 4.23 (bs, 1H, NH), 4.25 (s, 2H, CH2), 6.15–7.30 (m, 9H, Ar-H). MS (ESI): 213 (M+), 192.
C str.). 1H NMR (CDCl3): δ 3.82 (s, 3H, OCH3), 4.23 (bs, 1H, NH), 4.25 (s, 2H, CH2), 6.15–7.30 (m, 9H, Ar-H). MS (ESI): 213 (M+), 192.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str.) 1H NMR (CDCl3): δ 3.87 (s, 3H, OCH3), 3.94 (bs, 1H, NH), 4.22 (s, 2H, CH2), 6.55 (d, J = 9.06 Hz, 2H, Ar-H), 6.84 (d, J = 9.06 Hz, 2H, Ar-H), 7.06 (d, J = 9.06 Hz, 2H, Ar-H), 7.27 (d, J = 8.30 Hz, 2H, Ar-H). MS (ESI): 247 (M+), 249 (M+ + 2).
C str.) 1H NMR (CDCl3): δ 3.87 (s, 3H, OCH3), 3.94 (bs, 1H, NH), 4.22 (s, 2H, CH2), 6.55 (d, J = 9.06 Hz, 2H, Ar-H), 6.84 (d, J = 9.06 Hz, 2H, Ar-H), 7.06 (d, J = 9.06 Hz, 2H, Ar-H), 7.27 (d, J = 8.30 Hz, 2H, Ar-H). MS (ESI): 247 (M+), 249 (M+ + 2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O str.). 1H NMR (CDCl3, 200 MHz): δ 2.25 (s, 3H, CH3), 3.79 (d, J = 7.6 Hz, 2H, CH2), 4.95 (t, J = 7.6 Hz, 1H, CH), 6.84–8.0 (m, 13H, Ar-H), 8.10 (bs, 1H, NH). MS (ESI): 373 (M+).
O str.). 1H NMR (CDCl3, 200 MHz): δ 2.25 (s, 3H, CH3), 3.79 (d, J = 7.6 Hz, 2H, CH2), 4.95 (t, J = 7.6 Hz, 1H, CH), 6.84–8.0 (m, 13H, Ar-H), 8.10 (bs, 1H, NH). MS (ESI): 373 (M+).Conclusions
We have developed a novel catalytic system derived from amorphous carbon/silica composites and ionic liquid for carrying out Knoevenagel condensation, reductive amination and Michael addition in water. The catalyst allows reactions to be carried out in the most benign of reaction media and avoids most of the problems normally associated with the use of ionic liquids. The catalyst can be easily recycled without loss of activity. Simplicity of operation and the environmentally benign and legislatively robust nature of reaction conditions are the main advantages of the developed protocols.Acknowledgements
We thank the Director, IIIM Jammu for spectral and library facilities; Head, Sophisticated Analytical Instrumentation Facility, Punjab University Chandigarh for XRD, SEM and TEM; and Head, RSIC, IIT Roorkee for thermal analysis and elemental analysis. Financial support from UGC, New Delhi (Major Research Project, F 41-281/2012 (SR)) is gratefully acknowledged.Notes and references
- (a) C.-J. Li and T.-H. Chan, Organic Reactions in Aqueous Media, Wiley, New York, 1997 Search PubMed; (b) U. M. Lindström, Chem. Rev., 2002, 102, 2751 CrossRef PubMed; (c) C.-J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed; (b) M. Eissen, J. O. Metzger, E. Schmidt and V. Schneidewind, Angew. Chem., Int. Ed., 2002, 41, 414 CrossRef CAS; (c) I. T. Horváth, Acc. Chem. Res., 2002, 35, 685 (Special Topic Issue on Green Chem.) Search PubMed.
- (a) M. H. Valkenberg, C. deCastro and W. F. Hölderich, Appl. Catal., A, 2001, 215, 185 CrossRef CAS; (b) H. Hagiwara, Y. Sugawara, K. Isobe, T. Hoshi and T. Suzuki, Org. Lett., 2004, 6, 2325 CrossRef CAS PubMed; (c) Y. Gu, C. Ogawa, J. Kobayashi, C. Mori and S. Kobayashi, Angew. Chem., Int. Ed., 2006, 45, 7217 CrossRef CAS PubMed; (d) Y. Gu, C. Ogawa and S. Kobayashi, Org. Lett., 2007, 9, 175 CrossRef CAS PubMed.
