Controllable preparation of graphene oxide/metal nanoparticle hybrids as surface-enhanced Raman scattering substrates for 6-mercaptopurine detection
Wen Liang Fua,
Shu Jun Zhen*a and
Cheng Zhi Huang*ab
aKey Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, 400715, Chongqing, P.R. China. E-mail: zsj@swu.edu.cn; Fax: +86-23-68367257; Tel: +86-23-68254059
bKey Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, College of Pharmaceutical Sciences, Southwest University, 400715, Chongqing, P.R. China. E-mail: chengzhi@swu.edu.cn; Fax: +86-23-68367257; Tel: +86-23-68254659
First published on 11th March 2014
Abstract
In this contribution, a new simple and cost-effective strategy for the preparation of hybrids of graphene oxide (GO) and metal nanoparticles (MNPs) through the mediation of polyethyleneimine (PEI) molecules was reported. PEI molecules as cationic polymers could effectively attach onto the surface of GO for further negative MNP adsorption. By this process, gold and silver nanoparticles are assembled on GO with high efficiency. This method has also been successfully applied to the assembly of metal nanoparticles and carbon nanotubes (CNTs), indicating that this method is general. Furthermore, the as-prepared graphene oxide/silver nanoparticle (GO/AgNP) hybrids have been used as perfect surface enhanced Raman scattering (SERS) substrates with an enhancement factor of 1.5 × 105 and successfully applied for the sensitive and selective detection of 6-mercaptopurine (6MP) in pharmaceutical tablets with satisfactory results.
1. Introduction
In recent years, famous carbon nanomaterials including graphene and carbon nanotubes have been popularly used as surface enhanced Raman scattering (SERS) substrates through a chemical enhancement mechanism.1–4 However, the deficiency is their relatively weak Raman enhanced effect contrasting with an electromagnetic enhancement mechanism of noble metal nanomaterials, which restricts the analytical applications of carbon nanomaterials as SERS substrates. To address this issue, many researchers focus on assembling these famous carbon nanomaterials with metal nanoparticles (MNPs). For a combined chemical and electromagnetic enhancement mechanism, these hybrids can be applied for SERS detection with high sensitivity. Moreover, functionalized carbon nanomaterials have the ability to condense molecules to further improve the sensitivity in SERS detection because of their large surface areas and unique electronic structures.5 For example, Liu et al. successfully prepared graphene oxide and silver nanoparticle hybrids (GO/AgNPs) and used them in the sensitive detection of trinitrotoluene.6 Wang et al. and Huang et al. constructed GO or single walled carbon nanotubes (SWCNTs) with gold or silver nanoparticle assemblies for intracellular Raman mapping using the enhanced Raman signal of carbon nanomaterials.7,8 Thus, to develop effective methods, preparing carbon nanomaterials and MNP hybrids is essential.Up to now, the wet chemical methods for construction of carbon nanomaterials and MNP hybrids mainly include the in situ method and the self-assembled method. By the in situ method, the synthesis procedure is simple, and there is no need to add other linker molecules, but the morphology and density of the MNPs are difficult to control. In contrast, self-assembly is an ordinary method to fabricate the carbon nanomaterials and MNP hybrids because the loading ration and the morphology of the nanoparticles are tunable.5,9 However, most MNPs are synthesized with negatively charged reducing agents and capping agents, which makes it impossible for them to be anchored on negatively charged carbon nanomaterials directly. Therefore, various kinds of linker molecules, including protein,10 DNA,11,12 and organic small molecules,13,14 have been exploited to connect carbon nanomaterials and MNPs through different reactions, such as π–π stacking, chemical bond formation and electrostatic adsorption.9,11–13 But most reported assembly strategies are complex for adding more operating steps. In addition, the linker molecules, such as protein and DNA, are expensive, and some linker molecules need to be synthesized through complex organic synthesis steps. Therefore, it is necessary to fabricate a new assembly method, which is simple, cheap, and could be applied universally for the assembly of other materials.
