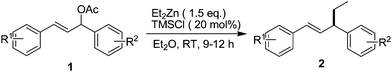Chlorotrimethylsilane (TMSCl): an efficient silicon-based Lewis acid mediator in allylic alkylation using a diethylzinc reagent†
Wen-Hui
Deng
,
Fei
Ye
,
Xing-Feng
Bai
,
Li
Li
*,
Tao
Song
,
Yun-Long
Wei
and
Li-Wen
Xu
*
Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, College of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou 311121, P. R. China. E-mail: liwenxu@hznu.edu.cn; licpxulw@yahoo.com; Fax: +86 2886 5135; Tel: +86 2886 8915
First published on 5th November 2013
Abstract
Chlorotrimethylsilane (TMSCl) was a highly efficient catalyst in the allylic alkylation of 1,3-diaryl-2-propenyl acetates with a diethylzinc reagent, in which various 1,3-diaryl-2-propenyl acetates are efficiently transferred to 1,3-diarylpent-1-enes in good to excellent yields.
Organosilicon compounds present key structural features in a plethora of non-naturally occurring molecules, and they are important reagents, catalysts, or substrates in organic synthesis.1 Especially in the past few decades, silicon-based Lewis acid has occupied an important position in catalysis and tremendous progress has been achieved in the application of silicon-based Lewis acids in organic synthesis.2 The behavior of these silicon-based Lewis acids were suggested primarily by the tendency of the silicon atom to expand its valence shell, giving rise to five- or six-coordinate intermediates, which allows one to consider organosilicon compounds as Lewis acids to promote organic transformations.3 Furthermore, silicon-based Lewis acids offer some advantages over traditional metal-based Lewis acid and Brønsted acid. For example, silicon-based Lewis acids are compatible with many synthetically valuable C-nucleophiles and heteroatoms, such as silyl enol ethers, indium or cuprates-based organometallic reagents, Grignard reagents, phosphines, and carbamates.4 Among many commercial available silicon-based Lewis acids, chlorotrimethylsilane (TMSCl) is one of the most useful reagents or activators in catalytic organic reactions, such as conjugate addition,5 multi-component reactions,6 Pictet–Spengler reaction,7 aza-Michael additions,8 etherification of aldehydes,9 cross-aldol condensation,10 nucleophilic addition,11 Friedel–Crafts reaction,12 and other transformations.13 However, its direct use as a silicon-based Lewis acid catalyst in organic reactions is rarely reported.14 As an extension of our previous studies on TMSCl-mediated and -catalyzed organic transformations, herein we reported a mild and convenient procedure for TMSCl-catalyzed allylic alkylation of 1,3-diaryl-2-propenyl acetate with diethylzinc reagent.
In recent years, catalytic allylic alkylation of organometallic reagents has received much attention and several methods have been developed for regioselective allylation of organolithium compounds, alkyl Grignard and alkylzinc reagents under copper catalysis or stoichiometric cuprates derived from these organometallic reagents.15–19 Nevertheless, the scope of allylic substrates providing the desired carbon–carbon bond formation remains limited to allyl halides and allylic picolinates as well as the use of metal catalysts in most cases. In addition, in many cases an excess of organometallic reagent as well as stoichiometric copper salt is required, which is particularly undesirable from the standpoint of modern organic synthesis.
Although the copper-catalyzed asymmetric allylic alkylations with organometallic reagents are known,16–19 it seemed that an allylic substitution of 1,3-diphenylallyl acetate with organozinc reagent as well as the synthesis of 1,3-diarylpent-1-enes could be developed. Interestingly, during our work on the development of catalytic allylic alkylation of organozinc reagent, we observed unexpectedly that chlorotrimethylsilane (TMSCl) was a highly efficient catalyst in the allylic alkylation of 1,3-diaryl-2-propenyl acetates with diethylzinc reagent. To the best of our knowledge, the subject of catalytic ability of TMSCl in copper-free allylic alkylation has not been reported to date.
