Insulin loaded iron magnetic nanoparticle–graphene oxide composites: synthesis, characterization and application for in vivo delivery of insulin
Kostiantyn Turcheniukab,
Manakamana Khanala,
Anastasiia Motorinaac,
Palaniappan Subramaniana,
Alexandre Barrasa,
Vladimir Zaitsevc,
Victor Kuncserd,
Aurel Lecad,
Alain Martoriatie,
Katia Cailliaue,
Jean-Francois Bodarte,
Rabah Boukherrouba and
Sabine Szunerits*a
aInstitut de Recherche Interdisciplinaire (IRI, USR CNRS 3078), Université Lille 1, Parc de la Haute Borne, 50 Avenue de Halley, BP 70478, 59658 Villeneuve d'Ascq, France. E-mail: Sabine.Szunerits@iri.univ-lille1.fr
bDepartment of Fine Organic Synthesis, Institute of Bioorganic Chemistry and Petrochemistry NAS of Ukraine, 1 Murmanska Str., 02660, Kiev, Ukraine
cTaras Shevchenko University, 60 Vladimirskaya str., Kiev, Ukraine
dNational Institute of Materials Physics, Atomistilor 105 bis, 077125 Magurele, Romania
eEA 4479, IFR 147, Université Lille 1, 59658 Villeneuve d'Ascq, France
First published on 11th November 2013
Abstract
One of the focal subjects in insulin delivery is the development of insulin formulations that protect the native insulin from degradation under acidic pH in the stomach. In this work we show, for the first time, that a graphene oxide (GO) based matrix can ensure the stability of insulin at low pH. GO and GO modified with 2-nitrodopamine coated magnetic particle (GO–MPdop) matrices loaded with insulin were prepared and the pH triggered release of the insulin was studied. The loading of insulin on the GO nanomaterials proved to be extremely high at pH < 5.4 with a loading capacity of 100 ± 3% on GO and 88 ± 3% on GO–MPdop. The insulin-containing GO matrices were stable at acidic pH, while insulin was released when exposed to basic solutions (pH = 9.2). Using Xenopus laevis oocytes as a model we showed that the meiotic resumption rate of GO and GO–MPdop remained unaltered when pre-treated in acidic conditions, while pre-incubated insulin (without GO nanomaterials) has lost almost entirely its maturation effect. These results suggest that GO based nanomatrices are promising systems for the protection of insulin.
1. Introduction
The use of nanomaterials as carriers of glycans,1,2 drugs,3,4 genes,5,6 and other biologically active compounds7 has become a widely investigated research field. Most recently, graphene, a two-dimensional nanomaterial, has been intensively explored as an alternative nanocarrier for biological materials due to its large surface area, rich surface chemistry and its potential for crossing the plasma membrane and promoting the cellular uptake of molecules.8,9 The interest in using graphene and graphene oxide (GO) for loading and release of chemical and biological molecules is in addition linked to the different ways the molecule can be linked to the graphene matrix: hydrogen bonding, hydrophobic, π–π stacking and electrostatic interactions can act as anchors that are sensitive to external stimuli (pH, temperature, chemical substances, electrical field, etc.), enabling controlled release.10–14 Since the pioneering work of Dai and colleagues13,15 on the use of PEGylated (PEG = polyethylene glycol) GO as a nanocarrier to load anticancer drugs via noncovalent physisorption and study its cellular uptake, several papers have been devoted to improving the loading efficiency and release of anticancer drugs such as doxorubicin (DOX)16 or to the preparation of multi-functionalized graphene nanomaterials.17–20 Besides graphene and GO, graphene/iron oxide nanoparticles composite materials have shown great promise as drug carriers4,20,21 and for the immobilization and enrichment of biomolecules.22 The magnetic particles modified graphene sheets were synthesized by in situ oxidation of Fe2+ salts to Fe3+ and deposited as Fe3O4 particles onto GO, being at the same time reduced to reduced graphene oxide (rGO).23 Other approaches exploited the strong complexation of the carboxylate anions of GO with FeCl3 and FeCl2, before precipitating Fe3O4 nanoparticles onto GO by treatment with sodium hydroxide.4,20It is worth mentioning that in the earlier reports the magnetic particles were not chemically protected and therefore, they were prone to corrosion upon immersion in cell culture media. Here, we report a different strategy for the preparation of GO–magnetic nanoparticles composite. It is based on the ex situ synthesis of chemically stabilized magnetic particles with 2-nitrodopamine, followed by their insertion onto the GO matrix (Fig. 1). This approach prevents any subsequent reduction of water soluble GO to water insoluble rGO and ensures the formation of a chemically stable GO–magnetic nanoparticles interface. This GO matrix is well suited for the uptake of biomolecules such as insulin.