Herein, the GO and MNP hybrids were fabricated by an electrostatic self-assembly strategy. In our method, polyethyleneimine (PEI) molecules served as linkers to prepare stable cationic polyelectrolyte-functionalized GO for further MNP anchoring (Scheme 1). PEI is a cationic polymer, which contains a high density of amino groups. It has been proved that PEI has good affinity with GO through electrostatic interactions,15,16 which reverses the charge of GO and allows for easy attachment of negatively charged MNPs. PEI is commercially available without the need to be synthesized, which makes the process simple and easy to operate. In addition, it has been certified that this method is quite facile and MNPs can be attached on GO with high density. More importantly, this method was suitable for assembling MNPs on single walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), confirming the good versatility of this method. The synthesized graphene oxide and silver nanoparticle hybrids (GO/AgNPs) could be used as efficient SERS substrates, which have been successfully applied for the sensitive and selective detection of the chemotherapy drug 6-mercaptopurine (6MP) in tablets.
 | ||
| Scheme 1 A scheme (not to scale) to illustrate the proposed preparation method of GO/MNP hybrids and their application in the detection of 6-mercaptopurine. | ||
2. Experimental
2.1 Chemicals and materials
All the chemicals were of analytical grade and used without further purification. Deionized distilled water (DI water) was used throughout. 6-Mercaptopurine, as a standard sample, was purchased from the National Institutes for Food and Drug Control. 6-Mercaptopurine tablets were purchased from Zhebei Pharmaceutical Co. Ltd. (Zhejiang, China). GO was supplied by XF Nano, INC. (Shanghai, China). Single-walled carbon nanotubes (SWCNTs) were supplied by Sigma, and multi-walled carbon nanotubes (MWCNTs) were supplied by Chengdu Organic Reagent Co. Ltd. (Chengdu, China). Polyethyleneimine (PEI) at molecular weight of 1.8 K was purchased from Aladdin. Other reagents including hydrogen tetrachloroaurate(III) tetrahydrate (HAuCl4·4H2O), silver nitrate (AgNO3), and reagents for selectivity experiments are commercially available. Britton–Robinson (BR) buffer (pH 2.0) was employed for acidity control.2.2 Characterization
Scanning electron microscopy (SEM) images were captured using an S-4800 scanning electron microscope (Hitachi, Japan). The absorption spectra were measured using a U-3010 UV-vis spectrophotometer (Hitachi, Japan). Transmission electron microscopy (TEM) images were captured using an S-4800 scanning electron microscope (Hitachi, Japan) equipped with a transmission accompaniment (HHTNT-539-9739, Hitachi). Zeta-potential measurements were performed using a Zetasizer Nano-ZS90 instrument (Malvern Inc). Raman spectra were recorded using a LabRam HR 800 spectrometer (HORIBA Jobin Yvon, France).2.3 Synthesis of GO and MNP hybrids
For preparation of the GO–PEI complex, a PEI solution (100 mg mL−1) was slowly added to a GO solution (1 mg mL−1) to make the weight ratio of GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEI 2
PEI 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under continuous stirring. Then the mixture was ultrasonicated for about 30 min, stirred overnight and washed 3 times with DI water by centrifugation and re-dispersed. For the procedure of assembling AgNPs or AuNPs to GO–PEI sheets, 25 μL GO–PEI aqueous solution was added to 1 mL AgNPs or AuNP solution and allowed to stand for several hours. The precipitate was washed several times and re-dispersed in 0.5 mL DI water.
1 under continuous stirring. Then the mixture was ultrasonicated for about 30 min, stirred overnight and washed 3 times with DI water by centrifugation and re-dispersed. For the procedure of assembling AgNPs or AuNPs to GO–PEI sheets, 25 μL GO–PEI aqueous solution was added to 1 mL AgNPs or AuNP solution and allowed to stand for several hours. The precipitate was washed several times and re-dispersed in 0.5 mL DI water.
The synthesis methods of 12 nm AgNPs and 13 nm AuNPs were based on the reported methods.11 For preparing AgNPs, 1 mL 50 mmol L−1 AgNO3 and 1 mL 5% (w/w) sodium citrate were added to 48 mL DI water under vigorous stirring. Then a small amount of NaBH4 solid was added to the mixture. Rapidly, the color of the solution changed to brown-yellow, indicating the formation of AgNPs. The AgNP aqueous solution was continuously stirred until the color no longer changed. Then, the AgNPs were centrifuged by 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min, the precipitate was removed and the supernatant was collected for further use. For the synthesis of AgNPs, 2 mL of 1% (w/w) HAuCl4·4H2O solution were added to 48 mL of DI water under stirring. The mixture was then heated to boiling under stirring, and 1 mL of 5% sodium citrate was added to the solution. Under continuous stirring and boiling, the mixture gradually changed to wine red color within 3 min. After boiling for another 20 min, the solution was cooled to room temperature under vigorous stirring.
000 rpm for 15 min, the precipitate was removed and the supernatant was collected for further use. For the synthesis of AgNPs, 2 mL of 1% (w/w) HAuCl4·4H2O solution were added to 48 mL of DI water under stirring. The mixture was then heated to boiling under stirring, and 1 mL of 5% sodium citrate was added to the solution. Under continuous stirring and boiling, the mixture gradually changed to wine red color within 3 min. After boiling for another 20 min, the solution was cooled to room temperature under vigorous stirring.
2.4 Procedure for SERS detection
For the determination of 6MP, 30 μL of the GO–AgNP aqueous suspension was mixed with 10 μL 6MP dispersed in BR buffer (pH 2.0) with the final concentrations of 2 μmol L−1, 4 μmol L−1, 6 μmol L−1, 8 μmol L−1, 10 μmol L−1, 12 μmol L−1. The mixture was allowed to stay for 1 hour to make the adsorption enough. Then, the samples in the solution phase were loaded into capillary tubes for SERS detection, with the laser beam focused on the center of a tube in both longitudinal and transverse directions. Laser wavelength: 532 nm; power: 28 mW; lens: 10× objective; acquisition time: 5 s. For enhancement factor detection, laser wavelength: 532 nm; power: 2.45 mW; lens: 50× objective; acquisition time: 5 s.2.5 6-Mercaptopurine tablet preparation
Ten tablets were weighed and ground into fine powder. The powder (50 mg) was transferred into a 100 mL measuring flask, and 50 mL 0.1 mol L−1 HCl solution was added. The mixture was heated to be dissolved, and then cooled to room temperature. HCl (0.1 mol L−1) was added to the mixture to the mark and filtered. An appropriate amount of filtrate was diluted to 100 times for UV-vis absorption determination.3. Results and discussion
3.1 Fabrication of carbon nanomaterials and MNP hybrids
Branched PEI molecules with positive charges, which are generally used in small molecules or gene delivery and release,17–19 can be directly adsorbed on the negatively charged carbon nanomaterials. In order to confirm the electrostatic assembly process occurring in the solution, zeta-potential measurements were performed before and after GO adsorption with PEI and MNPs. Fig. 1a showed that the average potential of pure GO solution was about −54.7 mV, indicating that numerous carboxyl groups and π electrons were scattered on the GO sheets. After assembly with PEI, the value sharply increased to +42.1 mV, which confirmed the effective attachment occurring between GO and PEI. PEI functionalized GO with a positive charge is a perfect building block for further decoration with negatively charged MNPs. After adding AgNPs to the GO–PEI mixture, the zeta-potential decreased to −35.6 mV, indicating that AgNPs indeed assembled onto the surface of GO through the mediation of PEI. Fig. 1b displayed the absorption spectra of GO/AgNPs and individual nanoparticles. Dispersive AgNPs have a characteristic absorption peak at 402 nm, and an 8 nm red shift occurred after GO/AgNP formation, which can be attributed to the distance between AgNPs that became smaller when the dispersive AgNPs were linked to GO sheets.12 The intensity of the absorption peak was reduced, also certifying that a small amount of AgNP aggregation occurred after AgNP adsorption onto the GO surface.12,20 | ||
| Fig. 1 Zeta-potential measurements of GO, GO–PEI and GO/AgNP hybrids in the aqueous solution (a); the UV-vis spectra of AgNPs and GO/AgNP hybrids (b); TEM pictures of GO/AgNPs (c) and (d). | ||
The morphologies of GO/AgNPs were further characterized by TEM imaging as shown in Fig. 1c and d. Although the GO sheets were as thin as a wafer and mostly transparent, the area and edge of the AgNP distribution led us to believe that the AgNPs indeed assembled on the GO surface. Some areas of GO/AgNPs showed a deep color, which was ascribed to the overlapping of loaded AgNPs. The results were also consistent with the red shift and reduction of the absorption peak. In addition, AgNPs were attached on the GO surface at a high density, confirming that the GO/AgNP hybrids had formed effectively. Similarly, gold nanoparticles (AuNPs) with negative charges could also connect with GO–PEI successfully. Fig. 2a and b showed that the zeta-potential and absorption spectra have a similar tendency to those of the AgNPs attached to GO–PEI. The TEM images of Fig. 2c and d also confirmed the successful assembly between AuNPs and GO.
 | ||
| Fig. 2 Zeta-potential measurements of GO, GO–PEI and GO/AuNP hybrids in the aqueous solution (a); the UV-vis spectra of AuNPs and GO/AuNP hybrids (b); TEM pictures of GO/AuNPs (c) and (d). | ||
To further test the generality of the self-assembly strategy, other carbon nanomaterials, including SWCNTs and MWCNTs, were employed to assemble with MNPs. Carboxylated SWCNTs and MWCNTs with high negative charges played a role similar to GO and could easily adsorb PEI molecules. As shown in Fig. 3a and b, MWCNTs have lengths of about 0.4–2 μm and diameters of 10–50 nm. AuNPs and AgNPs engaged in a compact layer around the MWCNTs, verifying the generality of the present assembly method. The result of SWCNTs/PEI reacting with metal nanoparticles was also shown in Fig. 3c and d, further confirming the versatility and efficiency of the present self-assembly strategy.
 | ||
| Fig. 3 SEM pictures of MWCNT/AgNPs (a), MWCNT/AuNPs (b), SWCNT/AgNPs (c) and SWCNT/AuNPs (d). The insets show the TEM images of the hybrid materials with high magnification. The scale bar = 50 nm. | ||
3.2 SERS property of the as-prepared GO/AgNP hybrids
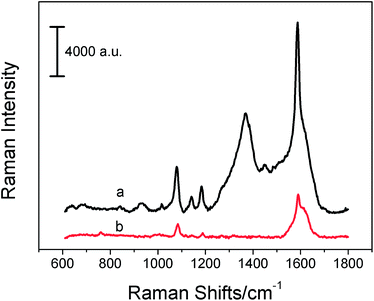
Because of the electromagnetic enhancement effect of AgNPs and AuNPs and the chemical enhancement effect of carbon nanomaterials, such nanocomposites can be potentially used as SERS substrates for sensitive analysis. To estimate the SERS activity of carbon nanomaterials and MNP hybrids, GO/AgNPs were chosen as typical substrates because enhancement factor (EF) calculations indicated a better enhancement effect for AgNPs than AuNPs.5 4-Mercaptobenzoic acid (pMBA) was used as a model Raman probe because it has been well characterized by SERS in the range of 610–1800 cm−1. Fig. 4 shows the characteristic Raman spectrum of solid pMBA and the SERS spectrum of pMBA at 5 × 10−7 mol L−1 in the presence of the GO/AgNP substrate. According to previous studies, the predominant bands were located at 1084, 1188, and 1590 cm−1, which were assigned to a1 modes of vCS, δCH, and vCC.21,22 In the SERS spectrum, the vCS band at 1084 cm−1 shifted to 1079 cm−1, due to Ag–S bond formation. The band δCH at 1188 cm−1 shifted to 1180 cm−1, and the vCC shifted from 1590 cm−1 to 1587 cm−1 after pMBA adsorption on the silver surface. To estimate the enhancement force of GO/AuNP hybrids for pMBA, we calculated the SERS EF values using the following equation:23,24| EF = (ISERSNbulk)/(IRamanNsurface) | (1) |
ISERS stands for the intensities of the vibrational mode of pMBA in the SERS spectra. IRaman stands for the same vibrational mode as ISERS in the normal Raman spectra of pMBA. Nbulk and Nsurface represent the number of pMBA molecules illuminated by the laser focus spot under normal Raman and SERS conditions. All of these values can be obtained from the SERS spectra and Raman spectra. Nsurface can be calculated according to the reported method.25 Supposing the molecules were uniformly dispersed on the substrates, the density of pMBA on the film was assumed to be 5 × 10−7 mol L−1 × 3 μL × NA/7.0 mm2 (the surface area of the substrate is 7.0 mm2), namely 1.3 × 1011 molecules per mm2.26 The laser spot has a 1 μm diameter and the surface area is about 7.9 × 10−7 mm−2, so Nsurface had a value of 1.0 × 105. Taking the laser spot (1 μm diameter), the penetration depth (about 2 μm), and the density of solid pMBA (1.06 g cm−3) into account,27 Nbulk had a value of 6.6 × 109 in the detected solid sample area. All the spectra were normalized for laser power and acquisition time. The EF at the band at 1079 cm−1 of pMBA can be calculated to be 1.5 × 105 for the GO/AgNP hybrids, and it is high enough to allow for ultrasensitive analytical detection.
3.3 GO/AgNPs as a SERS substrate for 6MP detection
As a chemotherapy drug, 6MP has been employed in the treatment of a variety of diseases for many years, including rheumatologic disorders, immune diseases, post-transplant immunosuppression, inflammatory bowel diseases, and lymphoblastic leukemia.28 However, some adverse effects are produced after taking more 6MP, including myelosuppression and bone-marrow suppression.29 Therefore, 6MP detection has biological significance. 6MP is a planar molecule that can attach on a metal surface with possible binding sites of N and S groups, and it has been proved that 6MP-modified gold nanoparticles exhibit enhanced drug delivery30 and can be applied in the biomedical field.30,31 A previous work has shown that 6MP can be efficiently adsorbed on AgNPs and exhibits fairly strong SERS signals.32 Therefore, we can construct a label-free and sensitive SERS method for determination of 6MP by using the assemblies of carbon nanomaterials and metal nanoparticles as substrates.As shown in Fig. 5, a series of SERS spectra of 5 μmol L−1 6MP on different substrates were acquired. It was found that in the liquid condition, the signal of 6MP could be present just with carbon nanomaterials and silver nanoparticle hybrids as substrates, owing to the stronger enhancement effect of AgNPs than AuNPs. Main vibrations of 6MP shown in the SERS spectrum were confirmed according to the reported work.33 The band at 1000 cm−1 is related to the Ag–S formation. The prominent feature of 6MP at 1280 cm−1 is assigned to the C–N stretching. The other prominent peaks of 6MP at 613, 681, 859, 1141, 1326, 1386, 1473, and 1585 cm−1 were also observed in the SERS spectra. In addition, it was found that the SERS intensity of 6MP in the presence of GO/AgNPs was higher than that in the presence of MWCNT/AgNP and SWCNT/AgNP hybrids; so GO/AgNPs were chosen as the SERS substrate for further 6MP detection.
 | ||
| Fig. 5 The SERS spectra of 5 μmol L−1 6MP on different substrates including GO/AuNPs (a), MWCNTs/AuNPs (b), SWCNTs/AuNPs (c); SWCNTs/AgNPs (d), MWCNTs/AgNPs (e), and GO/AgNPs (f). | ||
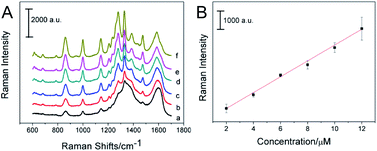
Choosing GO/AgNP hybrids as substrates, SERS spectra of the 6MP at a series of concentrations are shown in Fig. 6A. With increasing concentrations of 6MP, the intensity of the SERS spectra increased regularly, which means the Raman intensity is proportional to the number of 6MP molecules adsorbed on the GO/AgNPs. The SERS intensity of the vibration located at 1000 cm−1 was plotted as a function of 6MP concentration in Fig. 6B, which revealed a linear SERS response from 2 μmol L−1 to 12 μmol L−1 of 6MP (R2 = 0.977); the limit of detection (LOD) was calculated to be 1.05 μmol L−1. Considering that the as-prepared nanocomposites have a high enhancement effect, they can be used as potential SERS sensors to detect other pharmaceutical drugs.
To assess the selectivity of this SERS system for 6MP detection, some common potentially interfering substances, including metal ions (Na+, K+, Mg2+), carbohydrates (glucose, lactose, sucrose), amino acids (lysine, histidine), and purine analogues (ATP, GTP), were investigated. It was seen from Fig. 7 that none of these substances caused obvious Raman signal alteration of SERS spectra of 6MP. Even though these substances were coexistent with 6MP in solution, the SERS intensity of 6MP changed weakly, which verified the good selectivity of this method. It should be noted that a little stronger SERS signal was produced after adding 25 μmol L−1 of L-histidine to the GO/AgNP hybrids because it has its own SERS peak near 1000 cm−1. While L-histidine was coexistent with 6MP for SERS determination, no obvious alteration was observed relative to individual 6MP. Namely, L-histidine has little effect on the Raman signal of 6MP, which might be owing to the stronger combination between 6MP with AgNPs than L-histidine.
Finally, determination of 6MP in pharmaceutical tablets was performed by this method. The determination carried out by the SERS method was compared with the standard UV-method. The experimental results in Table 1 showed that there was no significant difference between the two methods. In order to evaluate whether or not the SERS method was valid in the determination, recovery studies of three concentrations with known amounts of 6MP were carried out on the tablet samples. It was found that the recoveries of these samples are between 92% and 114% (n = 3) (Table 2). These results proved that GO/AgNPs are appropriate SERS substrates for determination of 6MP in tablets.
| Normal | UV/method | RSD n = 3 (%) | SERS method | RSD n = 3 (%) |
|---|---|---|---|---|
| 50 mg | 53.3 mg | 4.4 | 48.4 mg | 2.5 |
| Sample | Add (μmol L−1) | Found (μmol L−1) | Recovery (%) | RSD (n = 3) |
|---|---|---|---|---|
| Tablet | 5.7 | 5.5 ± 0.2 | 92–99 | 3.5 |
| 7.7 | 8.1 ± 0.9 | 92–114 | 10.8 | |
| 9.7 | 9.6 ± 0.3 | 96–101 | 3.5 |
4. Conclusion
In conclusion, we have developed a new strategy to fabricate GO and MNP hybrids with the use of PEI as a linker molecule, and the as-prepared hybrids were successfully applied for 6MP detection. The assembly procedure is through an electrostatic assembly without any chemical modification, and the PEI linker is commercial without any organic synthesis reactions. Therefore, the assembly method is very simple. In addition, this method has been successfully applied for the assemblies of CNTs and MNPs. Thus, it has general uses and can be applied for the construction of other nano-hybrids. Using the obtained GO/AgNP nanocomposites as SERS substrates, a label-free, sensitive and selective method to detect 6MP in pharmaceutical tablets has been demonstrated. This detection method can be easily applied for other target detections, such as pharmaceuticals, small biological molecules and environmental pollutants, by using their own special Raman scattering as detection signals.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, no. 21035005, 21305113), the fund of State Key Laboratory of Electroanalytical Chemistry (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences) (SKLEAC201312), the fund of Chongqing Fundamental and Advanced Research Project (cstc2013jcyjA50008), the Fundamental Research Funds for the Central Universities (XDJK2012C057), the Research Fund for the Doctor Program of Southwest University (swu111077) and the special fund of Chongqing key laboratory (CSTC).Notes and references
- C.-C. Chang, K.-H. Yang, Y.-C. Liu, T.-C. Hsu and F.-D. Mai, ACS Appl. Mater. Interfaces, 2012, 4, 4700–4707 CAS.
- X. Ling, L. Xie, Y. Fang, H. Xu, H. Zhang, J. Kong, M. S. Dresselhaus, J. Zhang and Z. Liu, Nano Lett., 2010, 10, 553–561 CrossRef CAS PubMed.
- Z. Liu, S. Tabakman, S. Sherlocx, X. Li, Z. Chen, K. Jiang, S. Fan and H. Dai, Nano Res., 2010, 3, 222–233 CrossRef CAS PubMed.
- K. Zimny, T. Roques-Carmes, C. Carteret, M. J. Stébé and J. L. Blin, J. Phys. Chem. C, 2012, 116, 6585–6594 CAS.
- W. Ren, Y. Fang and E. Wang, ACS Nano, 2011, 5, 6425–6433 CrossRef CAS PubMed.
- M. Liu and W. Chen, Biosens. Bioelectron., 2013, 46, 68–73 CrossRef CAS PubMed.
- J. Huang, C. Zong, H. Shen, M. Liu, B. Chen, B. Ren and Z. Zhang, Small, 2012, 8, 2577–2584 CrossRef CAS PubMed.
- X. Wang, C. Wang, L. Cheng, S.-T. Lee and Z. Liu, J. Am. Chem. Soc., 2012, 134, 7414–7422 CrossRef CAS PubMed.
- C. Hu, J. Rong, J. Cui, Y. Yang, L. Yang, Y. Wang and Y. Liu, Carbon, 2013, 51, 255–264 CrossRef CAS PubMed.
- J. Liu, S. Fu, B. Yuan, Y. Li and Z. Deng, J. Am. Chem. Soc., 2010, 132, 7279–7281 CrossRef CAS PubMed.
- Y. Wang, S. J. Zhen, Y. Zhang, Y. F. Li and C. Z. Huang, J. Phys. Chem. C, 2011, 115, 12815–12821 CAS.
- L. Zhang, S. J. Zhen, Y. Sang, J. Y. Li, Y. Wang, L. Zhan, L. Peng, J. Wang, Y. F. Li and C. Z. Huang, Chem. Commun., 2010, 46, 4303–4305 RSC.
- F. A. He, J. T. Fan, F. Song, L. M. Zhang and H. L.-W. Chan, Nanoscale, 2011, 3, 1182–1188 RSC.
- X. Huang, X. Zhou, S. Wu, Y. Wei, X. Qi, J. Zhang, F. Boey and H. Zhang, Small, 2010, 6, 513–516 CrossRef CAS PubMed.
- X. Zhou, Z. Chen, D. Yan and H. Lu, J. Mater. Chem., 2012, 22, 13506–13516 RSC.
- J. Xiang and L. T. Drzal, ACS Appl. Mater. Interfaces, 2011, 3, 1325–1332 CAS.
- L. Feng, S. Zhang and Z. Liu, Nanoscale, 2011, 3, 1252–1257 RSC.
- X. Yang, G. Niu, X. Cao, Y. Wen, R. Xiang, H. Duan and Y. Chen, J. Mater. Chem., 2012, 22, 6649–6654 RSC.
- E. Dillon, M. S. Bhutani and A. R. Barron, J. Mater. Chem. B, 2013, 1, 1461–1465 RSC.
- S.-J. Li, Y. F. Shi, L. Liu, L.-X. Song, H. Pamg and J.-M. Du, Electrochim. Acta, 2012, 85, 628–635 CrossRef CAS PubMed.
- E.-Z. Tan, P.-G. Yin, T.-T. You, H. Wang and L. Guo, ACS Appl. Mater. Interfaces, 2012, 4, 3432–3437 CAS.
- K. L. Nagashree, R. Lavanya, C. Kavitha, N. S. V. Narayanan and S. Sampath, RSC Adv., 2013, 3, 8356–8364 RSC.
- X. Liu, L. Cao, W. Song, K. Ai and L. Lu, ACS Appl. Mater. Interfaces, 2011, 3, 2944–2952 CAS.
- R. Li, C. Han and Q.-W. Chen, RSC Adv., 2013, 3, 11715–11722 RSC.
- W. L. Fu, S. J. Zhen and C. Z. Huang, Analyst, 2013, 138, 3075–3081 RSC.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261–3266 CrossRef CAS PubMed.
- A. Kamińska, I. Dzięcielewski, J. L. Weyher, J. Waluk, S. Gawinkowski, V. Sashuk, M. Fiałkowski, M. Sawicka, T. Suski, S. Porowski and R. Hołyst, J. Mater. Chem., 2011, 21, 8662–8669 RSC.
- F. Alvarez, C. A. Grillo, P. L. Schilardi, A. Rubert, G. Benítez, C. Lorente and M. F. L. d. Mele, ACS Appl. Mater. Interfaces, 2013, 5, 249–255 CAS.
- J. Du, Y. Wang and W. Zhang, Chem.–Eur. J., 2012, 18, 8540–8546 CrossRef CAS PubMed.
- K. Ock, W. I. Jeon, E. O. Ganbold, M. Kim, J. Park, J. H. Seo, K. Cho, S.-W. Joo and S. Y. Lee, Anal. Chem., 2012, 84, 2172–2178 CrossRef CAS PubMed.
- J. Yang, Y. Cui, S. Zong, R. Zhang, C. Song and Z. Wang, Mol. Pharmaceutics, 2012, 9, 842–849 CrossRef CAS PubMed.
- H. Chu, H. Yang, S. Huan, G. Shen and R. Yu, J. Phys. Chem. B, 2006, 110, 5490–5497 CrossRef CAS PubMed.
- H. Yang, Y. Liu, Z. Liu, Y. Yang, J. Jiang, Z. Zhang, G. Shen and R. Yu, J. Phys. Chem. B, 2005, 109, 2739–2744 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |