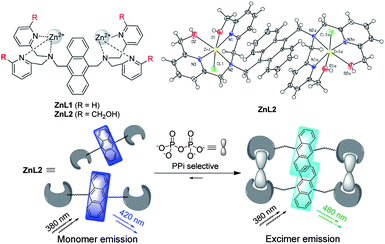
Highly selective and controllable pyrophosphate induced anthracene-excimer formation in water†
Feihu
Huang
and
Guoqiang
Feng
*
Key Laboratory of Pesticide and Chemical Biology of Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan 430079, P. R. China. E-mail: gf256@mail.ccnu.edu.cn
First published on 24th October 2013
Abstract
Introducing four –CH2OH groups to an anthracene bridged Zn–DPA complex was found to result in a new highly selective and controllable excimer-based fluorescent sensor for pyrophosphate in water.
The development of sensors for selective recognition and detection of phosphate anion derivatives is an important area of research due to their biological significance.1 Among them, pyrophosphate (PPi) is involved in many vital metabolic and bioenergetic processes such as DNA and RNA polymerization reactions and ATP hydrolysis,2 and its selective detection by small molecular probes has become a major research focus in the past decade. So far, many chemosensors for PPi have been developed, with fluorescent sensors being most attractive.3,4 In general, studies showed that sensors based on metal complexes especially zinc(II)–dipicolylamine (Zn–DPA) complexes5 are most efficient for PPi sensing in water. However, the discrimination of PPi from other phosphate derivatives, particularly ATP was often found difficult, as only a few sensors showed high selectivity for PPi over ATP.3
Sensors based on excimer formation for PPi are very attractive due to the considerably different wavelengths of the monomer and the excimer emissions.6 For example, the naphthalenetetracarboxdiimide-excimer based system reported by Yoon et al. can be considered as a potential fluorescent sensor for PPi as it shows high selectivity for PPi over ATP.6a However, this kind of sensor for PPi sensing in water is rather rare.6,7 The pyrene-excimer system reported by Hong et al. showed good sensing ability for PPi, but this system also showed considerable excimer emission in the presence of small amount of ATP.6b Although the formation of anthracene-excimer has also been often used in the design of chemosensors for anions, so far, anthracene-excimer based fluorescent sensor for PPi has not yet been reported.8
We are interested in PPi sensing9 and in introducing small functional groups to Zn–DPA complexes to enhance their properties.10 Recently, we found that both selectivity and sensitivity can be improved by introducing hydrogen bond donor amino or acetamido groups to a dinuclear Zn–DPA complex for PPi sensing.11 Herein, we report that the effect of introducing –CH2OH groups to enhance the selectivity of an anthracene bridged Zn–DPA complex for PPi sensing can be also dramatic. ZnL1 (Scheme 1) was selected in this study as the object of comparison, because this complex has been reported to show a selective response for phosphate derivatives over other various anions in aqueous solution, but not selective enough to well discriminate for a specific phosphate species among the phosphate family.12 After introducing four –CH2OH groups to ZnL1, we obtained a new complex ZnL2 (Scheme 1) and found that its sensing property was very different to that of ZnL1. Interestingly, ZnL2 was found to show a high selectivity for PPi over many other anions including ATP by formation of a unique anthracene-excimer in water (Scheme 1). Moreover, this excimer formation was found highly concentration and temperature dependent. Thus, our study provided a new highly selective and controllable anthracene-excimer based sensor for PPi.