 | ||
| Fig. 1 (A) Synthetic route of 2-nitrodopamine and functionalization of magnetic nanoparticles (MP) with 2-nitrodopamine. (B) Insulin loading on GO and GO–MPdop. (A). | ||
Insulin, a polypeptide composed of 51 amino acid residues and secreted by the pancreas, plays an important role in the control of blood glucose. Diabetic people suffer from low levels of insulin production and/or from abnormal resistance to the insulin hormone. Current treatment methods involve regular injections of insulin, which can be both painful and inconvenient. In order to overcome these hurdles, the oral route is considered as one of the most convenient means of drug uptake. However, oral administration of hydrophilic macromolecules such as insulin encounters (or faces) major problems such as hydrolysis in the low pH of gastric medium, splitting by proteinases in the stomach and weak penetration through the membrane of epithelial cells of the intestine.24,25 One of the most promising strategies to achieve oral insulin uptake is the use of microsphere systems, which act both as protease inhibitors by protecting the encapsulated insulin from enzymatic degradation and as permeation enhancers by effectively crossing the epithelial layer after oral administration.26–31 Behavior, toxicity and biocompatibility of nanomaterials in vivo is associated to size, surface of coating and administration routes.32 Nevertheless, oral administration appears as an appealing strategy. Indeed, a recent study underlined the biocompatibility of PEGylated GO derivatives after oral administration since the injected material exhibited a long-term retention but no toxicity.33
2. Experimental part
2.1. Materials
Graphite powder (<20 microns), hydrogen peroxide (H2O2), sulfuric acid (H2SO4), dimethylsulfoxide (DMSO), acetonitrile (CH3CN), ammonium hydroxide (NH4OH), iron(II) chloride tetrahydrate (FeCl2·4H2O), iron(III) chloride hexahydrate (FeCl3·6H2O), dopamine hydrochloride, sodium nitrite, insulin (from bovine pancreas, code 10516), dispase, and collagenase were purchased from Sigma-Aldrich and used as received. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from invitrogen.2.2. Preparation of graphene oxide (GO)
Graphene oxide (GO) was synthesized from graphite powder by a modified Hummers method.34 5 mg of the synthesized GO was dispersed in 1 mL of water and exfoliated through ultrasonication for 3 h. This aqueous suspension of GO was used as a stock solution in subsequent experiments.2.3. Synthesis of 2-nitrodopamine
2-Nitrodopamine was synthesized according to ref. 35. Dopamine hydrochloride (1.90 g, 10 mmol) and sodium nitrite (1.52 g, 22 mmol) were dissolved in water (25 mL) and cooled to 0 °C. Sulfuric acid (17.4 mmol in 10 mL of water) was added slowly to the mixture, and a yellow precipitate was formed. After stirring at room temperature overnight, the precipitate was filtered and recrystallized from water to give a product as a hemisulfate salt. Yield 1.9 g (77%). 1H NMR (DMSO-d6, 300 MHz, ppm): 3.10 (br s, 4H, CH2CH2), 6.85 (s, 1H), 7.47 (s, 1H).2.4. Preparation of 2-nitrodopamine modified magnetic particles (MPdop)
Magnetic particles (MP) were prepared as reported previously.36 FeCl2·4H2O (0.34 g, 1.7 mmol) and FeCl3·6H2O (0.95 g, 3.5 mmol) were dissolved in deareated water (20 mL) and subsequently added to a nitrogen-protected three-necked flask under sonication. The resulting mixture was heated at 50 °C for 30 min. Then concentrated ammonium hydroxide (2 mL) was added dropwise and kept at constant temperature (50 °C) for 30 min. The system was finally cooled to room temperature and the solid product was isolated via a non-uniform magnetic field generated by a Nd–Fe–B permanent magnet. The resulting Fe3O4 particles were washed six times with Milli-Q water to remove unreacted chemicals and then stored in water.A water dispersion of bare MP (10 mg mL−1, 1 mL) was mixed with 2-nitrodopamine (7 mg) and sonicated for 1 h at room temperature. The nitrodopamine modified MP (MPdop) were isolated by means of magnet and purified through six consecutive wash/precipitation cycles with water to ensure complete removal of unreacted dopamine. The precipitate was dried in an oven at 50 °C.
2.5. Preparation of GO–MPdop nanohybrid
2 mL of GO in water (2 mg mL−1) was sonicated for 1 h before 2 mL of MPdop (1 mg mL−1) were added and further sonicated for 2 h at 30 °C under N2. In a first step, the resulting precipitate was isolated by centrifugation at 13.500 rpm (20 min) and purified through two consecutive wash/centrifugation cycles at 13.500 rpm (20 min) with water. Further purification was achieved by magnetic separation to separate the magnetic GO–MPdop hybrid from the non-magnetic phase. This procedure yielded ≈5 mg of GO–MPdop hybrid.2.6. Loading of insulin onto GO and GO–MPdop hybrid
GO or GO–MPdop nanohybrid (150 μg mL−1) was sonicated with the desired concentration of insulin for 2 h and then stirred for 22 h at room temperature. All samples were centrifuged at 13.500 rpm for 30 min. The concentration of insulin in the supernatant was determined using a standard insulin concentration curve generated with a UV/Vis spectrophotometer at 275 nm from a series of insulin solutions of different concentrations.2.7. Release of insulin from GO and GO–MPdop hybrid
The release behavior of insulin from the GO–MPdop hybrid and GO was investigated at 37 °C under stirring at 40 ± 10 rpm by varying the pH. At predetermined time-points, samples were centrifuged at 13.500 rpm for 30 min and the supernatant was analyzed using a UV/Vis spectrometer at 275 nm. The precipitate was redispersed in 1 mL of fresh PBS and release studies were continued.2.8. Cell viability/cytotoxicity studies (MTT test) on HEK cells
HEK cells were seeded in 96 wells plate at a density of 3 × 104 cells per well at 37 °C. After 24 h of culture, the medium in the wells was replaced with fresh medium, containing the GO–MPdop insulin hybrid in varying concentrations. After incubation of the HEK cells for 24 hours, the medium was replaced and 10 μL of MTT (12 mM in sterile PBS) was added in each well and incubated for 4 h at 37 °C. Then medium was carefully removed and formed formazan crystals were solubilized with DMSO (50 μL). The absorbance of each well was read on a microplate reader (PHERAstar FS, BMG LABTECH) at 540 nm. Each condition was replicated for four times and wells without GO–MPdop insulin hybrid were taken as negative control.2.9. Biological assays: cytotoxicity and M-phase entry in Xenopus oocytes
Adult Xenopus females were purchased from University of Rennes I, France. After anesthetizing Xenopus females by immersion in MS222 solution (tricaine methane sulfonate, 1 g L−1), ovarian lobes were surgically removed and kept in ND96 physiological medium (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES–NaOH, pH 7.5). For follicular cells removal from oocytes, fragments of ovarian lobes were treated by dispase (3 h, 0.4 mg L−1), rinsed and bathed in collagenase (1 h, 0.4 mg L−1) to end defolliculation. Fully-grown stage VI oocytes were selected according to their morphology.37 The latter oocytes are arrested at the G2/M border of the first meiotic division and resume meiosis in response to hormonal stimulation in vitro upon progesterone or insulin addition in the medium.38,39 Oocytes were stored at 14 °C in ND96 medium until use.In the case of GO and GO–MPdop, the oocytes were pre-incubated for 30 min with the GO matrix before hormonal stimulation by insulin. In the case of GO–insulin and GO–MPdop–insulin, the matrix was directly added to the oocytes. The pH media were adjusted using HCl/NaOH solutions at pH 1, 2, 5.3, 7.4 and 9.2. Kinetic of Germinal Vesicle Breakdown (GVBD) was scored by the appearance of a white spot (WS) at the animal pole of the cell, which attests of the M-phase entry and meiosis resumption.