- (a) Y. Gu, A. Karam, F. Jérôme and J. Barrault, Org. Lett., 2007, 9, 3145 CrossRef CAS PubMed; (b) M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef CAS PubMed.
- P. Gupta and S. Paul, Green Chem., 2011, 13, 2365 RSC.
- P. Leelavathi and S. R. Kumar, J. Mol. Catal. A: Chem., 2005, 240, 99 CAS.
- T. I. Reddy and R. S. Varma, Tetrahedron Lett., 1997, 38, 1721 CrossRef CAS.
- Y. Goa, P. Wu and T. Tatsumi, J. Catal., 2004, 224, 107 CrossRef CAS PubMed.
- S. Nakamura, H. Hiro and T. J. Ohwada, J. Org. Chem., 2004, 69, 4309 CrossRef CAS PubMed.
- (a) B. Tamami and A. Fadavi, Catal. Commun., 2005, 6, 747 CrossRef CAS PubMed; (b) A. Ilangovan, S. Muralidharan and S. Maruthamuthu, J. Korean Chem. Soc., 2011, 55, 1000 CrossRef CAS; (c) F. Al-Omran, R. M. Mohareb and A. A. El-Khair, Molecules, 2011, 16, 6129 CrossRef CAS PubMed.
- M. A. Pasha and K. Manjula, J. Saudi Chem. Soc., 2010, 15, 283 CrossRef PubMed.
- R. Gupta, M. Gupta, S. Paul and R. Gupta, Bull. Korean Chem. Soc., 2009, 30, 2419 CrossRef CAS.
- N. N. Karade, S. V. Gampawar, S. V. Shinde and W. N. Jadhav, Chin. J. Chem., 2007, 25, 1686 CrossRef CAS.
- K. R. Reddy, K. Rajgopal, C. U. Maheswari and M. L. Kantam, New J. Chem., 2006, 30, 1549 RSC.
- S. Shanmuganathan, L. Greiner and P. D. de María, Tetrahedron Lett., 2010, 51, 6670 CrossRef CAS PubMed.
- B. A. Robichaud and K. G. Liu, Tetrahedron Lett., 2011, 52, 6935 CrossRef CAS PubMed.
- D. Jain, C. Khatri and A. Rani, Fuel Process. Technol., 2010, 91, 1015 CrossRef CAS PubMed.
- J. Mondal, A. Modak and A. Bhaumik, J. Mol. Catal. A: Chem., 2011, 335, 236 CrossRef CAS PubMed.
- J.-P. Li, J.-K. Qiu, H.-J. Li and G.-S. Zhang, J. Chin. Chem. Soc., 2011, 58, 1 CrossRef.
- Z. Gao, J. Zhou, F. Cui, Y. Zhu, Z. Hua and J. Shi, Dalton Trans., 2010, 39, 11132 RSC.
- Y.-F. Lai, H. Zheng, S.-J. Chai, P.-F. Zhang and X.-Z. Chen, Green Chem., 2010, 12, 1917 RSC.
- G. Li, J. Xiao and W. Zhang, Green Chem., 2011, 13, 1828 RSC.
- M. K. Pillai, S. Singh and S. B. Jonnalagadda, Synth. Commun., 2010, 40, 3710 CrossRef CAS.
- K. F. Shelke, S. B. Sapkal, K. S. Niralwad, B. B. Shingate and M. S. Shingare, Cent. Eur. J. Chem., 2010, 8, 12 CrossRef CAS PubMed.
- M. Góra, B. Kozik, K. Jamroży, M. K. Łucyński, P. Brzuzan and M. Woźny, Green Chem., 2009, 11, 863 RSC.
- (a) K. K. Senapati, C. Borgohain and P. Phukan, J. Mol. Catal. A: Chem., 2011, 339, 24 CrossRef CAS PubMed; (b) C. Su, Z.-C. Chen and Q.-G. Zhen, Synthesis, 2003, 555 CAS; (c) B. C. Ranu and R. Jana, Eur. J. Org. Chem., 2006, 3767 CrossRef CAS; (d) S. Zhao, X. Wang and L. Zhang, RSC Adv., 2013, 3, 11691 RSC; (e) A. Ying, H. Liang, R. Zheng, C. Ge, H. Jiang and C. Wu, Res. Chem. Intermed., 2011, 37, 579 CrossRef CAS PubMed; (f) R. V. Hangarge, D. V. Jarikote and M. S. Shingare, Green Chem., 2002, 4, 266 RSC.
- B. T. Chao and S. K. Kang, Tetrahedron, 2005, 61, 5725 CrossRef PubMed.
- H. Alinezhad, M. Tajbaksh and R. E. Ahangar, Monatsh. Chem., 2008, 139, 21 CrossRef CAS.
- H. Alinezhad, M. Tajbaksh, F. Salehian and K. Fazli, Tetrahedron Lett., 2009, 50, 659 CrossRef CAS PubMed.
- H. Alinezhad, M. Tajbaksh and M. Zare, Synth. Commun., 2009, 39, 2907 CrossRef CAS.
- H. Alinezhad, M. Tajbaksh and N. Mahdavi, Synth. Commun., 2010, 40, 951 CrossRef CAS.
- H. Alinezhad, M. Tajbaksh and N. Hamidi, Chin. Chem. Lett., 2010, 21, 7 CrossRef.
- R.-Y. Lai, C.-I. Lee and S.-T. Liu, Tetrahedron, 2008, 64, 1213 CrossRef CAS PubMed.
- Y.-B. Huang, W.-B. Yi and C. Cai, J. Fluorine Chem., 2010, 131, 879 CrossRef CAS PubMed.
- H. Alinezhad and Z. Tollabian, Bull. Korean Chem. Soc., 2010, 31, 1927 CrossRef CAS.
- A. Shokrolahi, A. Zali and M. H. Keshavarz, Green Chem. Lett. Rev., 2011, 4, 195 CAS.
- G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045 RSC.
- A. Arcadi, G. Bianchi, M. Chiarini, G. Anniballe and F. Marinelli, Synlett, 2004, 944 CrossRef CAS PubMed.
- S.-J. Ji and S.-Y. Wang, Synlett, 2003, 2074 CrossRef CAS.
- S. S. Ekbote, A. G. Panda, M. D. Bhor and B. M. Bhanage, Catal. Commun., 2009, 10, 1569 CrossRef CAS PubMed.
- C. S. Schwalm, M. A. Ceschi and D. Russowsky, J. Braz. Chem. Soc., 2011, 22, 623 CrossRef CAS PubMed.
- D. Das, M. Rahman, D. Kundu, A. Majee and A. Hajra, Can. J. Chem., 2010, 88, 150 CrossRef.
- (a) Y.-H. Gao, L. Yang, W. Zhou, L.-W. Xu and C.-G. Xia, Appl. Organomet. Chem., 2009, 23, 114 CrossRef CAS; (b) J.-K. Kobayashi, S.-I. Matsui, K. Ogiso, S. Hayase, M. Kawatsura and T. Itoh, Tetrahedron, 2010, 66, 3917 CrossRef CAS PubMed; (c) G. Chen and B. Fang, Bioresour. Technol., 2011, 102, 2635 CrossRef CAS PubMed; (d) J. S. Yadav, B. V. S. Reddy, A. K. Basak, B. Visali, A. V. Narsaiah and K. Nagaiah, Eur. J. Org. Chem., 2004, 546 CrossRef CAS; (e) D.-Z. Xu, Y. Liu, S. Shi and Y. Wang, Green Chem., 2010, 12, 514 RSC; (f) S. M. Ribeiro, A. C. Serra and A. M. d'A. R. Gonsalves, Appl. Catal., A: Gen, 2011, 399, 126 CrossRef CAS PubMed.
- R. S. Varma and R. Dahiya, Tetrahedron, 1998, 54, 6293 CrossRef CAS.
- Z.-H. Huang, J.-P. Zou and W.-Q. Jiang, Tetrahedron Lett., 2006, 47, 7965 CrossRef CAS PubMed.
- C.-J. Yu, Molecules, 2009, 14, 3222 CrossRef CAS PubMed.
- F. S. Prout, A. A. Abdel-Latif and M. R. Kamal, J. Chem. Eng. Data, 1963, 8, 597 CrossRef CAS.
- P. S. Reddy, S. Kanjilal, S. Sunitha and R. B. N. Prasad, Tetrahedron Lett., 2007, 48, 8807 CrossRef CAS PubMed.
- A. Heydari, S. Khaksar, M. Esfandyari and M. Tajbakhsh, Tetrahedron, 2007, 63, 3363 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45229h |
| This journal is © The Royal Society of Chemistry 2014 |