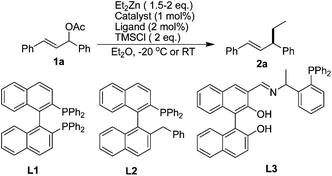
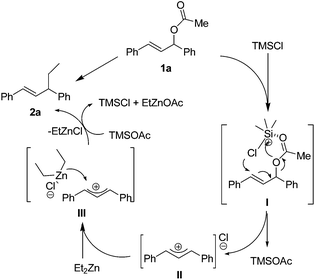
Initially, we commenced the investigation by testing the copper-catalyzed allylic substitution of 1,3-diphenylallyl acetate with organozinc reagent (Et2Zn) in the presence of chiral phosphine ligands. Beside famous BINAP (2,2′-bis(diphenylphophino)-1,1′-binaphthyl), a C2-axially chiral monophosphine L2 {Ph-NNP: (1-(2-benzylnaphthalen-1-yl)naphthalen-2-yl)diphenylphosphine} and L3 prepared from Ar-BINMOL,20 have been brought to light after their successful application in asymmetric catalysis. As illustrated in Table 1, unfortunately, the copper catalyst system derived from Cu(OTf)2 and BINAP almost has no activity for the allylic substitution (Entry 1 of Table 1, <5% yield). It should be mentioned that allylic alkylation of 1,3-diaryl-2-propenyl acetates with diethylzinc reagent was not occurred under catalyst-free reaction. Despite these negative results, we decided to investigate the effect of additive on the catalytic allylic alkylation of 1,3-diphenylallyl acetate with diethylzinc reagent. Inspired by Alexakis's copper catalysis in the allylic alkylation of allylic chloride and allylic bromide,17 we hypothesized that the addition of chlorotrimethylsilane (TMSCl) would be beneficial to the in situ formation 3-chloro-1,3-diphenylprop-1-ene and then accelerated the allylic alkylation of Et2Zn due to the cooperative catalytic activity of Cu(OTf)2 and TMSCl. As shown in Scheme 1, in our preliminary hypothesis, 1,3-diphenylallyl acetate could be converted to high reactive 3-chloro-1,3-diphenylprop-1-ene in the presence of TMSCl under the catalytic reaction conditions,21 which would led to the formation of key π-allylcopper intermediate (IV) after the oxidative addition of copper source.22 Meanwhile, it has been revealed that TMSCl could activated Cu(OTf)2 and other Lewis acid catalysts in many organic transformations.5–13
| Entry | Cat. | Ligand | TMSCl (eq.) | Temperature | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 mol% of transition metal catalyst, 1 mmol of 1,3-diphenylallyl acetate (1a), Et2Zn (1.0 M solution in hexane; 2.0 equiv. for entries 1–8 and 1.5 equiv. for 9–14), 2.0 equiv. TMSCl, and 2 mol% of racemic ligand or chiral ligand (L1–L3), at −20 °C. b Isolated yields after flash column chromatography. c The reaction was performed in THF. d The reaction was performed in DCM. e The reaction was performed in toluene. | ||||||
| 1 | Cu(OTf)2 | L1 | 0 | −20 °C | 36 | <5 |
| 2 | Cu(OTf)2 | L1 | 2.0 | −20 °C | 10 | 80 |
| 3 | Cu(OTf)2 | L2 | 2.0 | −20 °C | 10 | 90 |
| 4 | Cu(OTf)2 | L3 | 2.0 | −20 °C | 18 | >99 |
| 5 | Cu(OTf)2 | L3 | 0 | −20 °C | 36 | <5 |
| 6 | [Pd(η3-C3H3)Cl]2 | L3 | 2.0 | −20 °C | 10 | 40 |
| 7 | Ni(acac)2 | L3 | 2.0 | −20 °C | 10 | 60 |
| 8 | — | — | 2.0 | −20 °C | 12 | >99 |
| 9 | — | — | 1.0 | RT | 4 | 94 |
| 10 | — | — | 0.5 | RT | 5 | 95 |
| 11 | — | — | 0.2 | RT | 9 | 96 |
| 12 | — | — | 0.2 | RT | 12 | 50c |
| 13 | — | — | 0.2 | RT | 12 | 30d |
| 14 | — | — | 0.2 | RT | 12 | <5e |
 | ||
| Scheme 1 The hypothesis of TMSCl-mediated copper-catalyzed allylic substitution of organometallic reagents (M-R): double roles of TMSCl as reactive reagent and silicon-based Lewis acid catalyst. | ||
Fortunately, when 2.0 equiv. of TMSCl was added to the reaction mixture using Cu(OTf)2 as catalyst, the allylic alkylation proceeded smoothly and the yield of desired product 2a was dramatically improved to 80% (Entry 2). However, when chiral (R)-BINAP was used as ligand, there was no enantioselectivity. Next, we examined our ligands that designed and prepared recently20 in this copper-catalyzed allylic alkylation of 1,3-diphenylallyl acetate with organozinc reagent (Et2Zn) in the presence of 2.0 equiv. of TMSCl. As expectedly, the reaction proceeded smoothly to give the desired 2a in higher yield (Entry 3 and 4). Nevertheless, the enantioselectivity was not detected under both the reaction conditions. Notably, the absence of TMSCl was also led to no reaction when ligand L3 was used in this case (Entry 5). Comparably and interestingly, we found that both the palladium and nickel catalysts gave inferior activity in this reaction. More meaningfully, the allylic alkylation of 1,3-diphenylallyl acetate with organozinc reagent (Et2Zn) proceeded better in the presence of TMSCl without any catalysts and ligands (Entry 8). These reaction results showed that these transition metal catalysts and chiral ligands disturbed the interaction between TMSCl and substrates to some extent. Further evaluations of the amount of TMSCl highlighted the crucial role of this silicon-based Lewis acid in achieving high yield with 1.5 equiv. Et2Zn. As shown in Table 1 (Entries 9–11), the use of 20 mol% of TMSCl as catalyst or mediator gave rise to excellent yield in 9 hours at room temperature (Entry 11). By changing the diethylether (Et2O) to other solvents (Entries 12–14), such as THF, DCM, or toluene, we observed an obvious decrease of yield. Especially, TMSCl did not promote this reaction in toluene (Entry 14). Notably, a longer time was needed when 10 mol% of TMSCl was used in this reaction, and some unexpected and unidentified byproducts were detected in this case.
| Entry | R1 | R2 | Yield (%)b |
|---|---|---|---|
a
Reaction conditions: 1 mmol of 1,3-diarylallyl acetates (1), 1.5 equiv. Et2Zn (1.0 M solution in hexane), and 20 mol% of TMSCl, in Et2O, at room temperature.
b Isolated yields after flash column chromatography.
c The total yield of two isomers. It was difficult to be separated by flash column chromatography. The ratio of two isomers is almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1.3 1–1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||
| 1 | H | H | 2a: 96 |
| 2 | p-Br | p-Br | 2b: 96 |
| 3 | p-Me | p-Me | 2c: 94 |
| 4 | p-Cl | p-Cl | 2d: 96 |
| 5 | p-CF3 | p-CF3 | 2e: 56 |
| 6 | p-Cl | H | 2f: 97c |
| 7 | H | p-Cl | 2g: 97c |
| 8 | p-Br | H | 2h: 94c |
| 9 | H | p-Br | 2i: 91c |
| 10 | H | p-Me | 2j: 98c |
| 11 | p-Me | H | 2k: 95c |
Having established the utilization of 20 mol% TMSCl in diethylether at room temperature as the optimized conditions to perform the allylic alkylation reaction, we next extended allylic coupling of organozinc reagent (Et2Zn) with various 1,3-diarylallyl acetates (Table 2). As can be seen, the process can tolerate a variety of common functional 1,3-diarylallyl acetates (1a–k) with Et2Zn. The allylic alkylation of Et2Zn with 1,3-diarylallyl acetates (1a–k), bearing either electron-donating groups or electron-withdrawing groups on their aromatic rings proceeded smoothly in good to excellent yields. Unfortunately, an obvious limitation at the present stage of this method is the selectivity is low because the reactions with unsymmetrical 1,3-diarylallyl acetates (1f–k) containing two different substituents on aromatic rings would lead to a mixture of regioisomeric products (Entries 6–11, 2f–k). Nevertheless, these reaction results may suggest that the allylic addition involves the formation a π-allyl or carbenium intermediate.
For further investigation on the scope of this type allylic substrates for TMSCl-promoted allylic alkylation of Et2Zn, two different substrates 3 and 4 were synthesized from 1,3-diphenylprop-2-en-1-ol though silylation and allylic amidation.22 However, when 3 or 4 was employed in this reaction, desired product 2a was not detected. It may be due to low leaving abilities of the silicon-based group and benzamide group. Thus, treatment of TMSCl with allylic substrate 3 or 4 did not furnish the formation of a reactive intermediate, including π-allyl or carbenium intermediate (Scheme 2).
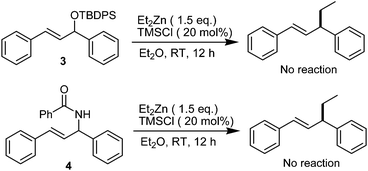 | ||
| Scheme 2 The evaluation of TMSCl-mediated allylic substitution of TBDPS-protected allylic substrate and allylic amide. | ||
As shown in Table 1, although the application of chiral phosphine ligands was unsuccessfully in the preparation of optically pure 1,3-diphenylpent-1-ene, further exploration led to the determination of TMSCl-promoted allylic alkylation of organozinc reagent for the facile preparation of 1,3-diarylpent-1-enes. To gain access to the asymmetric version of TMSCl-promoted allylic alkylation of Et2Zn with 1,3-diphenylallyl acetate, a chiral starting material 1a with 39% ee was prepared by our synthetic method reported recently.20c The stereochemistry of allylic substitution was examined next. Unfortunately, as shown in Scheme 3, the desired product 2a was obtained with no enantiomeric excess.
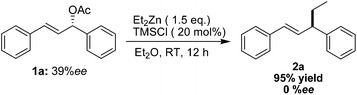 | ||
| Scheme 3 TMSCl-mediated allylic alkylation of chiral 1,3-diphenylallyl acetate (39% ee) with diethylzinc reagent. | ||
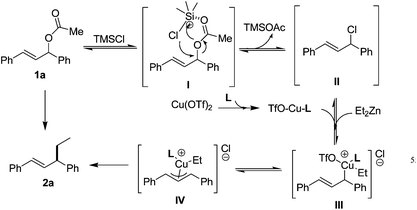
Based on these studies and previous findings on allylic alkylation of organometallic reagents,15 a mechanism of the TMSCl-promoted allylic alkylation of Et2Zn with 1,3-diarylallyl acetates could be proposed in Scheme 4. The initial step of present reaction can take place through possible activation of 1,3-diarylallyl acetate by Lewis acidic TMSCl, and the corresponding carbenium intermediate (II) was formed.23 It was also supported by the reaction result of catalytic allylic alkylation of chiral 1,3-diphenylallyl acetate (39% ee) with diethylzinc reagent (Scheme 3). Then the attack Et2Zn to the corresponding carbenium intermediate (II) was occurred as rate determining step.23a In the final step, elimination of EtZnCl and subsequent reaction with TMSOAc from this nucleophilic addition gives the final product 2a and began new cycle of silicon-based Lewis acid catalyst. In the controlled experiment, we have ever used strong Lewis acidic ZnCl2 as catalyst in this reaction that only trace product was obtained, which maybe support the formation of carbenium intermediate was promoted by powerful TMSCl.2 Although the mechanism of the TMSCl-mediated allylic alkylation of 1,3-diaryl-2-propenyl acetates with diethylzinc reagent has not been well established, it is potentially very useful and simple synthetic methods because various 1,3-diaryl-2-propenyl acetates are efficiently transferred to 1,3-diarylpent-1-enes in good to excellent yields.
In summary, we have disclosed that chlorotrimethylsilane (TMSCl) was a highly efficient catalyst in the allylic alkylation of 1,3-diaryl-2-propenyl acetates with diethylzinc reagent. In this reaction, various 1,3-diaryl-2-propenyl acetates are efficiently transferred to 1,3-diarylpent-1-enes in good to excellent yields. A plausible mechanism for this reaction involves the formation of carbenium intermediate activated by silicon-based Lewis acid. Although allylic alkylation of allylic substrates with organometallic reagents has been reported with copper catalysis, this is the first example that the simple silicon-based Lewis acid, TMSCl, could be applied in the intermolecular allylic alkylation of organozinc reagent. Additionally, the use of organosilicon compounds as catalyst opens up the possibility of performing the reaction of organometallic compounds with silicon-based Lewis acids. Further investigations in this direction are currently underway.
The authors gratefully thank the financial support of the National Natural Science Foundation of China (NSFC, no. 21173064), Zhejiang Provincial Natural Science Foundation of China (ZPNSFC, Q12B020037), and Program for Excellent Young Teachers in Hangzhou Normal University (HNUEYT, JTAS 2011-01-014).
Notes and references
- For selected reviews, see: (a) L. A. Paquette, Chem. Rev., 1986, 86, 733 CrossRef CAS; (b) E. Langkopf and D. Schinzer, Chem. Rev., 1995, 95, 1375 CrossRef CAS; (c) T. H. Chan and D. Wang, Chem. Rev., 1995, 95, 1279 CrossRef CAS; (d) C. E. Masse and J. S. Panek, Chem. Rev., 1995, 95, 1293 CrossRef CAS; (e) K. A. Horn, Chem. Rev., 1995, 95, 1317 CrossRef CAS; (f) J. Y. Corey and J. Braddock-Wilking, Chem. Rev., 1999, 99, 175 CrossRef CAS PubMed; (g) M. Suginome and Y. Ito, Chem. Rev., 2000, 100, 3221 CrossRef CAS PubMed; (h) L. W. Xu, Curr. Org. Chem., 2011, 15, 2742 CrossRef CAS , Special Issue; (i) L. W. Xu, L. Li, G. Q. Lai and J. X. Jiang, Chem. Soc. Rev., 2011, 40, 1777 RSC.
- A. D. Dillman and S. L. Ioffe, Chem. Rev., 2003, 103, 733 CrossRef PubMed.
- For selected examples, see: (a) H. Fujioka, K. Yahata, O. Kubo, Y. Sawama, T. Hamada and T. Maegawa, Angew. Chem., Int. Ed., 2011, 50, 12232 CrossRef CAS PubMed; (b) U. K. Tambar, S. K. Lee and J. L. Leighton, J. Am. Chem. Soc., 2010, 132, 10248 CrossRef CAS PubMed; (c) S. Kobayashi, H. Kiyohara and M. Yamaguchi, J. Am. Chem. Soc., 2011, 133, 708 CrossRef CAS PubMed; (d) R. Hrdina, C. E. Müller, R. C. Wende, K. M. Lippert, M. Benassi, B. Spengler and P. R. Schreiner, J. Am. Chem. Soc., 2011, 133, 7624 CrossRef CAS PubMed; (e) G. T. Notte, J. M. B. Vu and J. L. Leighton, Org. Lett., 2011, 13, 816 CrossRef CAS PubMed.
- (a) R. B. Othman, T. Bousquet, A. Fousse, M. Othman and V. Dalla, Org. Lett., 2005, 7, 2825 CrossRef PubMed; (b) B. C. Ranu, A. Saha and T. Mandal, Tetrahedron, 2009, 65, 2072 CrossRef CAS PubMed; (c) X. Bugaut and E. Roulland, Eur. J. Org. Chem., 2012, 908 CrossRef CAS; (d) M. P. Jennings and K. B. Sawant, Eur. J. Org. Chem., 2004, 3201 CrossRef CAS; (e) P. H. Lee, H. Ahn, K. Lee, S.-y. Sung and S. Kim, Tetrahedron Lett., 2001, 42, 37 CrossRef CAS.
- (a) B. C. Ranu and A. Das, Adv. Synth. Catal., 2005, 347, 712 CrossRef CAS; (b) L. W. Xu, M. S. Yang, H. Y. Qiu, G. Q. Lai and J. X. Jiang, Synth. Commun., 2008, 38, 1011 CrossRef CAS.
- (a) H. Mao, J. P. Wan and Y. Pan, Tetrahedron, 2009, 65, 1026 CrossRef CAS PubMed; (b) M. Krasavin, S. Tsirulnikov, M. Nikulnikov, V. Kysil and A. Ivachtchenko, Tetrahedron Lett., 2008, 49, 5241 CrossRef CAS PubMed; (c) V. Kysil, A. Khvat, S. Tsirulnikov, S. Tkachenko and A. Ivachtchenko, Tetrahedron Lett., 2009, 50, 2854 CrossRef CAS PubMed; (d) B. L. Yang, Z. T. Weng, S. J. Yang and S. K. Tian, Chem.–Eur. J., 2010, 16, 718 CrossRef CAS PubMed; (e) H. H. Li, D. J. Dong and S. K. Tian, Eur. J. Org. Chem., 2008, 3623 CrossRef CAS; (f) J. P. Wan and Y. Liu, Curr. Org. Chem., 2011, 15, 2758 CrossRef CAS.
- A. M. A. Shumaila, V. G. Puranik and R. S. Kusurkar, Tetrahedron, 2011, 67, 936 CrossRef CAS PubMed.
- L. Yang, L. W. Xu and C. G. Xia, Tetrahedron Lett., 2007, 48, 1599 CrossRef CAS PubMed.
- (a) M. S. Yang, L. W. Xu, H. Y. Qiu, G. Q. Lai and J. X. Jiang, Tetrahedron Lett., 2008, 49, 253 CrossRef CAS PubMed; (b) M. Izumi and K. Fukase, Chem. Lett., 2004, 34, 594 CrossRef.
- G. Sabitha, G. S. K. K. Reddy, K. B. Reddy and J. S. Yadav, Synthesis, 2004, 263 CrossRef CAS PubMed.
- (a) M. K. Pandey, A. Bisai, A. Pandey and V. K. Singh, Tetrahedron Lett., 2005, 46, 5039 CrossRef CAS PubMed; (b) T. Iwai, T. Ito, T. Mizuno and Y. Ishino, Tetrahedron Lett., 2004, 45, 1083 CrossRef CAS PubMed.
- N. Sakai, T. Hamajima and T. Konakahara, Tetrahedron Lett., 2002, 43, 4821 CrossRef CAS.
- For selected examples, see: (a) V. O. Iaroshenko, S. Ali, T. M. Babar, S. Dudkin, S. Mkrtchyan, N. Hasan, A. Villinger and P. Langer, Tetrahedron Lett., 2011, 52, 373 CrossRef CAS PubMed; (b) K. Bahrami, M. M. Khodaei, B. H. Yousefi and M. S. Arabi, Tetrahedron Lett., 2010, 51, 6939 CrossRef CAS PubMed; (c) P. Sarnpitak, S. Tsirulnikov and M. Krasavin, Tetrahedron Lett., 2012, 53, 6540 CrossRef CAS PubMed; (d) C. R. Liu, M. B. Li, C. F. Yang and S. K. Tian, Chem.–Eur. J., 2009, 15, 793 CrossRef CAS PubMed.
- (a) Y. Yoshihiro, N. Matsumoto, R. Hamasaki and Y. Tanabe, Tetrahedron Lett., 1999, 40, 4227 CrossRef; (b) G. Sabitha, G. S. K. K. Reddy, C. S. Reddy and J. S. Yadav, Synlett, 2003, 858 CrossRef CAS; (c) L. W. Xu, W. Zhou, L. Yang and C. G. Xia, Synth. Commun., 2007, 37, 3095 CrossRef CAS; (d) N. Azzi, A. R. K. Amiri, H. Ghafuri, M. R. Saidi and M. Bolourtchian, J. Iran. Chem. Soc., 2010, 7, 428–431 CrossRef; (e) H. M. Yang, L. Li, F. Li, K. Z. Jiang, J. Y. Shang, G. Q. Lai and L. W. Xu, Org. Lett., 2011, 13, 6508 CrossRef CAS PubMed.
- For selected examoples, see: (a) Y. Kiyotsuka and Y. Kobayashi, Tetrahedron, 2010, 66, 676 CrossRef CAS PubMed; (b) E. Erdik and M. Koçoğlu, J. Organomet. Chem., 2009, 694, 1890 CrossRef CAS PubMed; (c) Y. Kiyotsuka, Y. Katayama, H. P. Acharya, T. Hyodo and Y. Kobayashi, J. Org. Chem., 2009, 74, 1939 CrossRef CAS PubMed; (d) Y. kiyotsuka and Y. Kobayashi, J. Org. Chem., 2009, 74, 7489 CrossRef CAS; (e) E. Erdik and M. Koçoğlu, Appl. Organomet. Chem., 2006, 20, 290 CrossRef CAS; (f) P. Demel, M. Keller and B. Breit, Chem.–Eur. J., 2006, 12, 6669 CrossRef CAS PubMed; (g) H. Leuser, S. Perrone, F. Liron, F. F. Kneisel and P. Knochel, Angew. Chem., Int. Ed., 2005, 44, 4627 CrossRef CAS PubMed.
- (a) N. Krause and A. Gerold, Angew. Chem., Int. Ed., 1997, 36, 186 CrossRef CAS; (b) B. Breit, P. Demel, D. Grauer and C. Studte, Chem.–Asian J., 2006, 1, 586 CrossRef CAS PubMed.
- J. B. Langlois and A. Alexakis, Adv. Synth. Catal., 2010, 352, 447 CrossRef CAS.
- (a) M. Pérez, M. Fañanás-Mastral, P. H. Bos, A. Rudolph, S. R. Harutyunyan and B. L. Feringa, Nat. Chem., 2011, 3, 377 CrossRef PubMed; (b) M. Fañanás-Mastral, M. Pérez, P. H. Bos, A. Rudolph, S. R. Harutyunyan and B. L. Feringa, Angew. Chem., Int. Ed., 2012, 51, 1922 CrossRef PubMed; (c) V. Hornillos, M. Pérez, M. Fañanás-Mastral and B. L. Feringa, Chem.–Eur. J., 2013, 19, 5432 CrossRef CAS PubMed.
- M. Magrez, Y. L. Guen, O. Baslé, C. Crévisy and M. Mauduit, Chem.–Eur. J., 2013, 19, 1199 CrossRef CAS PubMed.
- For Ar-BINMOLs, see: (a) G. Gao, F. L. Gu, J. X. Jiang, K. Jiang, C. Q. Sheng, G. Q. Lai and L. W. Xu, Chem.–Eur. J., 2011, 17, 2698 CrossRef CAS PubMed; (b) G. Gao, X. F. Bai, H. M. Yang, J. X. Jiang, G. Q. Lai and L. W. Xu, Eur. J. Org. Chem., 2011, 5039 CrossRef CAS; (c) L. S. Zheng, K. Z. Jiang, Y. Deng, X. F. Bai, G. Gao, F. L. Gu and L. W. Xu, Eur. J. Org. Chem., 2013, 748 CrossRef CAS , For L2 (Ph-NNP), see: ; (d) L. S. Zheng, L. Li, K. F. Yang, Z. J. Zheng, X. Q. Xiao and L. W. Xu, Tetrahedron, 2013, 69, 8777 CrossRef CAS PubMed; (e) For Ar-BINMOL-derived L3 ligand, see: ; (f) F. Ye, Z. J. Zheng, W. H. Deng, L. S. Zheng, Y. Deng, C. G. Xia and L. W. Xu, Chem.–Asian J., 2013, 8, 2242 CrossRef CAS PubMed.
- (a) A. Streitwieser, E. G. Jayasree, S. S. H. Leung and G. S. C. Choy, J. Org. Chem., 2005, 70, 8486 CrossRef CAS PubMed; (b) A. Streitwieser, E. G. Jayasree and S. S. H. Leung, J. Org. Chem., 2008, 73, 9426 CrossRef CAS PubMed.
- (a) M. Yamanaka, S. Kato and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 6287 CrossRef CAS PubMed; (b) Z. Y. Jiang, C. H. Zhang, F. L. Gu, K. F. Yang, G. Q. Lai, L. W. Xu and C. G. Xia, Synlett, 2010, 1251 CAS.
- (a) M. Kalkan and E. Erdik, J. Phys. Org. Chem., 2013, 26, 256 CrossRef CAS; (b) V. Komanduri, F. Pedraza and M. J. Krische, Adv. Synth. Catal., 2008, 350, 1569 CrossRef CAS PubMed; (c) B. Qin, N. Yamagiwa, S. Matsunaga and M. Shibasaki, Angew. Chem., Int. Ed., 2007, 46, 409 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44742a |
| This journal is © The Royal Society of Chemistry 2014 |