2.10. Instrumentation
3. Results and discussion
3.1. Graphene oxide (GO)-2-nitrodopamine modified iron oxide nanoparticles (MPdop): synthesis and characterization
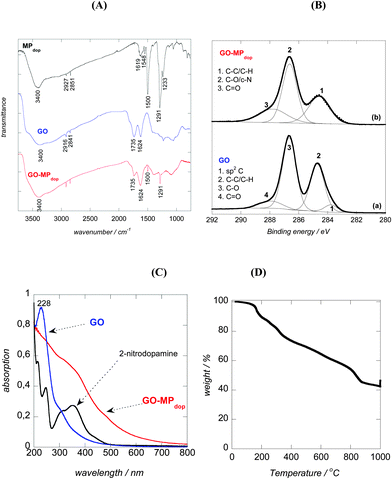
The synthesis of the GO–MPdop hybrid matrix and the chemical structure of the capping ligand employed are illustrated in Fig. 1. We synthesized Fe3O4 NPs using the co-precipitation reaction of Fe2+ and Fe3+ in alkaline media as reported previously by us.36 The magnetic particles were functionalized using 2-nitrodopamine as capping agent. The introduction of an electron withdrawing nitro group onto the catechol nucleus is known to result in a catecholate anchor far superior to dopamine.40–42 The higher oxidation potential of the 2-nitrodopamine ligand implies that nanoparticles surface degradation is diminished, insuring irreversible binding of ligand and good stability of the resulting nanostructures. These properties are crucial when using such particles for follow-up reactions and in biomedical applications.43 This procedure results in MPdop with a mean diameter of 15 ± 5 nm obtained from the analysis of several thousands of nanoparticles by transmission electron microscopy (TEM) images (Fig. 2A).The chemical composition of the 2-nitrodopamine modified particles (MPdop) was examined using FTIR spectroscopy (Fig. 3A). The MPdop nanostructures exhibit bands at 1291 and 1500 cm−1 corresponding to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations of the catechol system, bands at 1233 and 1548 cm−1 due to symmetric and asymmetric vibrations of NO2 group, and bands at 3367 and 1619 cm−1 due to the stretching and bending modes of primary amines. The bands at 2854 and 2920 cm−1 are due to CH stretching vibrations of the dopamine ligand. The MPdop particles were incorporated onto graphene oxide (GO) nanosheets by sonicating GO and MPdop (mass ratio 2/1) for 2 h (Fig. 1). MPdop nanoparticles adsorption on GO is believed to be driven by π–π stacking interactions between the 2-nitrodopamine ligand on the magnetic particles and sp2 rings on GO. Electrostatic interactions between the acid groups of GO and the amine groups of the 2-nitrodopamine ligand are unlikely as the pKa of 2-nitrodopamine is near pH 6.5, lower when compared to dopamine ligands with a pKa > 9.40,44 TEM measurements of GO–MPdop nanohybrid reveal the presence of spherical particles with 15 ± 5 nm in diameter as in the case of free magnetic particles (Fig. 2B). The hydrodynamic size of the hybrid is estimated to be 294 ± 68 nm (polydispersity index = 0.649 ± 0.113) with a surface charge of −50.4 ± 1 mV.
C vibrations of the catechol system, bands at 1233 and 1548 cm−1 due to symmetric and asymmetric vibrations of NO2 group, and bands at 3367 and 1619 cm−1 due to the stretching and bending modes of primary amines. The bands at 2854 and 2920 cm−1 are due to CH stretching vibrations of the dopamine ligand. The MPdop particles were incorporated onto graphene oxide (GO) nanosheets by sonicating GO and MPdop (mass ratio 2/1) for 2 h (Fig. 1). MPdop nanoparticles adsorption on GO is believed to be driven by π–π stacking interactions between the 2-nitrodopamine ligand on the magnetic particles and sp2 rings on GO. Electrostatic interactions between the acid groups of GO and the amine groups of the 2-nitrodopamine ligand are unlikely as the pKa of 2-nitrodopamine is near pH 6.5, lower when compared to dopamine ligands with a pKa > 9.40,44 TEM measurements of GO–MPdop nanohybrid reveal the presence of spherical particles with 15 ± 5 nm in diameter as in the case of free magnetic particles (Fig. 2B). The hydrodynamic size of the hybrid is estimated to be 294 ± 68 nm (polydispersity index = 0.649 ± 0.113) with a surface charge of −50.4 ± 1 mV.