ZnL2 can be readily prepared from its corresponding ligand L2 and zinc nitrate in aqueous methanol solution. The X-ray crystal structure of ZnL2 in Scheme 1 clearly shows that it is a dinuclear zinc complex. Detailed synthetic procedures and structure characterizations are given in the ESI.†
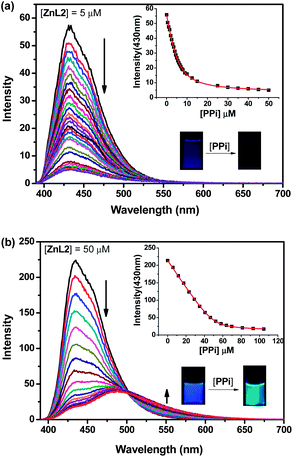
The sensing ability of ZnL2 was first evaluated by measuring its fluorescence intensity changes upon addition of various anions in 10 mM HEPES buffer (pH = 7.2, HEPES = 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid) at 25 °C. Since ZnL2 gives strong fluorescence at 430 nm (Φ = 0.31, relative to 5 μM quinine sulphate in 1 N H2SO4), 5 μM concentration of ZnL2 was first used. As shown in Fig. 1a and Fig. S2–S3 (ESI†), upon addition of PPi, ATP and citrate, the fluorescence of ZnL2 gradually quenched. This quenching effect may reflect an enhancement of photo-induced electron transfer (PET) from the amine to the adjacent anthracene upon these anions bind to ZnL2.13 In contrast, addition of ADP caused very small changes to the fluorescence of ZnL2 and addition of AMP caused a slightly intensified fluorescence to ZnL2, while the effects of addition of other common anions such as PO43−, HPO42−, H2PO4−, F−, Cl−, Br−, I−, ClO4−, AcO−, CO32−, HCO3−, SO42−, HSO4−, NO3−, N3−, NO2−, SCN−, S2O32−, S2O72− to the fluorescence of ZnL2 were found almost negligible even they were added an excess amount of more than 100 equivalents (Fig. S4–S6, ESI†). These initial experiments indicate that ZnL2 is most responsive to PPi, ATP and citrate, which is quite different to the reported sensing property of ZnL1.12
Surprisingly, when ZnL2 was used at a concentration of 50 μM instead, it showed an unexpected high selectivity for PPi over these tested anions, as a unique response of ZnL2 to PPi with appearance of an anthracene-excimer emission was observed. As shown in Fig. 1b, addition of PPi (0–105 μM) to 50 μM ZnL2 in 10 mM HEPES buffer (pH = 7.2) at 25 °C gradually quenched the fluorescence of ZnL2 at 430 nm, meanwhile a new emission peak at 480 nm appeared. This new emission peak is corresponding to the formation of an anthracene-excimer.8 As a result, the blue fluorescence of ZnL2 changed to blue-green after PPi addition, which is highly naked-eye observable under a 365 nm UV light (Fig 1b, inserted). In contrast, no excimer emissions were found to appear when other anions including ATP and citrate were added to the 50 μM ZnL2 solution under the same conditions (Fig. S7–S11, ESI†). These results clearly show that ZnL2 became highly selective for PPi over these anions including ATP when it was used at 50 μM concentration. The selectivity can be well illustrated with a plot of the emission ratiometric responses of ZnL2 between 550 and 430 nm (I550/I430) against various anions shown in Fig. 2 and the emission colour changes of ZnL2 upon addition of these anions shown in Fig. S12 (ESI†).
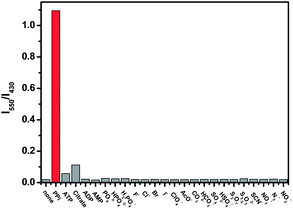 | ||
| Fig. 2 A plot of the emission ratiometric responses of ZnL2 (50 μM) between 550 and 430 nm (I550/I430) against different anions (10 equiv.). λex = 380 nm, dem = dex = 2.5 nm. | ||
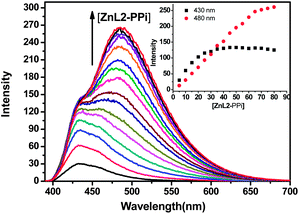
Combining the above results, it is likely that the PPi induced formation of anthracene-excimer is concentration dependent. Thus, different concentration of ZnL2 and PPi were mixed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and the fluorescence of the resulting solutions were measured at 25 °C. As shown in Fig. 3, as the concentration of ZnL2–PPi increased from 5 μM to 80 μM, the dominant fluorescent wavelength of the solution gradually shifted from 430 nm to 480 nm, concomitant with the emission colors of the solution gradually changed from blue to blue-green (Fig. S13, ESI†). These experiments strongly indicate that the excimer formation is highly dependent on the concentration of ZnL2–PPi and is favourable at higher concentrations. Plotting of the fluorescence intensity changes as a function of the concentration of ZnL2–PPi show that the intensity at 430 nm reached a plateau at 40 μM of ZnL2–PPi in the solution (Fig. 3, inserted), which maybe the critical concentration of ZnL2–PPi to dominantly form the anthracene-excimer species under the test conditions. In addition, we also found that the PPi induced anthracene-excimer formation become more difficult when the buffer concentration is increased to 100 mM, and is tending to be hindered when high concentration of salts (NaNO3 was used) are present in the test buffer solution (Fig. S14 and S15, ESI†).
1 ratio, and the fluorescence of the resulting solutions were measured at 25 °C. As shown in Fig. 3, as the concentration of ZnL2–PPi increased from 5 μM to 80 μM, the dominant fluorescent wavelength of the solution gradually shifted from 430 nm to 480 nm, concomitant with the emission colors of the solution gradually changed from blue to blue-green (Fig. S13, ESI†). These experiments strongly indicate that the excimer formation is highly dependent on the concentration of ZnL2–PPi and is favourable at higher concentrations. Plotting of the fluorescence intensity changes as a function of the concentration of ZnL2–PPi show that the intensity at 430 nm reached a plateau at 40 μM of ZnL2–PPi in the solution (Fig. 3, inserted), which maybe the critical concentration of ZnL2–PPi to dominantly form the anthracene-excimer species under the test conditions. In addition, we also found that the PPi induced anthracene-excimer formation become more difficult when the buffer concentration is increased to 100 mM, and is tending to be hindered when high concentration of salts (NaNO3 was used) are present in the test buffer solution (Fig. S14 and S15, ESI†).
The effect of the temperature on the fluorescence of the ZnL2–PPi solution (50 μM) was also investigated, and it was found that the formation of this PPi induced anthracene-excimer is also highly temperature dependent. As shown in Fig. 4, at 20 °C, the major emission band of the ZnL2–PPi solution is centred at 480 nm of the excimer emissions. However, upon the temperature of the test solution gradually increased from 20 °C to 90 °C, the emission of the anthracene-excimer at 480 nm gradually decreased and shifted to the anthracene monomer emission at 430 nm, accompanied by a visual emission color change from blue-green to blue under a UV light. This process was found fully reversible when the temperature of the test solution was gradually reduced back to 20 °C (Fig. S16, ESI†). Therefore, the PPi induced anthracene-excimer formation in this study is also controllable by temperature and low temperature is favourable for its formation.
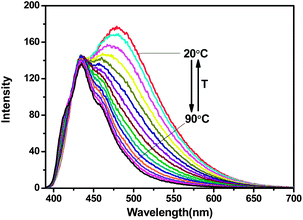 | ||
| Fig. 4 The effect of temperature on the fluorescence of ZnL2–PPi (50 μM) in 10 mM HEPES buffer solution (pH = 7.2). λex = 380 nm, dex = 5 nm, dem = 2.5 nm. | ||
To shed further light on this sensing behaviour, the binding patterns of ZnL2 with PPi was investigated by Job's plot, 31P NMR and Mass analysis. Job's plot showed that ZnL2 binds PPi in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. S17, ESI†). Nonlinear least-squares fitting the fluorescent titration curve in Fig. 1 gave the apparent association constants (Ka) between ZnL2 and PPi to be about 5.39 × 105 M−1 (R2 > 0.999, Fig. 1a) and 7.68 × 105 M−1 (R2 > 0.999, Fig. 1b) when ZnL2 was used at 5 μM and 50 μM, respectively, which are fairly comparable.1431P NMR studies showed that the P signal of PPi in the presence of ZnL2 slightly shifted, but still appeared only one peak, indicating the resulting complex is symmetrical (Fig. S18, ESI†). Mass analysis of the ZnL2–PPi solution supports the excimer formation and suggests formation of a 2 + 2 complex.6a As shown in Fig. S19†, the peak at m/z = 1023 corresponds to [C88H90N12O22P4Zn4]2+ (= [2ZnL2 + 2PPi]2+), which is confirmed by the isotopic distribution calculations and further proved by high resolution mass analysis (Fig. S20, ESI†). Therefore, the PPi selective sensing process by ZnL2 is most likely happened as shown in Scheme 1, while other anions could not induce the formation of such excimer.
1 stoichiometry (Fig. S17, ESI†). Nonlinear least-squares fitting the fluorescent titration curve in Fig. 1 gave the apparent association constants (Ka) between ZnL2 and PPi to be about 5.39 × 105 M−1 (R2 > 0.999, Fig. 1a) and 7.68 × 105 M−1 (R2 > 0.999, Fig. 1b) when ZnL2 was used at 5 μM and 50 μM, respectively, which are fairly comparable.1431P NMR studies showed that the P signal of PPi in the presence of ZnL2 slightly shifted, but still appeared only one peak, indicating the resulting complex is symmetrical (Fig. S18, ESI†). Mass analysis of the ZnL2–PPi solution supports the excimer formation and suggests formation of a 2 + 2 complex.6a As shown in Fig. S19†, the peak at m/z = 1023 corresponds to [C88H90N12O22P4Zn4]2+ (= [2ZnL2 + 2PPi]2+), which is confirmed by the isotopic distribution calculations and further proved by high resolution mass analysis (Fig. S20, ESI†). Therefore, the PPi selective sensing process by ZnL2 is most likely happened as shown in Scheme 1, while other anions could not induce the formation of such excimer.
In summary, we found that introducing four –CH2OH groups to an anthracene bridged dinuclear Zn–DPA complex could change its sensing property dramatically. By this approach, a new complex ZnL2 was found to show high selectivity for PPi over ATP and other common anions via formation of a unique and controllable anthracene-excimer towards addition of PPi in neutral aqueous solution. This study may imply a simple but effective method to improve a sensor's sensing property. Further studies to explore the effect of introducing other functional groups to the DPA unit are under progress in our laboratory.
Acknowledgements
We thank the National Natural Science Foundation of China (Grants no. 21172086, 20902033 and 21032001) for financial support. This work was also sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.Notes and references
- A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603 CrossRef CAS PubMed.
- J. K. Heinonen, Biological Role of Inorganic Pyrophosphate, Kluwer Academic Publishers, Norwell, 2001 Search PubMed.
- S. K. Kim, D. H. Lee, J.-I. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23 CrossRef CAS PubMed.
- Some recent sensors for PPi: (a) W. Zhu, X. Huang, Z. Guo, X. Wu, H. Yu and H. Tian, Chem. Commun., 2012, 48, 1784 RSC; (b) X. Liu, H. T. Ngo, Z. Ge, S. J. Butler and K. A. Jolliffe, Chem. Sci., 2013, 4, 1680 RSC; (c) X. Su, C. Zhang, X. Xiao, A. Xu, Z. Xu and M. Zhao, Chem. Commun., 2013, 49, 798 RSC.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928 RSC.
- (a) H. N. Lee, Z. Xu, S. K. Kim, K. M. K. Swamy, Y. Kim, S.-J. Kim and J. Yoon, J. Am. Chem. Soc., 2007, 129, 3828 CrossRef CAS PubMed; (b) H. K. Cho, D. H. Lee and J.-I. Hong, Chem. Commun., 2005, 1690 RSC; (c) F. Schmidt, S. Stadlbauer and B. König, Dalton Trans., 2010, 39, 7250 RSC; (d) N. Ahmed, B. Shirinfar, I. S. Youn, A. Bist, V. Suresh and K. S. Kim, Chem. Commun., 2012, 48, 2662 RSC.
- An earlier system can be found, but this system was examined only in methanol, see: S. Nishizawa, Y. Kato and N. Teramae, J. Am. Chem. Soc., 1999, 121, 9463 CrossRef CAS.
- Anthracene-excimer was used to sense other phosphate species, examples see: (a) D. Zhang, X. Jiang, H. Yang, Z. Su, E. Gao, A. Martinez and G. Gao, Chem. Commun., 2013, 49, 6149 RSC; (b) K.-H. Chen, J.-S. Yang, C.-Y. Hwang and J.-M. Fang, Org. Lett., 2008, 10, 4401 CrossRef CAS PubMed.
- S. Yang, G. Feng and N. H. Williams, Org. Biomol. Chem., 2012, 10, 5606–5612 CAS.
- (a) G. Feng, D. Natale, R. Prabaharan, J. C. Mareque-Rivas and N. H. Williams, Angew. Chem., Int. Ed., 2006, 45, 7056 CrossRef CAS PubMed; (b) G. Feng, J. C. Mareque-Rivas and N. H. Williams, Chem. Commun., 2006, 1845 RSC; (c) G. Feng, J. C. Mareque-Rivas, R. T. M. de Rosales and N. H. Williams, J. Am. Chem. Soc., 2005, 127, 13470 CrossRef CAS PubMed.
- F. Huang and G. Feng, J. Org. Chem., 2012, 77, 11405 CrossRef CAS PubMed.
- (a) A. Ojida, Y. Mito-oka, K. Sada and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 2454 CrossRef CAS PubMed; (b) A. Ojida, Y. Mito-oka, M.-a. Inoue and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 6256 CrossRef CAS PubMed; (c) T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 141 RSC.
- L. G. Pathberiya, N. Barlow, T. Nguyen, B. Graham and K. L. Tuck, Tetrahedron, 2012, 68, 9435 CrossRef CAS.
- K a for ATP and citrate can be found in the ESI, see Fig. S2–S3 and Fig. S7–S8. A comparison of Ka for ZnL1 and ZnL2 to several anions can be found in Table S1 in the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: synthesis of ZnL2 and additional spectra. CCDC 949746. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra45681a |
| This journal is © The Royal Society of Chemistry 2014 |