The application of a cytochrome P450 enzyme eluted from encapsulated biomaterials for the catalysis of enantioselective oxidation†
Hiroyuki Nagaoka*
Sanyo Shokuhin Co., Ltd. R & D, 555-4 Asakura, Maebashi, Gunma 371-0811, Japan. E-mail: hnagaoka@sanyofoods.co.jp; Fax: +81-27-220-3477; Tel: +81-27-220-3471
First published on 18th February 2014
Abstract
A membrane-bound enzyme (ME) eluted from encapsulated pea protein (PP) under aeration is applicable for the kinetic resolutions of turnover: each enantiomer of rac-1-(6-methoxynaphthalen-2-yl)ethanol (rac-1) can be selectively synthesized (>99% ee; ∼50% chemical yield), utilizing two types of biomaterials: (1) a PEG (1000/4000 = 2/1)-aggregated ME (AGME: synthesizing highly enantiopure R-(−)-1) and (2) a glutaraldehyde (GA)/a PEG (4000)-coated ME (a CMME: synthesizing S-(+)-1 (S-naproxen precursor)). Both reactions occur in the absence of an added cofactor (e.g., NAD(P)) in aqueous media. The specific activities of AGME and CMME were determined to be 0.8 ± 0.03 mU (mean ± SD) mg−1 min−1 and 0.6 ± 0.02 mU (mean ± SD) mg−1 min−1, respectively, and the species exact nature engaged in the key reaction was consistent with that of a heme-binding protein (HBP) based on an N-terminal sequence comparison, which showed 93% similarity with a 20.853 kDa hemophore HasA gene product [Pseudomonas fluorescens Pf-5, a plant commensal bacterium]. The PP-HBP can be regenerated via a successive asymmetric catalytic event using an incorporated iron electron-transfer system in the presence of oxygen—a process seemingly similar to that utilized by the oxygen-driven cytochrome P450 enzyme (Cyt-P450: cysteine – Fe2+ + O2 → Fe3+–O–O− → Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or rac-2) → Fe2+ + H2O). The use of a raw biomaterial as an ME-catalytic system with an incorporated redox cofactor for asymmetric oxidation overcomes the apparent difficulties in working with pure dehydrogenase enzyme/redox cofactor systems for biotransformation.
O (oxidizing rac-1 or rac-2) → Fe2+ + H2O). The use of a raw biomaterial as an ME-catalytic system with an incorporated redox cofactor for asymmetric oxidation overcomes the apparent difficulties in working with pure dehydrogenase enzyme/redox cofactor systems for biotransformation.
Introduction
Biocatalysis has emerged as an important tool for the industrial synthesis of bulk chemicals, pharmaceuticals, agricultural intermediates and food ingredients; however there are some limitations associated with its application to microbial synthesis.1 A review covering the well-known alcohol dehydrogenase (ADH) enzyme system that incorporates redox cysteine disulfide bonds, redox zinc, and a redox cofactor (such as NAD(P) or FAD) has been published, and the high cost and instability of the redox cofactors in enzymatic synthesis justifies efforts to regenerate them.2 On the other hand, the use of heme-binding proteins (HBPs) incorporating an iron electron-transfer system for the asymmetric oxidation (with oxygen) of secondary alcohols in organic synthesis has not been published, and the use of such a system overcomes the tedious nature of the pure dehydrogenase enzyme/redox cofactor systems for microbial biotransformation and the difficulties of working with them. It has been reported, however, that an oxygen-driven cytochrome P450 enzyme (Cyt-P450, a HBP) that depends on oxygen instead of a redox cofactor (NAD(P)H) (ref. 3a–c) as a detoxification system catalyzes hydroxylation, epoxidation and dehalogenation, generating a reactive oxygen species via an iron electron-transfer system,3d and that cytochrome c oxidase (CytcO, a HBP)3e incorporates the electron-transfer system for the reduction of oxygen to water to enable proton pumping across cell membranes.3f Furthermore, the hemophore HasA secreted by host HBPs (e.g., ABC transporters, CytcO, and P450)4a enables heme uptake across the cell outer membrane and spontaneously transforms it into the HasR receptor at the heme-binding site.4b Cytochrome P450 enzymes are found in all organisms, and compared with bacteria HBP and animal HBP (P450: ∼20 and 60 different forms, respectively), plants make several forms because they synthesize unusual pigments and exotic toxins to protect themselves;4d thus, the development of a new method for the purification and characterization of a new plant-HBP system may be enabled.The use of biomaterials as membrane-bound enzyme (ME)-catalytic systems incorporating redox cofactors for asymmetric oxidation/reduction reactions has been studied previously.5 In particular, the ME eluted from biomaterials (i.e., pea protein (PP)) encapsulated with calcium alginate gel (PP gel) is available for the synthesis and enantiomeric resolution of m- and p-substituted racemic aryl methyl carbinols.6 Other biomaterials used instead of proteins include young wheat or barley leaves, wheat bran, Artemisia vulgaris indica, wakame seaweed, carrots, and pumpkins.6 It has also been shown previously that, besides CLMEb being particularly activated by a reaction solution of 50 mM glycine–NaOH (pH 9.0–10.0), an iron electron-transfer system may be incorporated as an ME-catalytic system for asymmetric oxidation, with further advantages that neither NAD(P) nor FAD is required for colorimetric analysis and that the oxygen dependency can be observed in a reaction tube without any cap.7 Therefore, CLMEb was synthetically applied as a new biocatalyst for the asymmetric synthesis of S-(+)-1-(6-methoxynaphthalen-2-yl)ethanol (rac-1: a naproxen precursor,8 >99% ee) in buffered aqueous media with a cosolvent (DMSO). However, no supporting data characterizing both the initial oxidant and the redox catalytic center have been reported, and the poor activity of CLMEb requires an improvement to be practically useful in oxidative synthesis with respect to (1) the need for buffered aqueous media and (2) the characterization of both the initial oxidant and the redox catalyst center.
In this study, CLMEb (48 h) was improved, leading to MEa (a CMME: 13 h), a form not containing any traces of (NH4)2SO4, and a new method reconstructed for purification was done as follows (see the details for the ME-purification process and each activities in Scheme 1(a) and (b)). The specific activity of AGME/CMME for oxidative synthesis without an added redox cofactor in water was determined to be 0.8 ± 0.03 mU/0.6 ± 0.02 mU (mean ± SD, where U is unit and 1 U = 1 μmol (min−1 mg−1)). The asymmetric oxidation is attributed to the HBP that eluted from the encapsulated PP, and this conclusion is supported by an N-terminal sequence comparison, which showed a high similarity with the HBP from a PP, a 20.853 kDa hemophore HasA gene product. Therefore, the hemophore itself or its host HBPs may be regenerated by successive asymmetric catalytic events using an incorporated iron electron-transfer system in the presence of oxygen,3a–c a process seemingly similar to that utilized by oxygen-driven Cyt-P450: cysteine – Fe2+ + O2 → Fe3+–O–O− → Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or rac-2) → Fe2+ + H2O. Thus, these features of this ME-catalytic system incorporating a redox cofactor for asymmetric oxidation are clarified herein.
O (oxidizing rac-1 or rac-2) → Fe2+ + H2O. Thus, these features of this ME-catalytic system incorporating a redox cofactor for asymmetric oxidation are clarified herein.
Results and discussion
This study aims to (1) show the improvement in CLMEb (48 h) that leads to PEG (4000)-ME (a CMME: 13 h), a form not containing any traces of (NH4)2SO4; (2) calculate the CLMEa specific activity (unit mg−1 min−1); and (3) characterize the ME initial oxidant and redox catalyst center.Clarification of the contents of the CMMEs by physicochemical verification
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or rac-2) → Fe2+ + H2O), resulting in water solubility (PP → ME).3 This was also supported by the consistency of the ML FTIR instrumental analysis because of promoting a reactive oxygen species (cysteine – Fe3+–O–O− → Fe4+
O (oxidizing rac-1 or rac-2) → Fe2+ + H2O), resulting in water solubility (PP → ME).3 This was also supported by the consistency of the ML FTIR instrumental analysis because of promoting a reactive oxygen species (cysteine – Fe3+–O–O− → Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O),4a–c leading to the appearance of sulfate (SO: a superoxoiron–cysteinate)4e in the range 950–1250 cm−1 (see ESI Fig. S2, S3, and Table S1†).4 Furthermore, a comparison of the PEG (4000)-ME (not treated with (NH4)2SO4) with CLMEb showed that the former contained four times the level of iron (215 ppm, PEG (4000)-ME vs. 59 ppm, CLMEb), suggesting that the activity of PEG (4000)-ME should be approximately four times greater than that of CLMEb if an iron electron-transfer system is incorporated (see Table 2 and see ESI Fig. S4†). These results indicate that the initial ME-redox oxidant may be more active because of the presence of a greater proportion of incorporated Fe. The results also show that there were a few differences in the Fe content of PEG (4000)-ME (215 ± 5 ppm) and PP (179 ± 4 ppm) (see Table 1), and that no activities were observed.5,6 This means that, during the encapsulation of PP under aeration, an incorporated iron electron-transfer system is both purified/concentrated/solubilized and activated by removing the inhibitors present in the aggregates or complexes from the PP, such as metallic cations (Zn2+), chelating agents and surfactant from PP (see ESI Tables S2–S6†).7
O),4a–c leading to the appearance of sulfate (SO: a superoxoiron–cysteinate)4e in the range 950–1250 cm−1 (see ESI Fig. S2, S3, and Table S1†).4 Furthermore, a comparison of the PEG (4000)-ME (not treated with (NH4)2SO4) with CLMEb showed that the former contained four times the level of iron (215 ppm, PEG (4000)-ME vs. 59 ppm, CLMEb), suggesting that the activity of PEG (4000)-ME should be approximately four times greater than that of CLMEb if an iron electron-transfer system is incorporated (see Table 2 and see ESI Fig. S4†). These results indicate that the initial ME-redox oxidant may be more active because of the presence of a greater proportion of incorporated Fe. The results also show that there were a few differences in the Fe content of PEG (4000)-ME (215 ± 5 ppm) and PP (179 ± 4 ppm) (see Table 1), and that no activities were observed.5,6 This means that, during the encapsulation of PP under aeration, an incorporated iron electron-transfer system is both purified/concentrated/solubilized and activated by removing the inhibitors present in the aggregates or complexes from the PP, such as metallic cations (Zn2+), chelating agents and surfactant from PP (see ESI Tables S2–S6†).7
| Sample | IC analysis (wt%) and ICP-AES analysis (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Fe | Residues | |
| a CLMEb was subjected to “(NH4)2SO4” precipitation.b PEG (4000)-ME underwent centrifugation and was not subjected to “(NH4)2SO4” precipitation.c Highest wt% indicates the reminder of “(NH4)2SO4” following the precipitation process.d Lowest wt% indicates that no “(NH4)2SO4” remained, but a higher oxygen (O2) content was detected for the PP. | |||||||
| PP | 48.83 | 8.56 | 12.76 | 1.78 | 6.06 | 179 ± 4 (≒4 μmol) | — |
| CLMEba | 26.31 | 7.36 | 16.09c | 12.06c | 23.15c | 59 ± 2 (≒1 μmol) | (NH4)2SO4 |
| PEG (4000)-MEb | 46.04 | 8.34 | 6.92d | 1.59d | 7.85 | 215 ± 5 (≒4 μmol) | — |
| Forms (20 mg) | Product | Reactions (rac-1 or rac-2) | Mineralse | ||||
|---|---|---|---|---|---|---|---|
| Solvent | Time (h) | Unitf (mg min) | Substrate (%) | Ca2+ (%) | Fe2+ (ppm) (Fe/mol) | ||
| a CLMEa prepared without “(NH4)2SO4” precipitation. CLMEb prepared with “(NH4)2SO4” precipitation.b Chemical yield (%).c Distilled water.d 50 mM glycine–NaOH (pH 9.0) or 50 mM Tris–HCl (pH 8.0).e Mineral values were determined by ICP-AES; mean ± SD (n = 4).f Mean ± SD (n = 4). | |||||||
| CLMEba | S-1, S-2, ≥78%ee, ∼50% CYb | Bufferd | 48 | Low | 0.02 | 0.45 ± 0.05 | 59 ± 2 (≒1 μmol) |
| CLMEaa | S-1, S-2, ≥99%ee, ∼50% CYb | D.W.c | 16 | 0.6 ± 0.02 mU | ≥0.06 | 2.4 ± 0.2 | 220 ± 5 (≒4 μmol) |
| PEG (4000)-ME | S-1, S-2, ≥99%ee, ∼50% CYb | D.W. | 14 | 0.6 ± 0.02 mU | ≥0.06 | 2.6 ± 0.2 | 215 ± 5 (≒4 μmol) |
| AGME | R-1, R-2, ≥99%ee, ∼50% CYb | D.W. | 9 | 0.8 ± 0.03 mU | ≥0.06 | 2.5 ± 0.2 | 213 ± 5 (≒4 μmol) |
The determination of the specific activity of the MEs (unit mg−1 min−1)
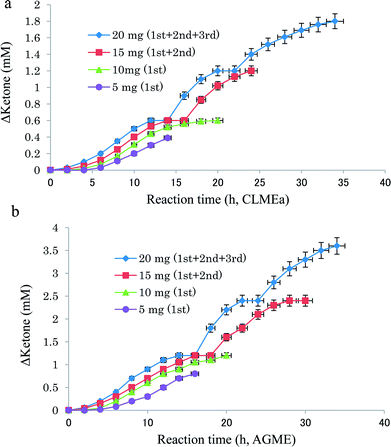
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm) was added in three portions at 0, 12, and 20 h. Fig. 1a and b show the dependence of the product ketone concentration (mM) on the reaction time (h) for various quantities of CLMEa and AGME, respectively, and Fig. 2a and b show the dependence of the instant velocity (mM h−1) on the quantity of CLMEa and AGME (mg), respectively. The unit indicates that the specified quantity of enzyme is capable of oxidizing 1 μmol of rac-1 per minute. These results suggest that the activity of CLMEa is approximately 0.6 mU ± 0.02 (mean ± SD) mg−1 min−1, and the activity of AGME is approximately 0.8 mU ± 0.03 (mean ± SD) mg−1 min−1, as shown in Fig. 2a and b, respectively. The isomer (S)-1 (1.8 mM; 99% ee, ∼50% yield) was obtained from rac-1 (3.6 mM) using CLMEa (20 mg), while ketone-1 (3.6 mM; ∼95% yield) was obtained from rac-1 (3.6 mM) using AGME via the asymmetric oxidation of S-(+)-1, leaving highly enantiopure R-(−)-1 (>99% ee: ∼50% chemical yield, 9 h). Overall, the unit of activity for these conversions in comparison to the well-known ADH alternative (100–1000 units) appears to be low; however, the novel aspect of these new biocatalysts is that they do not depend on the redox cofactor NAD(P) but on oxygen.7 Thus, the difficulties of working with a pure dehydrogenase enzyme/redox cofactor system have been shown to be surmountable (see ESI Table S7†).
000 ppm) was added in three portions at 0, 12, and 20 h. Fig. 1a and b show the dependence of the product ketone concentration (mM) on the reaction time (h) for various quantities of CLMEa and AGME, respectively, and Fig. 2a and b show the dependence of the instant velocity (mM h−1) on the quantity of CLMEa and AGME (mg), respectively. The unit indicates that the specified quantity of enzyme is capable of oxidizing 1 μmol of rac-1 per minute. These results suggest that the activity of CLMEa is approximately 0.6 mU ± 0.02 (mean ± SD) mg−1 min−1, and the activity of AGME is approximately 0.8 mU ± 0.03 (mean ± SD) mg−1 min−1, as shown in Fig. 2a and b, respectively. The isomer (S)-1 (1.8 mM; 99% ee, ∼50% yield) was obtained from rac-1 (3.6 mM) using CLMEa (20 mg), while ketone-1 (3.6 mM; ∼95% yield) was obtained from rac-1 (3.6 mM) using AGME via the asymmetric oxidation of S-(+)-1, leaving highly enantiopure R-(−)-1 (>99% ee: ∼50% chemical yield, 9 h). Overall, the unit of activity for these conversions in comparison to the well-known ADH alternative (100–1000 units) appears to be low; however, the novel aspect of these new biocatalysts is that they do not depend on the redox cofactor NAD(P) but on oxygen.7 Thus, the difficulties of working with a pure dehydrogenase enzyme/redox cofactor system have been shown to be surmountable (see ESI Table S7†).
The asymmetric redox activity of the ME-dried, AGME and PEG (4000)-ME
The results for the ME-dried, AGME and PEG (4000)-ME are summarized in Table 3 and the microscope photographs of the ME-surfaces, that are surrounded by 2.6%-Ca2+ or Ca2+-compound (see Table 2), are summarized in ESI Scheme S1.† This implies not only that, with regards to the ability of the reactant to enter the MEb surface (e.g., CLMEb), the over 20% (v/v) of (NH4)2SO4 surrounded may stem from a multi-subunit species because of a tendency to dissociate upon dilution, resulting in a longer half-life albeit with less activity at the start, but also that a ME-active site in CMMEs (e.g., CLMEa) may be affected by the solubility/ability of the reactant to enter beyond the metallic surface with highly polymerized compound treatment (see ESI Fig. S5 and S6†). These results indicate that, while the ME-redox protein is stable to colloid aggregation similar to highly polymerized systems: CM-cellulose, PEG (4000), and PG-starch (see Scheme 1(a)), the ME-active site may be effectively concentrated with the PP-heme during centrifugation (see Table 2), and such a compound-modified treatment may be well justified as a method for improving the activity (i.e., enantioselectivity) and solvent pH independence (i.e., operational stability). Overall, the concentration of the product ketone decreased to less than 0.6 mM due to its volatility (asymmetric oxidation was performed in a tube without a cap).7 40 °C (with magnetic stirring at 700 rpm) was the optimum temperature in these oxidation reactions because of the preventing volatility of the products and/or reactants.| MEs (20 mg) | Timesa (h) | Substrate (1.2 mM) | Solventc [4.0 mL] | Products | ||
|---|---|---|---|---|---|---|
| Comp. | OP/%eeb | |||||
| a Reaction time.b Optical purity determined by HPLC.c That including 0.6% (v/v) DMSO as the cosolvent.d 50 mM glycine–NaOH (pH 9.0). CLMEa is produced without (NH4)2SO4, while CLMEb is produced with (NH4)2SO4.e ME-dried was prepared by freeze-drying after centrifuging the PP-gel extract (see Scheme 1).f Chemical yield (%).g Distilled water. | ||||||
| 1 | CLMEa | 16 | Rac-1 | D.W.g | S-1 | >99 |
| 16 | Rac-2 | S-2 | >99 | |||
| 2 | CLMEb | 48 | Rac-1 | D.W. | S-1 | 5.8 |
| 48 | Rac-1 | Bufferd | S-1 | >99 | ||
| 48 | Rac-2 | D.W. | S-2 | 5.2 | ||
| 48 | Rac-2 | Buffer | S-2 | >99 | ||
| 3 | PEG (4000)-ME | 13 | Rac-1 | D.W. | S-1 | >99 |
| 13 | Rac-2 | S-2 | >99 | |||
| 4 | CMC-me | 14 | Rac-1 | D.W. | S-1 | >99 |
| 13 | Rac-2 | S-2 | >99 | |||
| 5 | PGS-me | 14 | Rac-1 | D.W. | S-1 | >99 |
| 14 | Rac-2 | S-2 | >99 | |||
| 6 | AGME | 9 | Rac-1 | D.W. | R-1 | >99 |
| 9 | Rac-2 | R-2 | >99 | |||
| 7 | ME-driede | 22 | Rac-1 | D.W. | S-1 | >99 |
| 20 | Rac-2 | S-2 | >99 | |||
In addition, AGME (treated with PEG (MW: 1000/4000 = 2/1) aggregation) was used for the oxidative conversion of rac-1 to the corresponding ketone (∼95% chemical yield: >16 h) via the selective oxidation of S-(+)-1, leaving highly enantiopure R-(−)-1 (>99% ee: ∼50% chemical yield, <9 h) (see Table 3). Therefore, the turnover of the ME-active site may be affected by a different PEG molecular size (MW: 1000 or 4000) such that each enantiomer can be selectively synthesized by utilizing either AGME (PEG (1000/4000 = 2/1)-aggregated ME) or CMME (specifically, CLMEa).
The sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the ME-redox protein for MW determination
To determine the nature of the ME-redox protein that catalyzes asymmetric oxidation, the ME-suspension (10 mL) eluted from the PP-gel, which was incubated for 48 h, was first separated into 60 fractions (acquired in 18 mm test tubes with 3.0 mL portions in each tube) using a gel-filtration system and the absorbance was monitored at 280 nm (see ESI Fig. S7–S9†). These 60 fractions (3.0 mL portions) were each added to 0.48 mL of an aqueous solution of rac-2 (0.8 mM) containing 1.03% (v/v) DMSO. The resulting mixtures were then incubated at 40 °C for 48 h with magnetic stirring at 700 rpm and subjected to SDS-PAGE analysis (see Fig. 3). The results of the analysis were compared to those for sample F (an aqueous solution containing the gel-filtered ME-redox protein (fraction 36)), and it was determined that the ME-redox protein molecular mass is approximately 20 kDa measured as a single band (ME-redox protein band) in correspondence with the respective marker, as shown in Fig. 3 (see ESI Tables S9 and S10†).These results indicated that, when the suspension (10 mL) is directly injected onto the column, gel-filtration alone can provide a single band for F. In addition, it was found that different concentrations of sample F (ME-redox protein), sample A, and sample B (A and B are the suspensions for producing CLMEa) had to be evaluated for the ME-redox protein concentration; therefore, their concentrations were determined by the Bradford method (see ESI Table S8†). This result also indicated that approximately 5–7% of the ME-redox protein is present per CMME. The results of both the peptide mass fingerprinting (PMF) and the liquid chromatography-mass spectrometry-ion trap-time-of-flight (LCMS-IT-TOF) analyses indicated that the remaining components (>90%) of CMME (i.e., the labeled bold bands 1 through 6) mainly consist of debris from the damaged cell membranes and cell walls of PP, such as the extracellular ligand-binding receptor (band 4) and oligopeptide ABC transporter substrate-binding protein (band 5), as shown in Fig. 3 (see ESI Tables S9 and S10†).
These results therefore confirm that hemophore HasA (band 7) is secreted by the ABC transporter (band 5) that exists in the extracellular matrix and/or outer membrane (band 4) of PP.4a Furthermore, as previously reported, the ABC transporters appear to have a molecular weight greater than that of the hemophore,4c (i.e., band 5/band 7 = approximately 55.4 kDa/20.8 kDa).
The identification of the ME-redox oxidant via physicochemical analysis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or rac-2) → Fe2+ + H2O).3,4,14
O (oxidizing rac-1 or rac-2) → Fe2+ + H2O).3,4,14
| a X: may be Cys (C) but not detected.b X: many amino acids were detected.c YP 262445.1: the accession hit on the query sequence was limited between the query coverage (>93%) and E value (2 × 10−11), a 20.853 Da HasAp gene product [hemophore: Pseudomonas fluorescens Pf-5] from plant commensal bacteria, which can inhibit the rhizosphere and produce secondary metabolites that suppress soil-borne plant pathogens.9d Red amino acids indicate “hits” between fraction 36 and YP 262445.1c. The squares indicate the heme-binding site: His-32 (bearing loop), Tyr-75 (axial heme ligand) and His-83 (hydrogen ligand). |
|---|
 |
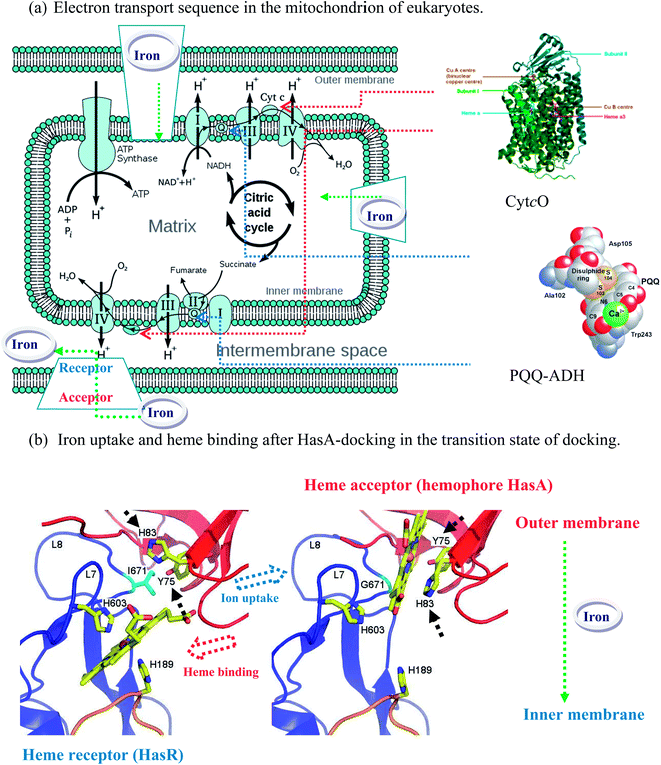 | ||
| Scheme 2 (a) The cell membrane protein containing a pyrroloquinoline quinone ADH (PQQ-ADH)17 with incorporated CytcO characteristics,18 and (b) crystal structures of the outer membrane receptor (HasR in blue) and excreted heme-binding proteins (hemophore HasA in red): The Serratia marcescens homophore HasA, a 188-redidue protein, is bound by axial coordination with HasA–His-32 (bearing loop), HasA–Tyr-75 (axial heme ligand), and HasA–His-83 (hydrogen ligand).4b | ||
In addition to the hemophore HasA of living organisms, it has also been reported that the membrane-bound enzymes of HBPs (e.g., plant-HBPs and/or bacterial-HBPs) produce secondary metabolites that enable the detoxification of many organic compounds as energy sources and are resistant to the toxic effects of antibiotics;9 e.g., cytochrome-P450s, the pea plant (Pisum sativum) and the soil bacterium BM-3 (Bacillus megaterium) catalyze the α-hydroxylation of carboxylic acids in the presence of oxygen in aqueous media.15 In addition, there are several reports describing membrane-bound enzymes of HBPs with incorporated iron (Fe), such as (1) peroxidase,16 (2) pyrroloquinoline quinone ADH (PQQ-ADH),17 and (3) CytcO.18 The oxidation reactions revealed in these studies16–18 demonstrate (1) a peroxide dependence, (2) a primary alcohol dependence, and (3) an oxygen independence. Obviously, the peroxidase process (H2O2 + HBP–H2 → 2H2O + HBP) is not comparable to HBP oxidation due to peroxide dependence. Therefore, the regeneration system for HBP can be considered to be similar in nature to that of (2) primary-alcohol-dependent ADH (PQQ-ADH: namely, CC1) with incorporated (3) cytochrome c oxidase characteristics (namely, CC2). This system is depicted as follows: (2) 4Fe2+–CC1 + 8Hin+ + O2 → 4Fe3+–CC1 + 2H2O + 4Hout+ in conjunction with (3) 2Fe2+–CC2 ⇄ ketones + 2Fe3+-reduced-CC2 (existing in the cell membrane protein, see Scheme 2). It is supposed that, because the sequence of hemophore HasA is an axis (i.e., bone) secreted by the HBP host proteins (e.g., ABC transporters, PQQ-ADH, and CytcO), the PP-HBP (the hemophore itself and/or its host HBPs) with the incorporated iron electron-transfer system may be enabled in the presence of oxygen (i.e., Fe2+ + O2 → Fe3+–O–O− → Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or rac-2) → Fe2+ + H2O),3a–c which is observed when the O2 concentration is varied, as a Cyt-P450 from PP that depends on oxygen instead of a redox cofactor (e.g., NAD(P)H).3,4,14 Thus, the reason why these new biocatalysts do not depend on a redox cofactor (e.g., NAD(P)) but instead on oxygen, may be clarified here,7 although there are no supporting studies specifically related to systems for the asymmetric oxidation of secondary alcohols in organic synthesis.3,4 Compared with bacteria and animals, plant-P450s make several (a few hundred) forms;4d thus, the new plant-HBP system similar to a Cyt-P450 can be enabled as a member of cytochrome P450 family.3,4
O (oxidizing rac-1 or rac-2) → Fe2+ + H2O),3a–c which is observed when the O2 concentration is varied, as a Cyt-P450 from PP that depends on oxygen instead of a redox cofactor (e.g., NAD(P)H).3,4,14 Thus, the reason why these new biocatalysts do not depend on a redox cofactor (e.g., NAD(P)) but instead on oxygen, may be clarified here,7 although there are no supporting studies specifically related to systems for the asymmetric oxidation of secondary alcohols in organic synthesis.3,4 Compared with bacteria and animals, plant-P450s make several (a few hundred) forms;4d thus, the new plant-HBP system similar to a Cyt-P450 can be enabled as a member of cytochrome P450 family.3,4
The equations indicate that the HBP-redox catalytic center (i.e., the hemophore itself or its host HBPs) can be regenerated for successive catalytic events only in the presence of O2 and the incorporated iron electron-transfer system (Cyt-P450: cysteine – Fe2+ + O2 → Fe3+–O–O− → Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or rac-2) → Fe2+ + H2O).3,4 Further explorations of the complete gene sequence of the PP-HasA homolog for studies at the molecular level are being actively pursued in this laboratory and the results will be reported in due course.19 The novel aspects of these results include not only a new method for the purification and characterization of the compound-modified HBP system and a proposal for the HBP pathway, but also the use of a raw biomaterial as an ME-catalytic system incorporating a redox cofactor to perform asymmetric oxidation. Therefore, it is expected that biomaterials containing HBP would be available not only as food but also as biological catalysts for the synthesis of optically active alcohols using environmentally friendly systems that promote industrial sustainability.
O (oxidizing rac-1 or rac-2) → Fe2+ + H2O).3,4 Further explorations of the complete gene sequence of the PP-HasA homolog for studies at the molecular level are being actively pursued in this laboratory and the results will be reported in due course.19 The novel aspects of these results include not only a new method for the purification and characterization of the compound-modified HBP system and a proposal for the HBP pathway, but also the use of a raw biomaterial as an ME-catalytic system incorporating a redox cofactor to perform asymmetric oxidation. Therefore, it is expected that biomaterials containing HBP would be available not only as food but also as biological catalysts for the synthesis of optically active alcohols using environmentally friendly systems that promote industrial sustainability.
Materials and methods
The preparation of CLMEa, CLMEb and AGME
PP (10 g) was added to 200 mL of 0.75% aq. sodium alginate and encapsulated with CaCl2 (500 mL, 39 g L−1). The PP-gel was then exposed to air for 5 h, and the resulting ME-suspension was extracted at 40 °C with distilled water (200 mL) in a 500 mL Erlenmeyer flask with rotary shaking at 150 rpm for over 40 h. For AGME production, the ME precipitate (wet: 16 g) was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min) and then dissolved in a 5% (v/v) PEG (MW: 1000/4000 = 2/1)/50 mM glycine–NaOH solution (pH 9.0, 100 mL) and stored for over 20 h to allow aggregation, after centrifugation, the precipitate was freeze-dried under vacuum and crushed using a ball mill. For CLMEa production, the ME-precipitate (wet: 16 g) was dissolved in a 50 mM glycine–NaOH solution (pH 9.0, 100 mL), and then a 25% (v/v) aqueous GA solution (2 mL) was added to obtain an overall 0.5% (v/v) GA solution for cross-linking, which was allowed to take place for over 20 h. After centrifuging the mixture (10
000 rpm for 10 min) and then dissolved in a 5% (v/v) PEG (MW: 1000/4000 = 2/1)/50 mM glycine–NaOH solution (pH 9.0, 100 mL) and stored for over 20 h to allow aggregation, after centrifugation, the precipitate was freeze-dried under vacuum and crushed using a ball mill. For CLMEa production, the ME-precipitate (wet: 16 g) was dissolved in a 50 mM glycine–NaOH solution (pH 9.0, 100 mL), and then a 25% (v/v) aqueous GA solution (2 mL) was added to obtain an overall 0.5% (v/v) GA solution for cross-linking, which was allowed to take place for over 20 h. After centrifuging the mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min), the resulting precipitate was freeze-dried under vacuum and crushed using a ball mill. For CLMEb production, the ME-solution (200 mL), precipitated using 30% (w/v) aq. (NH4)2SO4, was cross-linked at 0.25% (v/v) GA via the addition of a 25% (v/v) aq. GA solution (2.0 mL), and the resulting mixture was stored for over 20 h. After centrifuging the mixture (10
000 rpm for 10 min), the resulting precipitate was freeze-dried under vacuum and crushed using a ball mill. For CLMEb production, the ME-solution (200 mL), precipitated using 30% (w/v) aq. (NH4)2SO4, was cross-linked at 0.25% (v/v) GA via the addition of a 25% (v/v) aq. GA solution (2.0 mL), and the resulting mixture was stored for over 20 h. After centrifuging the mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min), the resulting precipitate was freeze-dried under vacuum and then crushed using a ball mill. An RLE-II 203 instrument was utilized to freeze-dry the samples under vacuum [−50 °C/10 Pa (1 h) → 5 °C min−1 → +50° C/10 Pa (22 h)].
000 rpm for 10 min), the resulting precipitate was freeze-dried under vacuum and then crushed using a ball mill. An RLE-II 203 instrument was utilized to freeze-dry the samples under vacuum [−50 °C/10 Pa (1 h) → 5 °C min−1 → +50° C/10 Pa (22 h)].
The preparation of PEG (4000)-ME, CMC-ME and PGS-ME
The protocol for the purification/preparation of PEG (4000)-ME, CMC-ME and PGS-ME is as follows: (1) the formation of the PP-gel by the encapsulation of the PP (10 g) with a calcium alginate gel prepared from a 0.75% aq. sodium alginate solution (200 mL D.W) and a 39 g L−1 CaCl2 solution (500 mL) (PP-gel); (2) the aeration of the PP-gel in air for several hours; (3) the extraction of the contents by rotary shaking using hot water to obtain an ME-suspension; (4) the separation of the precipitate from the suspension by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min); (5) coating the precipitate (wet: 5.0 g) via the addition of PEG (MW 4000), CM-cellulose, or pregelatinized starch (500 mg) dissolved in 50 mM glycine–NaOH (pH 9.0) buffer (50 mL); and (6) the preparation of the coated powder (approximately 1.0 g) via freeze-drying and crushing using a ball mill.
000 rpm, 10 min); (5) coating the precipitate (wet: 5.0 g) via the addition of PEG (MW 4000), CM-cellulose, or pregelatinized starch (500 mg) dissolved in 50 mM glycine–NaOH (pH 9.0) buffer (50 mL); and (6) the preparation of the coated powder (approximately 1.0 g) via freeze-drying and crushing using a ball mill.
The confirmation of the composition of PEG (4000)-ME, CLME and PP
The measurement of the metal content was conducted by adding the protein powder (0.5 g) to a solution of purified water (50 mL) and 60% HNO3 (Kokusan, 10 mL) in a 100 mL beaker followed by the digestion of the resulting mixture (heating on a hotplate for 1 h). The digested sample was diluted to 50 mL with purified water, and the metal content was measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES; Shimadzu, ICPS-7500). Elemental analysis of the samples (2 mg) was performed on a Perkin-Elmer PE 2400 Series II analyzer.The determination of the specific activity of the CMMEs and AGME in biocatalytic reactions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm) and CMME (20 mg) were combined in an 18 mm × 15 mL test tube in distilled water (4.0 mL), and the reactions were performed at 40 °C with magnetic stirring at 700 rpm. Subsequently, the reaction mixture was centrifuged at 3500 rpm (5 min) and then extracted by adding n-hexane (4.0 mL). The ee was calculated for either rac-1 (0.8 mM or 1.2 mM) or rac-2 (0.8 mM or 1.2 mM), which were separated using either a Daicel Chiralcel OB-H column ((S)-isomer/(R)-isomer/product ketone = 7.8/8.8/11.6 min) or a Daicel Chiralpak AS-H column ((S)-isomer/(R)-isomer/product ketone = 7.5/8.25/9.5 min) connected to an HPLC LC-10A system (Shimadzu). The analytical conditions were as follows: mobile phase, n-hexane/IPA: 9/1, flow rate: 1.0 mL min−1, temperature: 30 °C, wavelength: UV 254 nm. The stereochemistry of the isolated optically active alcohol was identified as reported previously5 by comparing the specific rotation values (+ or −) obtained using a polarimeter.
000 ppm) and CMME (20 mg) were combined in an 18 mm × 15 mL test tube in distilled water (4.0 mL), and the reactions were performed at 40 °C with magnetic stirring at 700 rpm. Subsequently, the reaction mixture was centrifuged at 3500 rpm (5 min) and then extracted by adding n-hexane (4.0 mL). The ee was calculated for either rac-1 (0.8 mM or 1.2 mM) or rac-2 (0.8 mM or 1.2 mM), which were separated using either a Daicel Chiralcel OB-H column ((S)-isomer/(R)-isomer/product ketone = 7.8/8.8/11.6 min) or a Daicel Chiralpak AS-H column ((S)-isomer/(R)-isomer/product ketone = 7.5/8.25/9.5 min) connected to an HPLC LC-10A system (Shimadzu). The analytical conditions were as follows: mobile phase, n-hexane/IPA: 9/1, flow rate: 1.0 mL min−1, temperature: 30 °C, wavelength: UV 254 nm. The stereochemistry of the isolated optically active alcohol was identified as reported previously5 by comparing the specific rotation values (+ or −) obtained using a polarimeter.The determination of the specific activity (unit mg−1 min−1) of the CMMEs and AGME
The asymmetric oxidation activity of CLMEa (5, 10, 15, and 20 mg) and the oxidation activity of AGME (5, 10, 15, and 20 mg) with rac-1 (1.2 mM) in water (4.0 mL D.W.) and DMSO (<1.0%) with magnetic stirring (700 rpm) for 34 h was measured. During the 34 h incubation period at 40 °C, the substrate solution (30 μL, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm) was added in three portions at 0, 12 and 20 h. Fig. 1 shows the dependence of the concentration of the product ketone (Δketone: mM) on the reaction time (h) for different quantities of CLMEa (or AGME). Fig. 2 shows the dependence of the instant velocity (mM h−1) of CLMEa (or AGME) on the quantity of CLMEa (or AGME) (mg). This unit indicates the quantity of enzyme (mg) that is capable of oxidizing 1 μmol of rac-1 per minute. The formulas utilized for calculating the instant velocity (IV, mM h−1), activity (AC, mmol per (4 mL min)), and the specific activity SA, unit (mmol per (4 mL min)) are as follows: IV = Δketone (mM) ÷ time (h), AC = IV (mM h−1) × 0.004 (4 mL L−1) × 1000 (M mM−1) ÷ 60 (min h−1), and SA = AC (mol per (4 mL min)) ÷ CLMEa (or AGME) (mg).
000 ppm) was added in three portions at 0, 12 and 20 h. Fig. 1 shows the dependence of the concentration of the product ketone (Δketone: mM) on the reaction time (h) for different quantities of CLMEa (or AGME). Fig. 2 shows the dependence of the instant velocity (mM h−1) of CLMEa (or AGME) on the quantity of CLMEa (or AGME) (mg). This unit indicates the quantity of enzyme (mg) that is capable of oxidizing 1 μmol of rac-1 per minute. The formulas utilized for calculating the instant velocity (IV, mM h−1), activity (AC, mmol per (4 mL min)), and the specific activity SA, unit (mmol per (4 mL min)) are as follows: IV = Δketone (mM) ÷ time (h), AC = IV (mM h−1) × 0.004 (4 mL L−1) × 1000 (M mM−1) ÷ 60 (min h−1), and SA = AC (mol per (4 mL min)) ÷ CLMEa (or AGME) (mg).
The separation of the ME-redox protein from the suspension
The identification of the ME-redox proteins by physicochemical analysis
Conclusion
This study aimed to clarify the exact nature of all of the species engaged in the biocatalytic oxidation sequence, including that of the ME eluted from encapsulated PP under aeration in both its PEG (1000/4000 = 2/1)-aggregated ME (AGME) and compound-modified form (CMME). The AGME complex represents a new biocatalyst enabling the oxidation of rac-(+)-1-(6-methoxynaphthalen-2-yl)ethanol (rac-1) to the corresponding ketone-1 (∼95% yield) via the asymmetric oxidation of S-(+)-1, leaving highly enantiopure R-(−)-1 (>99% ee, ∼50% chemical yield); furthermore, CMME enables the kinetic resolution of S-(+)-1 (an S-(+)-naproxen precursor; >99% ee, ∼50% yield) via the selective oxidation of R-(−)-1 in aqueous media. The activities of both AGME and CMME were found to be 0.8 ± 0.03 mU (mean ± SD) mg−1 min−1 and 0.6 ± 0.02 mU (mean ± SD) mg−1 min−1, respectively. The asymmetric oxidation activity is attributed to an HBP that is native to the pea plant. The N-terminal sequence comparison showed a 93% similarity with the 20.853 kDa HasAp gene product [hemophore HasA, Pseudomonas fluorescens Pf-5] HBP. The PP-HBP (the hemophore itself or its host HBPs) performs successive asymmetric catalytic events (i.e., regeneration) via an incorporated iron electron-transfer system in the presence of oxygen (Cyt-P450: cysteine – Fe2+ + O2 → Fe3+–O–O− → Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (oxidizing rac-1 or -2) → Fe2+ + H2O). Our findings open up new possibilities for the use of these materials not only as food but also as biological catalysts for the synthesis of optically active alcohols in an environmentally friendly manner, thereby promoting industrial sustainability.
O (oxidizing rac-1 or -2) → Fe2+ + H2O). Our findings open up new possibilities for the use of these materials not only as food but also as biological catalysts for the synthesis of optically active alcohols in an environmentally friendly manner, thereby promoting industrial sustainability.
Abbreviations
| ME | Membrane-bound enzyme |
| GA | Glutaraldehyde |
| FD | Freeze-dried |
| Has | Heme acquisition system |
| NAD(P) | Nicotinamide adenine dinucleotide |
| FAD | Flavin adenine dinucleotide |
| PP-gel | Calcium-alginate-gel-containing PP |
| CLME | Cross-linked ME |
| CMME | A compound-modified ME |
| AGME | PEG (MW: 1000/4000 = 2/1)-aggregated ME |
| S-1 | S-(+)-1-(6-Methoxynaphthalen-2-yl)ethanol |
| S-2 | S-(+)-1-(2-Naphthyl) ethanol |
| HBP | Heme-binding protein |
| PQQ-ADH | Pyrroloquinoline quinone alcohol dehydrogenase |
| CytcO | Cytochrome c oxidase |
| Cyt-P450 | Oxygen-driven cytochrome P450 enzyme |
| P450 | Cytochrome P450 enzyme |
Acknowledgements
We thank Prof. Kohtaro Kirimura and Keisuke Udagawa from Waseda University for the valuable advice and warm encouragement and we thank GM. Koh Ueda from Wako Pure Chemical Industries, Ltd. for placing SanCat-R on the market (i.e., the CMMEs: code no. 355-34211 (for 1 g) and code no. 351-34213 (for 5 g), respectively).References
- H. E. Schoemaker, D. Mink and M. G. Wubbolts, Science, 2003, 299, 1694 CrossRef CAS PubMed.
- (a) D. S. Auld and T. Bergman, Cell. Mol. Life Sci., 2008, 65, 3961 CrossRef CAS PubMed; (b) M. N. Giles, B. A. Watts, I. G. Giles, H. F. Fry, A. J. Littlechild and C. Jacob, Chem. Biol., 2003, 10, 677 CrossRef; (c) M. N. Giles, I. G. Giles and C. Jacob, Biochem. Biophys. Res. Commun., 2003, 300, 1–4 CrossRef; (d) B. S. Sobolov, D. M. Leonida, B. A. Malik, I. K. Voivodov, F. McKinney, J. Kim and J. Fry, J. Org. Chem., 1996, 61, 2125 CrossRef.
- (a) D. S. Lee, H. Yamada, H. Sugimoto, I. Matsunaga, H. Ogura, K. Ichihara, S. Adachi, S. Y. Park and Y. Shiro, J. Biol. Chem., 2003, 278, 9761 CrossRef CAS PubMed; (b) O. Shoji, T. Fujishiro, H. Nakajima, M. Kim, S. Nagano, Y. Shiro and Y. Watanabe, Angew. Chem., Int. Ed., 2007, 46, 3656 CrossRef CAS PubMed; (c) T. H. Yosca, J. Rittle, C. M. Krest, E. L. Onderko, A.-R. K. Beham and M. T. Green, Science, 2013, 342, 825 CrossRef CAS PubMed; (d) P. R. Ortiz de Montellano, Cytochrome P450: Structure, Mechanism, and Biochemistry, Kluwer Academic/Plenum, New York, Boston, Dorcrecht, London, Mosow, 2004, 3rd edn Search PubMed; (e) B. E. Prage, S.-C. Pawelzik, S. L. Busenlehner, K. Kim, R. Morgenstern, P.-J. Jakobsson and R. N. Armstrong, Biochemistry, 2011, 50, 7684 CrossRef PubMed; (f) A.-L. Johansson, J. Carlsson, M. Hogbom, J. P. Hosler, R. B. Gennis and P. Brzezinski, Biochemistry, 2013, 52, 827 CrossRef CAS PubMed.
- (a) M. L. Oldham, D. Khare, F. A. Quiocho, A. L. Davidson and J. Chen, Nature, 2007, 450, 515 CrossRef CAS PubMed; (b) S. Krieg, F. Huche, K. Diederichs, N. Izadi-Pruneyre, A. Lecroisey, C. Wandersman, P. Delepelaire and W. Welte, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(4), 1045 CrossRef CAS PubMed; (c) N. Izadi-Pruneyre, N. Wolff, V. Redeker, C. Wandersman, M. Delepierre and A. Lecroisey, J. Bacteriol., 1999, 261, 562 CAS; (d) F. P. Guengerich, Chem. Res. Toxicol., 2001, 14, 611 CrossRef CAS PubMed , for more information on cytochrome P450 from a genomics perspective, please check the Protein of the Month at the European Bioinformatics Institute; (e) A. R. McDonald, M. R. Bukowski, E. R. Farquhar, T. A. Jackson, K. D. Koehntop, M. Sook Seo, R. F. De Hont, A. Stubna, J. A. Halfen, E. Muonck, W. Nam and L. Que Jr, J. Am. Chem. Soc., 2010, 132, 17118 CrossRef CAS PubMed.
- (a) H. Nagaoka and H. Kayahara, Biosci., Biotechnol., Biochem., 1999, 63, 1991 CrossRef CAS; (b) H. Nagaoka and H. Kayahara, Biosci., Biotechnol., Biochem., 2000, 64, 781 CrossRef CAS; (c) H. Nagaoka, H. Kayahara and Y. Wakabayashi, Biosci., Biotechnol., Biochem., 2001, 65, 634 CrossRef CAS.
- (a) H. Nagaoka, Biotechnol. Prog., 2003, 19, 1149 CrossRef CAS PubMed; (b) H. Nagaoka, Curr. Top. Nutraceutical Res., 2003, 1, 281 CAS; (c) H. Nagaoka, Biotechnol. Prog., 2004, 20, 128 CrossRef CAS PubMed; (d) H. Nagaoka, Biotechnol. Prog., 2005, 21, 405 CrossRef CAS PubMed.
- (a) H. Nagaoka, K. Udagawa and K. Kirimura, Biotechnol. Prog., 2012, 28, 953 CrossRef CAS PubMed; (b) T. Ukai, Y. Matsumura and R. Urade, J. Agric. Food Chem., 2008, 56, 1122 CrossRef CAS PubMed; (c) I. Kenji, M. Satoshi, S. Mamoru, N. Atushi, Y. Eiki, O. Kengo and I. Koreaki, Cell, 2006, 127, 789 CrossRef PubMed.
- V. V. Thakur and A. Sudalai, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2005, 44, 557 Search PubMed.
- I. T. Paulsen, C. M. Press, J. Ravel, D. Y. Kobayashi, G. S. Myers, D. V. Mavrodi, R. T. DeBoy, R. Seshadri, Q. Ren, R. Madupu, R. J. Dodson, A. S. Durkin, L. M. Brinkac, S. C. Daugherty, S. A. Sullivan, M. J. Rosovitz, M. L. Gwinn, L. Zhou, D. J. Schneider, S. W. Cartinhour, W. C. Nelson, J. Weidman, K. Watkins, K. Tran, H. Khouri, E. A. Pierson, L. S. Pierson 3rd, L. S. Thomashow and J. E. Loper, Nat. Biotechnol., 2005, 23(7), 873 CrossRef CAS PubMed.
- (a) L. Cope, S. Thomas, Z. Hrkal and E. Hansen, Infect. Immun., 1998, 66, 4511 CAS; (b) C. Wandersman and I. Stojiljkovic, Curr. Opin. Microbiol., 2000, 3, 215 CrossRef CAS.
- (a) V. Braun and H. Killmann, Trends Biochem. Sci., 1999, 24, 104 CrossRef CAS; (b) S. Letoffe, K. Omori and C. J. Wandersman, J. Bacteriol., 2000, 182(16), 4401 CrossRef CAS.
- (a) J. M. Ghigo, S. Letoffe and C. Wandersman, J. Bacteriol., 1997, 179, 3572 CAS; (b) H. Cwerman, C. Wanderson and F. Biville, J. Bacteriol., 2006, 188, 3357 CrossRef CAS PubMed.
- D. P. Chimento, R. J. Kadner and M. C. Wiener, Proteins: Struct., Funct., Bioinf., 2005, 59, 240 CrossRef CAS PubMed.
- C. Barjon, K. Wecker, P. Izadi-Pruneyre and P. Delepelaire, J. Bacteriol., 2007, 189, 5379 CrossRef CAS PubMed.
- (a) A. Waldemar, B. Wilhelm, H. Jenny, H. Hans-Ulrich, L. Michael, S. Alexander, R. S. Chantu and S. Peter, J. Am. Chem. Soc., 1998, 120, 11044 CrossRef; (b) M. Landwehr, L. Hochrein, R. C. Otey, A. Kasrayan, E. J. Backvall and H. F. Amold, J. Am. Chem. Soc., 2006, 128, 6058 CrossRef CAS PubMed.
- (a) H. Shimizu, D. J. Schuller, W. N. Lanzilotta, M. Sundaramoorthy, D. M. Arciero, A. B. Hooper and T. L. Poulos, Biochemistry, 2001, 40, 13483 CrossRef CAS PubMed; (b) J. E. Erman and L. B. Vitello, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 2002, 1597, 193 CrossRef CAS; (c) X. Liu, Y. Dong, A. Zhang, L. Wang and L. Feng, Microbiology, 2009, 155, 2078 CrossRef CAS PubMed; (d) A. M. Hays Putnam, Y. T. Lee and D. B. Goodin, Biochemistry, 2009, 48, 1 CrossRef CAS PubMed.
- P. R. Afolabi, F. Mohammed, K. Amaratunga, S. L. Majekodunmi, S. L. Dales, R. Gill, D. Thompson, J. B. Cooper, S. P. Wood, P. M. Goodwin and C. Anthony, Biochemistry, 2001, 40, 9799 CrossRef CAS PubMed.
- (a) J. M. Zee and D. M. Glerum, Biochem. Cell Biol., 2006, 84, 859 CrossRef CAS PubMed; (b) E. B. Prage, S.-C. Pawelzik, L. S. Busenlehner, K. Kim, R. Morgenstern, P.-J. Jakobsson and R. N. Armstrong, Biochemistry, 2011, 50, 7684 CrossRef CAS PubMed; (c) A.-L. Johansson, J. Carlsson, M. Hogbom, J. P. Hosler, R. B. Gennis and P. Brzezinski, Biochemistry, 2013, 52, 827 CrossRef CAS PubMed.
- The protein sequence based on a BLAST query sequence analysis of cycle no. for YP 262445.1 is available on the internet on NCBI resource: http://www.ncbi.nlm.nih.gov/protein/70732682http://www.ncbi.nlm.nih.gov/protein/70732682 and the full length gene sequences is available to the web on PATRIC resource: VBIPseFlu72549_5489: http://patricbrc.vbi.vt.edu/portal/portal/patric/Feature?cType=feature%26cId=19880237http://patricbrc.vbi.vt.edu/portal/portal/patric/Feature?cType=feature%26cId=19880237.
Footnote |
| † Electronic supplementary information (ESI) available: for a description of the material see SI Fig. S1–S8, Tables S1–S10 and Scheme S1. See DOI: 10.1039/c3ra45936e |
| This journal is © The Royal Society of Chemistry 2014 |