We have shown, recently, that dopamine and its derivatives are excellent reducing agents of GO.45,46 In case of an irreversible binding of the 2-nitrodopamine capping agent to the magnetic particles, reduction of GO is unlikely to occur, as the 1,2-diols of the catechol linker are not oxidizable to their corresponding quinine structure. The FTIR spectra of GO before and after loading with MPdop particles are shown in Fig. 3A. In the case of GO, the bands at 1734 and 1624 cm−1 correspond to v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of –COOH and the skeletal vibration of unoxidized graphite domains, respectively. Loading 2-nitrodopamine modified magnetic particles on GO resulted in a comparable FTIR spectrum as for GO with additional bands at 1291 and 1500 cm−1 corresponding to C–O and C
O) of –COOH and the skeletal vibration of unoxidized graphite domains, respectively. Loading 2-nitrodopamine modified magnetic particles on GO resulted in a comparable FTIR spectrum as for GO with additional bands at 1291 and 1500 cm−1 corresponding to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations of the catechol system. To gain further information on the chemical composition of the resulting GO–MPdop nanomaterials, X-ray photoelectron spectroscopy (XPS) analysis was performed. The C1s core level XPS spectrum of GO nanosheets is displayed in Fig. 3B. It can be deconvoluted into four components with binding energies at about 283.8, 284.7, 286.7 and 287.9 eV assigned to sp2-hybridized carbon, C–H/C–C, C–O and C
C vibrations of the catechol system. To gain further information on the chemical composition of the resulting GO–MPdop nanomaterials, X-ray photoelectron spectroscopy (XPS) analysis was performed. The C1s core level XPS spectrum of GO nanosheets is displayed in Fig. 3B. It can be deconvoluted into four components with binding energies at about 283.8, 284.7, 286.7 and 287.9 eV assigned to sp2-hybridized carbon, C–H/C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, respectively. Deposition of MPdop onto GO did not alter the C1s spectrum significantly showing contributions at 284.7, 286.7 and 287.9 eV due to C–C/C–H, C–O/C–N and C
O species, respectively. Deposition of MPdop onto GO did not alter the C1s spectrum significantly showing contributions at 284.7, 286.7 and 287.9 eV due to C–C/C–H, C–O/C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moieties, respectively. The presence of GO rather than rGO is in line with the irreversible binding of the 2-nitrodopamine ligand to the magnetic particles. The success of the incorporation of MPdop particles is furthermore confirmed by the presence of 14.2 mass% of iron.
O moieties, respectively. The presence of GO rather than rGO is in line with the irreversible binding of the 2-nitrodopamine ligand to the magnetic particles. The success of the incorporation of MPdop particles is furthermore confirmed by the presence of 14.2 mass% of iron.
The UV/Vis absorption spectra of GO, 2-nitrodopamine and GO–MPdop hybrid are depicted in Fig. 3C. GO dispersed in water exhibits a maximum absorption at 228 nm, attributed to the π–π* transition resulting from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the aromatic skeleton, and a broad shoulder at ∼297 nm due to the n–π* transition of C
C bonds of the aromatic skeleton, and a broad shoulder at ∼297 nm due to the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from carboxylic acid functions. The UV/Vis spectrum of the free 2-nitrodopamine ligand exhibits a prominent peak at 352 nm. In the case of the GO–MPdop hybrid, a broad peak between 350 and 390 nm with a maximum at ∼370 nm was observed due to the presence of MPdop.
O bonds from carboxylic acid functions. The UV/Vis spectrum of the free 2-nitrodopamine ligand exhibits a prominent peak at 352 nm. In the case of the GO–MPdop hybrid, a broad peak between 350 and 390 nm with a maximum at ∼370 nm was observed due to the presence of MPdop.
Thermogravimetric analysis was carried out to understand better the binding nature of MPdop and insulin to GO. Fig. 3D indicates gradual decomposition of the hybrid with two stages of weight loss at 150 °C and 320 °C indicating the decomposition of functional groups from insulin and graphene oxide. Large weight loss at 850 °C is accounted for by breakdown of coordination bond between nitrodopamine and Fe3O4 nanoparticles.4,23,47
The magnetic properties of the MPdop and once onto the GO matrix were in addition determined. Indeed, it has been shown by Finotelli et al.,31 that insulin could be released from alginate/chitosan beads containing magnetic nanoparticles by the use of a magnetic field. While not investigated here, the magnetic properties were nevertheless determined. The hysteresis loop of the MPdop sample obtained at 300 K in a field range of ± 20 kOe (above the pseudo-saturation) is shown in Fig. 4A. In the inset of the same figure is presented the magnetization at increasing temperature obtained in a field of 80 Oe after cooling the sample in zero field. The well known zero field cooling (ZFC) procedure gives rise to a magnetization curve specific to nanoparticulate systems, with a maximum at a blocking temperature of about 250 K. It means that above such a temperature (e.g. at 300 K), the MPdop behave superparamagnetically, in agreement with the zero coercive field shown by the hysteresis loop. The specific saturation magnetization of MPdop at 300 K is about 60 emu g−1,43 being about 10% lower than that of naked (uncoated) magnetic nanoparticles34 at the same temperature (these values are lower than the specific spontaneous magnetization of bulk magnetite, due to both thermal effects related to the reported magnetization at 300 K as well as due to an expected more defective structure related to size effects). However, the lower saturation magnetization in the MPdop as compared to naked MP has to be related to a diminished relative weight of the magnetic ions in the sample, due to the presence of the additional 2-nitrodopamine surfactant.
The hysteresis loop of the GO–MPdop sample obtained in similar conditions as for sample MPdop is shown in Fig. 4B. In the down inset of the same figure is also presented the ZFC magnetization curve in 80 Oe, which is clearly different from that of the MPdop sample. It is worth mentioning the increase of the magnetization at lower temperature (e.g. from 150 K down to 10 K), suggesting the presence of a ferromagnetic-like interaction among the nanoparticles as compared to the antiferromagnetic dipolar type usually observed in non-diluted nanoparticulate systems. Such interactions seem to be present also at higher temperatures, leading to a consistent shift of the blocking temperature well above 290 K. If the nature of such unusual interactions requires additional studies of the GO–MPdop mixtures, the consistent difference of the ZFC curve of the GO–MPdop sample as compared to the ZFC curve of the MPdop sample clearly proves the formation of the GO–MPdop hybrid with specific properties induced by the strong interactions of the nanoparticles via the GO support. In the upper inset of the same Fig. 4B is shown the hysteresis loop of the GO substrate, collected in similar conditions as for the GO–MPdop hybrid. It is observed that in the maximum field of 20 kOe, the magnetization of GO (0.008 emu g−1) is 1000 times lower than that of the hybrid sample (about 8 emu g−1) and therefore can be clearly neglected. Hence, the saturation magnetization of the GO–MPdop hybrid is just 13% from the saturation magnetization of MPdop sample, inferring an equivalent (13 mass%) of loading magnetic material in the analyzed sample. This is in accordance with XPS analysis where a 14.3 mass% of iron was determined.
3.2. Insulin loading and release
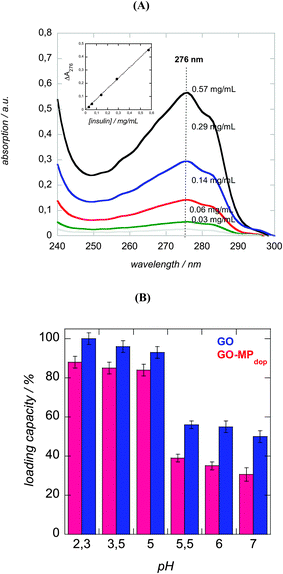
The kind of interactions of GO, MPdop and insulin is of uttermost importance not only for the construction of a stable GO–MPdop hybrid but also for insulin loading and release strategies. As discussed above, 2-nitrodopamine ligands are used as capping agent for the formed magnetic particles. The linkage insuring an irreversible binding of the ligand. Indeed, no degradation of the MPdop particles size and chemical composition was observed upon immersion for 4 h into aqueous solutions of low pH (pH = 1), as might be observed under biological conditions. The interaction of the dopamine ligand to the iron oxide nanoparticles is not disrupted in the lower pH range. Interaction of the dopamine-capped MP particles with the GO matrix is mostly over π–π stacking interactions between the hexagonal cells of graphene and the aromatic ring structure of dopamine. As the diol functions of the used catechol are not available, the formation of ortho-quinol structures is inhibited in this case and a further covalent binding not feasible.45The loading capacity of insulin onto GO–MPdop can be evaluated by measuring the concentration of insulin using UV/Vis spectra at 275 nm in solution before and after insulin loading. The difference corresponds to insulin loaded onto the GO–MPdop matrix (Fig. 5A). Indeed, due to the high UV/Vis absorbance of GO occurring in the same spectral area as insulin, a direct determination of the insulin concentration on the GO–MPdop matrix is not possible. The insulin loading capacity of the GO–MPdop nanohybrid was calculated according to eqn (1):
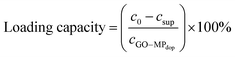 | (1) |
Many studies have shown that aromatic molecules including chemotherapy drugs such as doxorubicin can be loaded onto the surface of graphene via π–π stacking interactions.12,15,21 In the case of insulin, a polypeptide composed of 51 amino acid residues, electrostatic forces will additionally affect insulin loading as the isoelectronic point (pI) of insulin is reported to be 5.4.27 The influence of the pH on the loading of a fixed concentration of insulin onto GO–MPdop is displayed in Fig. 5B. Using a loading time of 24 h, increased insulin loading was observed at pH < 5.5 in accordance with an insulin pI = 5.4.27 Below pH 5.4, insulin is positively charged and interacts more strongly with the negatively charged GO–MPdop matrix. This interaction is weakened at pH > 5 and at pH 7 the loading capacity of insulin is 3 times lower as only π–π stacking interactions and/or hydrophilic interactions will prevail between insulin and GO–MPdop. The loading capacity of GO–MPdop is as high as 88 ± 3%, although some areas of the multifunctional GO has been previously occupied with magnetic particles. Indeed, this results in a decrease in the loading capacity when compared to GO (100 ± 3%), but is still remarkably high when compared to other nanostructures.47,48 For mesoporous silica insulin loading of 15% was reported,47 while poly(lactide-ethylene glycol) nanoparticles showed a maximal insulin loading of 58.8%.48
To understand better which of the materials, GO or MPdop is more effectively loading insulin, the loading capacitance of MPdop was in addition determined. No detectable amounts of insulin could be determined by the colorometic assay, indicating that all the insulin reacts with the GO matrix rather than the magnetic particles.
Insulin release from GO and GO–MPdop hybrid at pH 5 was analyzed by incubating the matrices at 37 °C at different pH while shaking. Fig. 6 shows the cumulative release of insulin from GO (Fig. 6A) and GO–MPdop (Fig. 6B) matrices as a function of pH. At pH = 2, even though a small release of insulin is observed, probably due to weakly bound insulin, GO and GO–MPdop appear to have a high insulin retention capacity. Comparable behavior was observed on core–shell poly(ethylene glycol)polyhedral oligosilsesquioxane nanoparticles.30
 | ||
| Fig. 6 Insulin release from GO (A) and GO–MPdop (B) for different pH and at different time points (error bars are based on triplicate measurements). | ||
Following a pH change to 5, insulin release is initiated and sustained for the first 90 min. The amount of insulin released is highly pH dependent with about 28 ± 3% of insulin released at pH 9 for GO–MPdop and 40 ± 3% for GO. The insulin release from the GO–MPdop hybrid turns to be less successful than from GO alone, which likely accounts for some levels of interaction between insulin and nanoparticles within the hybrid. The release at pH 9 is most likely due to electrostatic repulsion between negatively charged insulin and negatively charged GO and GO–MPdop. The release is fast and low when compared to mesoporous silica nanoparticles with a maximal release of 77% at pH 8.5 after 10 h.47 It is comparable to poly(lactide-ethylene glycol) nanoparticles with a release of 59% after 10 days,48 or alginate/chitosan microcapsules with a release of 18% in the first hours and about 45% after 3 days.31
The high insulin retention capacity at low pH, comparable to that of gastric pH suggests that insulin is well protected on GO and GO–MPdop hybrid, while at intestinal pH (pH 6–7) insulin is activated and released. We thus investigated, if GO and GO–MPdop hybrids could be used as potential carriers for an insulin drug delivery system.
3.3. Cell viability assay of GO and GO–MPdop
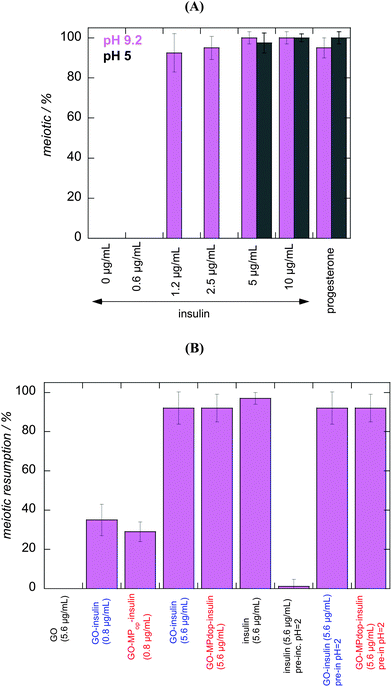
Two different cell viability assays were performed to obtain information about the cytotoxicity of the GO–MPdop hybrid. The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diephenyltetrazolium bromide) assay is a simple colorimetric assay to measure cell cytotoxicity, proliferation or viability and used in this work. As seen in Fig. 7A under the investigated concentration range of GO–MPdop and GO–MPdop–insulin no cytotoxicity to HEK cells is observed.In Xenopus laevis large number of oocytes are easily obtained at all stages of maturation, making this organism an excellent model for studying the role of insulin and insulin growth factors on the development of the organism.49,50 Fully grown Xenopus laevis oocytes are physiologically arrested at the prophase of the first meiotic divisions. These oocytes must resume meiosis and proceed to the metaphase of meiosis II before fertilization is possible. The process which enables fertilization and drive the oocyte from prophase of first meiotic division to a block in metaphase of second meiotic division, termed maturation, is triggered in vivo by a preovulatory gonadotropin surge followed by follicular production of progesterone.39,51–53 In addition to progesterone and other hormones, both insulin and insulin-like growth factor-1 (IGF-1) can induce meiotic resumption and oocyte maturation.36,39 Fig. 7A shows photographs of Xenopus laevis oocytes (stage VI) before and after treatment with insulin (10 μg mL−1) at pH 9.2. A typical white spot, attesting for the germinal vesicle breakdown (GVBD), is observed under the binocular at the animal pole of the oocytes. We use the monitoring of oocyte meiotic resumption in this study for testing their viability and responsiveness towards insulin after being released from GO and GO–MDdop nanostructures. To ensure that GO and GO–MDdop nanostructures without insulin have no cytotoxic effect on Xenopus oocytes, the fully-grown stage VI oocytes were exposed for 24 h to increasingly high concentrations of GO and GO–MPdop at pH 7.4. Fig. 7B shows the meiotic resumption rate upon insulin induction and indicates that exposure to GO and GO–MPdop even at high concentrations is not toxic for oocytes, showing a comparable meiotic resumption rate when the Xenopus laevis oocytes were not pre-incubated with the nanostructures. The meiotic resumption rate of Xenopus laevis oocytes upon injection of insulin (50 μg mL−1) at different pH was investigated to insure that insulin release at higher pH would have an important influence on oocytes. As seen from Fig. 7C, no significant changes in meiotic resumption rates were observed when insulin induction was performed at pH above 5. However, at pH = 2, the meiotic resumption rate is significantly decreased, indicating deviations from native insulin most likely linked to conformational changes that have occurred in the polypeptide chains of insulin.54
3.4. Meiotic resumption rates of Xenopus laevis oocytes upon addition of GO–insulin and GO–MPdop–insulin
The dose effect of insulin at pH 5 and 9.2 on the meiotic resumption rate of oocytes was investigated. As seen in Fig. 8A, at pH = 9.2, the minimal insulin concentration resulting in high rates of meiotic resumption is around 1.2 μg mL−1. Below this concentration level, no meiotic resumption was observed. At pH 5, this concentration limit was shifted to higher insulin concentrations. A comparable concentration range was thus chosen for the insulin loaded GO and GO–MPdop nanostructures. Fig. 8B compares the meiotic resumption rates of a variety of different experimental set-ups. GO (5.6 μg mL−1) and insulin (5.6 μg mL−1) were used as negative and positive controls in this comparative experiment. GO–insulin and GO–MPdop–insulin nanostructures showed a dose-dependence response: while at a concentration of 0.8 μg mL−1, both matrices exhibited only low meiotic resumption rates at pH 9.2, concentrations higher than 5.6 μg mL−1 resulted in high levels of meiotic resumption as for free insulin. While this behaviour is expected, a surprisingly different meiotic resumption behaviour was observed once insulin, GO–insulin and GO–MPdop–insulin were pre-incubated for 5 h at pH = 2. For insulin, the meiotic resumption rate was highly decreased in line with the observation in Fig. 7C. However, acid pre-treated GO–insulin and GO–MPdop–insulin nanostructures did not show any altered meiotic resumption characteristics. This in vitro experiment proves that the insulin incorporated onto the nanostructures is not affected by the low pH, and the GO “protects” insulin from acidic degradation. The GO–insulin and GO–MPdop–insulin nanostructures might be thus considered as novel insulin formulations next to microcapsules, polymers and others.26,31,48,54,55 The appealing character of GO–insulin and GO–MPdop–insulin nanostructures is that the nanocomposites are easy to prepare and can be produced on a larger scale. The incorporation of magnetic particles does not alter the meiotic resumption profile of Xenopus laevis oocytes, used as model system here. The attractiveness of the incorporation of the magnetic particles is that insulin controlled release can be enhanced in the presence of a magnetic field, as previously demonstrated by Finotelli et al. using alginate/chitosan beads containing magnetic particles.314. Conclusions
In this study, we have demonstrated that graphene oxide matrices can be easily loaded with different carriers. In our case 2-nitrodopamine coated magnetic particles and/or insulin was incorporated onto the GO nanosheets. The insulin loading capacity on the GO nanomaterials was pH-dependent, but proved to be extremely high at pH lower than 5.4 with 100 ± 3% and 88 ± 3% loading on GO and GO–MPdop, respectively. Insulin-loaded on GO matrices was stable at acidic pH, but was released when exposed to basic solutions (pH = 9.2). Insulin retained its native structure when released from the matrix. In addition, the insulin loaded on GO and GO–MPdop were strongly resistant to acidic pH, as for that encountered in the gastric environment. These results open new avenues for further investigations of the potential application of insulin loaded on GO matrices for treatment of patients with insulin deficiency.Acknowledgements
A.B, R.B and S.S. gratefully acknowledge financial support from the Centre National de Recherche Scientifique (CNRS), the Université Lille 1, the Nord Pas de Calais region, and the Institut Universitaire de France (IUF). Support from the European Union through a FP7-PEOPLE-IRSES (PHOTORELEASE) is acknowledged. Support from the Romanian project PNII IDEI 75/2011 is gratefully acknowledged.References
- A. Barras, F. A. Martin, O. Bande, J. S. Baumann, J.-M. Ghigo, R. Boukherroub, C. Beloin, A. Siriwardena and S. Szunerits, Nanoscale, 2013, 5, 2307 RSC.
- M. Durka, K. Buffet, J. Iehl, M. Holler, J.-F. Nierengarten, J. taganna, J. Bouckaert and S. P. Vicent, Chem. Commun., 2011, 47, 1321 RSC.
- J. J. Shi, A. R. Votruba, O. C. Farokhzad and R. Langer, Nano Lett., 2010, 10, 3223 CrossRef CAS PubMed.
- X. Yang, X. Zhang, Y. Ma, Y. Huan, Y. Wang and Y. Chen, J. Mater. Chem., 2009, 19, 2710 RSC.
- X. Yang, G. Niu, X. Cao, Y. Wen, R. Xiang, H. Duan and Y. Chen, J. Mater. Chem., 2012, 22, 6649 RSC.
- L. Zhang, Z. Lu, Q. H. Zhao, J. Hunag, H. Shen and Z. J. Zhang, Small, 2011, 7, 460 CrossRef CAS PubMed.
- K. E. Sapsford, W. R. Algar, L. Berti, K. Boeneman Gemmill, B. J. Casey, E. Oh, M. H. Stewart and I. L. Medintz, Chem. Rev., 2013, 113, 1904 CrossRef CAS PubMed.
- H. Y. Mao, S. Laurent, W. chen, O. Akhavan, M. Imani, A. A. Ashkarran and M. Mahmoudi, Chem. Rev., 2013, 113, 3407–3424 CrossRef CAS PubMed.
- K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530 RSC.
- Y. Pan, H. Bao, N. G. Sahoo, T. Wu and L. Li, Adv. Funct. Mater., 2011, 21, 2754 CrossRef CAS.
- U. Dembereldorj, M. Kim, S. Kim, E.-O. Ganbold, S. Y. Lee and S.-W. Joo, J. Mater. Chem., 2012, 22, 22845 RSC.
- W. Zhang, Z. Guo, D. Huang, Z. Liu, X. Guo and H. Zhong, Biomaterials, 2011, 32, 8555 CrossRef CAS PubMed.
- Z. Liu, J. T. Robinson, X. M. Sun and H. J. Dai, J. Am. Chem. Soc., 2008, 130, 10876 CrossRef CAS PubMed.
- J. Hong, N. J. Shah, A. C. Drake, P. C. DeMuth, J. B. Lee, J. Chen and P. T. Hammond, ACS Nano, 2012, 6, 81 CrossRef CAS PubMed.
- X. M. Sun, Z. Liu and K. Welsher, et al., Nano Res., 2008, 1, 203 CrossRef CAS PubMed.
- X. Yang, X. Zhang, Z. Liu, Y. Ma, Y. Huang and Y. Chen, J. Phys. Chem. C, 2008, 112, 17554 CAS.
- W. Miao, G. Shim, S. Lee, S. Lee, Y. S. Choe and Y.-K. Oh, Biomaterials, 2013, 34, 3402 CrossRef CAS PubMed.
- L. Zhang, J. Xia, Q. Zhao, L. H. Liu and Z. Zhang, Small, 2010, 6, 537 CrossRef CAS PubMed.
- Y. Yang, Y.-M. Zhang, Y. Chen, D. Zhao, J.-T. Chen and Y. Liu, Chem.–Eur. J., 2012, 18, 4208 CrossRef CAS PubMed.
- X. Yang, Y. Wang, X. Huang, Y. Ma, Y. Huang, R. Yang, H. Duan and Y. Chen, J. Mater. Chem., 2011, 21, 3448 RSC.
- X. Ma, H. Tao, K. Yang, L. Feng, L. Chen, X. Shi, Y. Li, L. Guo and Z. Liu, Nano Res., 2012, 5, 199 CrossRef CAS.
- G. Cheng, Y.-L. Liu, Z.-G. Wang, J.-L. Zhang, D.-H. Sun and J.-Z. Ni, J. Mater. Chem., 2012, 22, 21998 RSC.
- Y. Xue, H. Y. Chen, D. Yu, S. Wang, M. Yardeni, Q. Dai, M. Guo, Y. Liu, F. Lu, J. Qu and L. Dai, Chem. Commun., 2011, 47, 11689 RSC.
- M. Goldberg and I. Gomez-Orellana, Nat. Rev. Drug Discovery, 2003, 2, 289 CrossRef CAS PubMed.
- N. A. Peppas and N. J. Kavimandan, Eur. J. Pharm. Sci., 2006, 29, 183 CrossRef CAS PubMed.
- F. Wang, Y. Chen and H. A. E. Benson, Open Drug Delivery J., 2008, 2, 1 CrossRef CAS.
- F. Cui, K. Shi, L. Zhang, A. Tao and Y. Kawashima, J. Controlled Release, 2006, 114, 242 CrossRef CAS PubMed.
- G. P. Carino and E. Mathiowitz, Adv. Drug Delivery Rev., 1999, 35, 249 CrossRef CAS.
- D. P. Huynh, M. K. Nguyen, B. S. Pi, M. S. Kom, S. Y. Chae, K. C. Lee, B. S. Kim, S. W. Kim and D. S. Lee, Biomaterials, 2008, 29, 2527 CrossRef CAS PubMed.
- K.-O. Kim, B.-S. Kim and I.-S. Kim, J. Biomater. Nanobiotechnol., 2011, 2, 201 CrossRef CAS.
- P. V. Finotelli, D. Da Silva, M. Sola-Penna, A. Malta Rossi, M. Farina, L. R. Andrada, A. Y. Takeuchi and M. Rocha-Leao, Colloids Surf., B, 2010, 81, 206 CrossRef CAS PubMed.
- J. Liu, L. Cui and D. Losic, Acta Biomater., 2013, 9, 9243–9257 CrossRef CAS PubMed.
- K. Yang, H. Gong, X. Shi, J. Wan, Y. Zhang and Z. I. Liu, Biomaterials, 2013, 34, 2787 CrossRef CAS PubMed.
- O. Fellahi, M. R. Das, Y. Coffinier, S. Szunerits, T. Hadjersi, M. Maamache and R. Boukherroub, Nanoscale, 2011, 3, 4662 RSC.
- M. Rodenstein, S. Zürcher, S. G. Tosatti and N. D. Spencer, Langmuir, 2010, 26, 16211 CrossRef CAS PubMed.
- M. Mazur, A. Barras, V. Kuncer, A. Galatanu, V. Zaitzev, P. Woisel, J. Lyskawa, W. Laure, A. Siriwardena, R. Boukherroub and S. Szunerits, Nanoscale, 2013, 5, 2692 RSC.
- J. N. Dumont, J. Morphol., 1972, 136, 153 CrossRef CAS PubMed.
- F. Baert, J. F. Bodart, B. Bocquet-Muchembled, A. Lescuyer-Rousseau and J. P. Vilain, J. Biol. Chem., 2003, 278, 49714 CrossRef CAS PubMed.
- M. El-Etr, S. Schorderet-Slatkine and E. E. Baulieu, Science, 1979, 205, 1397 CAS.
- E. Amstad, A. U. Gehring, H. Fisher, V. V. Nagaiyanallur, G. Hähner, M. Textor and E. Reimhult, J. Phys. Chem. C, 2011, 115, 683 CAS.
- E. Amstad, T. Gillich, I. Bilecka, M. Textor and E. Reimhult, Nano Lett., 2009, 9, 4042 CrossRef CAS PubMed.
- E. Amstad, M. Textor and E. Reimhult, Nanoscale, 2011, 3, 2819 RSC.
- S. J. Clarke, C. A. Hollmann, Z. Zhang, S. Suffern, S. E. Bradforth, N. M. Dimitrijevic, W. G. Minarik and J. L. Nadeau, Nat. Mater., 2006, 5, 409 CrossRef CAS PubMed.
- A. K. L. Yuen, G. A. Hutton, A. F. Masters and T. Maschmeyer, Dalton Trans., 2012, 41, 2545 RSC.
- I. Kaminska, M. R. Das, Y. Coffinier, J. Niedziolka-Jonsson, J. Sobczak, P. Woisel, J. Lyskawa, M. Opallo, R. Boukherroub and S. Szunerits, ACS Appl. Mater. Interfaces, 2012, 4, 1016 CAS.
- I. Kaminska, W. Qi, A. Barras, J. Sobczak, J. Niedziolka-Jonsson, P. Woisel, J. Lyskawa, W. Laure, M. Opallo, M. Li, R. Boukherroub and S. Szunerits, Chem.–Eur. J., 2013, 19, 8673 CrossRef CAS PubMed.
- L. Sun, X. Zhang, C. Zhen, Z. s. Wu and C. Li, J. Phys. Chem. B, 2013, 117, 3852 CrossRef CAS PubMed.
- L. Tomar, C. Tyagi, M. Kumar, P. Kumar, H. Singh, Y. E. Choonara and V. Pillay, Int. J. Nanomed., 2013, 8, 505 CrossRef CAS PubMed.
- L. Scavo, A. R. Shuldiner, J. Serrano, R. Dashner, J. Roth and F. De Pablo, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 6214 CrossRef CAS.
- I. B. Dawid, T. D. Sargent and F. Rosa, Curr. Top. Dev. Biol., 1990, 24, 2 CrossRef.
- L. Zhu, N. Ohan, Y. Agazie, C. Cummings, S. Farah and X. J. Li, Endocrinology, 1997, 139, 949 CrossRef.
- Y. Mazui and H. J. Clarke, Int. Rev. Cytol., 1979, 57, 185 CrossRef.
- L. D. Smith, Development, 1989, 107, 685 CAS.
- K. Yoshida, K. Sato and J.-I. Anzai, J. Mater. Chem., 2010, 20, 1546 RSC.
- R. A. Shimkunas, E. Robinson, R. Lam, S. Lu, X. H. Xu, X.-Q. Zhang, H. Huang, E. Osawa and D. Ho, Biomaterials, 2009, 30, 5720 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |