Palladium-catalysed regioselective aroylation and acetoxylation of 3,5-diarylisoxazole via ortho C–H functionalisations†
Arghya Banerjee,
Anupam Bera,
Sourav Kumar Santra,
Srimanta Guin and
Bhisma K. Patel*
Department of Chemistry, Indian Institute of Technology Guwahati, 781 039, Assam, India. E-mail: patel@iitg.ernet.in; Fax: +91-3612690762
First published on 29th November 2013
Abstract
The higher directing ability of N over O in 3,5-diarylisoxazole is demonstrated during the construction of C–C and C–O bonds. Out of the four ortho sp2 C–Hs and one internal sp2 C–H in 3,5-diarylisoxazoles, regioselective aroylation and acetoxylation take place at one of the ortho-C–Hs proximal to the N atom using Pd(OAc)2 as the catalyst in the presence of suitable oxidants and solvents.
Introduction
Recently, a number of C–H functionalisation methods with a high level of accuracy and predictability for systematising the synthesis of a diverse array of complex organic molecules have been reported.1 Among the various methods, the cross-dehydrogenative coupling (CDC)2 and directing group-assisted approaches3 are the two most powerful strategies for the site selective C–H functionalisations. Donor heteroatoms like nitrogen (N) and oxygen (O) have been employed to direct the metallation at the proximal site for achieving the desired ortho-C–H functionalisations. In this field, Pd-catalysed directing group-assisted C–H functionalisation methods have received much recent attention for the construction of C–C or C–X (X = heteroatom) bonds.4Transition metal catalysed ortho-aroylations have been achieved using aldehydes,5 benzyl alcohols6 or even inert alkylbenzenes7 as aroyl surrogates assisted by N and O bearing directing groups such as 2-phenylpyridine,5a,b,6a,7a anilides,5c,d,e,6b,7b,c benzamides,5f O-methyl oxime,5g,7a and 2-arylbenzazoles.5h Recently, a variety of ortho-aroylation strategies have been demonstrated using different directing groups via the decarboxylation of alpha-keto acids.8 In a similar fashion, various directing group-containing substrates have also been employed for Pd-catalysed oxidative C–O bond formation, in particular o-hydroxylation or o-acetoxylation.9
It is pertinent to mention here that there are numerous examples of sp2 N atoms in heterocycles acting as chelating ligands in ortho-directed functionalisations.5–10 However, the directing (chelating) ability of sp2 oxygen11 in ketones and acids and sp3 oxygen12 in ethers and alcohols can hardly be ignored. 3,5-Diarylisoxazole possessing both N and O-chelating atoms in a rigid framework may provide a competitive cyclometallated intermediate either through N or O atoms by anchoring the metal selectively to one of the proximal ortho C–H bonds. There is only one instance where 3,5-diarylisoxazole is ortho-arylated using phenylboronic acid as the arylating partner in the presence of stoichiometric Pd salt.13 Apart from this, there is not a single report on catalytic ortho C–H functionalisation using this moiety. The 3,5-diarylisoxazole scaffold is an important pharmacophore having a diverse array of biological activities.14 The reductive N–O bond cleavage of 3,5-disubstituted isoxazoles leads to various bifunctional molecules15 that can be utilized for the synthesis of other important heterocycles.16 Thus, any further functionalisation of this important moiety is likely to generate further interest. There are two o-hydrogens, each proximal to a N and O atom in 3,5-diarylisoxazole that can be activated (o-palladated). Furthermore, functionalisation of the isoxazolyl internal sp2 C4–H by a non-directing metallation path cannot be ruled out. o-C–H functionalisation in isoxazoles having dual-directing atoms such as N and O has not been investigated so far. Thus, it would be interesting to see whether the palladation would occur through the –N donor ortho-site or –O donor ortho-site or at the C4 position in 3,5-diarylisoxazoles.
Results and discussion
An initial experiment for the aroylation of 3,5-diphenylisoxazole was performed using an aromatic aldehyde as the aroyl source. Thus 3,5-diphenylisoxazole (1) (1 equiv.) was reacted with an equivalent of benzaldehyde (a) in the presence of Pd(OAc)2 (5 mol%) and oxidant tert-butylhydroperoxide (TBHP, 1 equiv.) in toluene solvent. A product having a lower retention factor (Rf) was isolated with a mere yield of 18% (entry 1, Table 1). Spectroscopic (1H and 13C NMR) analysis of the product (1a) showed the presence of an aroyl group and retention of the internal C4 hydrogen in its 1H NMR at 6.54 ppm. Thus, the aroylation is taking place most probably at one of the ortho positions proximal to either the N or O donor site of the isoxazole moiety. In the pursuit to improve the yield of the product (1a), the reaction was again performed under identical conditions using 1,4-dioxane as the solvent. No product formation (entry 2, Table 1) was observed and the starting material was recovered completely. Switching the solvent to acetic acid provided an improved yield of 27% (entry 3, Table 1). Further screening of solvents such as DMSO, DMF, and dichloroethane (DCE) (entries 4–6, Table 1) revealed DCE to be the better solvent giving a moderate yield (41%) of the product. The use of 10 mol% of the catalyst increased the yield up to 53% (entry 7, Table 1). Improvement in the yield up to 64% (entry 8, Table 1) was observed when the oxidant quantity was increased to 1.2 equivalents. No further enhancement in the yield was observed even when the catalyst loading was increased to 15 mol % (entry 9, Table 1). Other palladium(II) catalysts such as PdBr2, PdCl2 and Pd(TFA)2 were found to be not so effective compared with Pd(OAc)2 (entries 10–12, Table 1). The use of hydrogen peroxide (H2O2) and benzoyl peroxide (PhCOO)2 as oxidants were unsuccessful in giving the anticipated product.| Entry | Catalyst (mol%) | Solvent | Oxidant | Yieldab (%) |
|---|---|---|---|---|
| a Isolated yield of o-aroylated product (1a) after 12 h, TBHP (1.2 equiv.) was added in 4 portions at 3 h intervals.b All reactions were performed under an air atmosphere using 1.2 equiv. of benzaldehyde at 110 °C. | ||||
| 1 | Pd(OAc)2 (5.0) | Toluene | TBHP (1.0) | 18 |
| 2 | Pd(OAc)2 (5.0) | 1,4-Dioxane | TBHP (1.0) | 00 |
| 3 | Pd(OAc)2 (5.0) | AcOH | TBHP (1.0) | 27 |
| 4 | Pd(OAc)2 (5.0) | DMSO | TBHP (1.0) | 00 |
| 5 | Pd(OAc)2 (5.0) | DMF | TBHP (1.0) | 00 |
| 6 | Pd(OAc)2 (5.0) | DCE | TBHP (1.0) | 41 |
| 7 | Pd(OAc)2 (10.0) | DCE | TBHP (1.0) | 53 |
| 8 | Pd(OAc)2 (10.0) | DCE | TBHP (1.2) | 64 |
| 9 | Pd(OAc)2 (15.0) | DCE | TBHP (1.2) | 65 |
| 10 | PdCl2 (10.0) | DCE | TBHP (1.2) | 8 |
| 11 | PdBr2 (10.0) | DCE | TBHP (1.2) | 16 |
| 12 | Pd(TFA)2 (10.0) | DCE | TBHP (1.2) | 22 |
The scope and generality of this aroylation reaction was further explored using various aromatic aldehydes and 3,5-diarylisoxazoles. The 3-aryl ring of 3,5-diarylisoxazoles possessing electron-donating substituents such as p-Me (2) and p-OMe (3) coupled efficiently with benzaldehyde (a) giving the corresponding o-aroylated products (2a) and (3a), respectively, in moderate yields. However, the presence of electron-withdrawing substituents such as o-NO2, m-F and p-Cl in the 3-aryl ring of 3,5-diarylisoxazole were completely unproductive under these present reaction conditions. Due to the deactivating nature of 3-phenyl rings, they are reluctant to form a palladacycle intermediate, thereby preventing the aroylation process.
Aromatic aldehydes possessing electron-donating substituents such as p-Me (b), p-tBu (c) and p-OMe (d) served as excellent aroyl precursors toward the aroylation of 3,5-diphenylisoxazole (1) giving products (1b), (1c) and (1d), respectively, in moderate yields. The structure of the product (1d) has been confirmed by X-ray crystallographic analysis (Fig. 1).17 As can be clearly seen from the crystal structure of (1d), an aroyl moiety has been incorporated into the 3-aryl ring of 3,5-diarylisoxazole (1) proximal to the N atom. Aromatic aldehydes possessing electron-withdrawing substituents such as m-Cl (e), p-Ph (f), p-CO2Me (g) and 2,6 di-Cl (h) underwent reactions giving better yields of their o-aroylated products (1e), (1f), (1g) and (1h) compared with the aldehydic substrates possessing electron-donating substituents as shown in Table 2. The structure of the product (1g) has been reconfirmed by X-ray crystallography (Fig. 2).18 Here again, the aroylation is directed by the N atom in the 3,5-diarylisoxazole. The fused aromatic aldehyde (i) provided a better yield of o-aroylated product (1i) compared to heteroaromatic aldehydes (j) and (k) in giving their corresponding products (1j) and (1k). The reductive N–O bond cleavage of 3,5-disubstituted isoxazoles provides β-aminoenones or 1,3-diketones,15d thus these ortho-aroylated products may be useful precursors to triketones.
 | ||
| Fig. 1 ORTEP molecular diagram of (1d).17 | ||
| a Yields of pure isolated products after silica gel column chromatography. |
|---|
 |
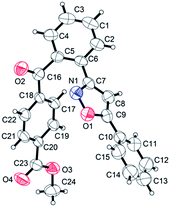 | ||
| Fig. 2 ORTEP molecular diagram of (1g).18 | ||
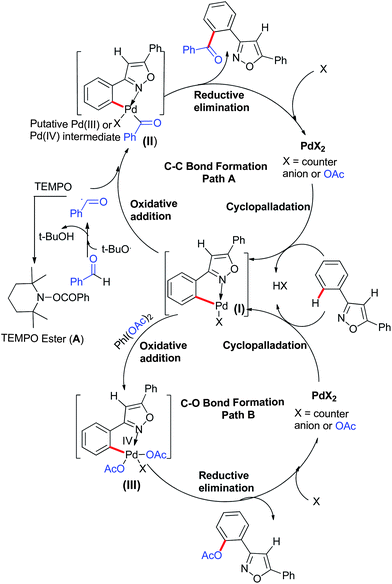
Substrate-directed Pd-catalysed oxidative C–O bond formation, viz. o-hydroxylation and o-acetoxylation, has been well explored.9 Analogous to the C–C bond formation in 3,5-diarylisoxazoles directed by the N atom, it is also of interest to see whether a C–O bond can similarly be constructed. From the literature reports, it is well established that directing group-promoted hydroxylation/acetoxylation proceeds better with a combination of diacetoxyiodobenzene (DIB), AcOH and Pd(OAc)2.9 To reassess the better directing ability of N in 3,5-diarylisoxazoles for o-acetoxylation, substrate (1) (1 equiv.) was treated with Pd(OAc)2 (5.0 mol%), DIB (1.2 equiv.) in AcOH (2 mL) at 110 °C, which provided the o-acetoxylated product (1a′) in 44% isolated yield. With this initial result in hand, further optimisations in terms of solvent, catalyst and their quantities were examined. Details of the optimisation reactions that were performed are summarised in Table 3. The most surprising aspect of the optimisation reaction is the use of the combination of solvents AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (3
toluene (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which gave the best possible yield. The other ratios of AcOH
1), which gave the best possible yield. The other ratios of AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene [(1
toluene [(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (2
1), (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] tried were found to be less effective. Thus all further reactions were performed taking the substrate (1 equiv.), DIB (1.2 equiv.), Pd(OAc)2 (0.1 equiv.) in a mixture of AcOH
2)] tried were found to be less effective. Thus all further reactions were performed taking the substrate (1 equiv.), DIB (1.2 equiv.), Pd(OAc)2 (0.1 equiv.) in a mixture of AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (3
toluene (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at a temperature of 110 °C.
1) at a temperature of 110 °C.
| Entry | Catalyst (mol%) | Solvent | Oxidant | Yieldab (%) |
|---|---|---|---|---|
a Isolated yield of o-acetoxyted product (1a′) after 12 h.b All reactions were performed under an air atmosphere at 110 °C.c AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene used in a 3 toluene used in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 1 ratio. |
||||
| 1 | Pd(OAc)2 (5.0) | AcOH | DIB (1.2) | 44 |
| 2 | Pd(OAc)2 (5.0) | 1,4-Dioxane | DIB (1.2) | 00 |
| 3 | Pd(OAc)2 (5.0) | Toluene | DIB (1.2) | 00 |
| 4 | Pd(OAc)2 (5.0) | DMSO | DIB (1.2) | 00 |
| 5 | Pd(OAc)2 (5.0) | DMF | DIB (1.2) | 00 |
| 6 | Pd(OAc)2 (5.0) | DCE | DIB (1.2) | 00 |
| 7 | Pd(OAc)2 (10.0) | AcOH | DIB (1.2) | 45 |
| 8 | Pd(OAc)2 (10.0) | AcOH | DIB (1.5) | 53 |
| 9 | Pd(OAc)2 (10.0) | AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
DIB (1.2) | 62c |
| 10 | Pd(OAc)2 (15.0) | AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
DIB (1.2) | 66c |
| 11 | PdBr2 (10.0) | AcOH | DIB (1.2) | 27 |
| 12 | Pd(TFA)2 (10.0) | AcOH | DIB (1.2) | 30 |
| 13 | PdCl2 (10.0) | AcOH | DIB (1.2) | 18 |
The above optimised conditions were then applied toward the o-acetoxylation of various 3,5-diphenylisoxazoles. It was observed that electron-donating substituents such as p-Me (2) and p-OMe (3) present in the 3-aryl ring of 3,5-diarylisoxazole afforded their corresponding o-acetoxylated products (2a′) and (3a′) in good yields. The electron-withdrawing substituent of o-NO2 (4) present in the 3-aryl ring of 3,5-diarylisoxazole underwent a slow reaction giving a poor yield (43%) of the product (4a′). Other substrates possessing electron-withdrawing substituents such as m-F and p-Cl were completely unproductive under these optimised reaction conditions. Barring the exception of o-NO2 (4) for o-acetoxylation, the results of o-aroylation and o-acetoxylation are identical (Table 2 and 4) for substrates possessing electron-withdrawing groups in their 3-aryl rings. The substrate having p-NO2 did not yield any product supporting our argument for the unreactive nature of a substrate having deactivating groups for o-acetoxylation and o-aroylation reactions. The unusual reactivity of (4) which, in spite of possessing a deactivating o-NO2 group, gives an o-acetoxylated product (4a′) can not be ascertained at this moment. Interestingly, 3-benzyl-5-phenylisoxazole afforded the desired ortho-acetoxylated product (5a′) in a modest yield. The reaction in the latter case is expected to proceed via a 6-membered palladacycle intermediate.
| a Yields of pure isolated products after silica gel column chromatography. |
|---|
 |
Recently alkylbenzenes have been demonstrated to be the synthetic equivalent of an aroyl moiety during substrate directed o-aroylation.7 In this case, even though an alkylbenzene (toluene) is used as the solvent, no aroylation was observed. This is because of the inability of toluene to be converted into an alcohol and aldehyde under the reaction conditions. It is clear from Table 2 that the presence of electron-withdrawing substituents results in completely unproductive reactions, while the presence of electron-donating substituents facilitates the reactions. Thus, if an electron-withdrawing substituent is placed in the 3-aryl ring (towards the N side) and an electron-donating group in the 5-aryl ring (towards the O side), can oxygen in 3,5-diarylisoxazole direct the o-aroylation? With this objective the designed substrate (9), Fig. 3, was subjected to the present o-aroylation conditions but no aroylation whatsoever was observed suggesting the inability of O as directing group in this case.
Although we have not thoroughly probed the mechanisms of these reactions, the mechanisms are expected to be similar to those recently proposed by us5,9e–h,l and others for these C–C and C–O bond forming reactions (Scheme 1). The presence of a radical scavenger such as 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) greatly suppressed the rate of this o-aroylation reaction and only a trace amount (8%) of ortho-aroylated product was formed along with the formation of a benzoyl-TEMPO adduct (A) in 66% isolated yield. Thus, a plausible mechanism can be depicted for this o-aroylation reaction. The first step of the o-aroylation reaction involves the cyclopalladation to form an intermediate (I) towards the N side, i.e. 3-aryl ring in 3,5-diarylisooxazole. The aroyl radical generated in situ by the reaction of an aldehyde and TBHP undergoes oxidative addition with the intermediate (I) to form either a reactive Pd(IV)19 or a dimeric Pd(III)20 intermediate (II) (Scheme 1, path A). In the final stage, reductive elimination affords C–C bond formation leading to the ortho-aroylated product and regenerating the Pd(II) catalyst, which maintains the subsequent catalytic cycle (Scheme 1, path A). The C–O bond forming path (o-acetoxylation) is illustrated in (Scheme 1, path B). This mechanism is identical to our Pd(II) catalysed o-hydroxylation of 2-arylbenzothiazole and compounds with other directing groups.9e–h,l
Conclusions
In conclusion, we have demonstrated the higher directing ability of N over O in 3,5-diarylisoxazole via a chelation-assisted approach in accomplishing site selective ortho-functionalisations. In spite of the higher acidic character of the isoxazolyl, sp2 C4–H directed o-metallation is preferred over the non-directed metallation pathway. This is the first report of catalytic o-functionalisation of this scaffold for the construction of C–C and C–O bonds.Experimental section
General remarks
All the reagents were commercial grade and purified according to the established procedures. Organic extracts were dried over anhydrous sodium sulphate. Solvents were removed in a rotary evaporator under reduced pressure. Silica gel (60–120 mesh size) was used for the column chromatography. Reactions were monitored by TLC on silica gel 60 F254 (0.25 mm). NMR spectra were recorded in CDCl3 with tetramethylsilane as the internal standard for 1H NMR (400 MHz, 600 MHz) and CDCl3 solvent as the internal standard for 13C NMR (100 MHz, 150 MHz). HRMS spectra were recorded using ESI mode (TOF). IR spectra were recorded neat or in KBr.(A) General experimental procedure for the preparation of phenyl(2-(5-phenylisoxazol-3-yl)phenyl)methanone (1a) from 3,5-diphenylisoxazole (1)
An oven-dried flask was charged with Pd(OAc)2 (22 mg, 0.10 mmol), benzaldehyde (127 mg, 1.2 mmol), 3,5-diphenylisoxazole (1) (221 mg, 1.0 mmol) and 1,2-dichloroethane (2 mL). The reaction vessel was then subjected to reflux in an oil bath preheated to 110 °C and stirring was maintained for the stipulated period of time. TBHP (1.2 mmol) was then added in 4 equal portions over a period of 12 h. The progress of the reaction was monitored by TLC. The reaction mixture was then cooled and admixed with water (5 mL). The product was extracted with ethyl acetate (3 × 10 mL) and the combined organic layer was washed with saturated sodium bicarbonate (NaHCO3) solution. The organic layer was dried over anhydrous sodium sulphate (Na2SO4), and evaporated under reduced pressure. Further purification of the crude product was done through silica gel column chromatography (8% EtOAc–hexane) to yield the pure phenyl(2-(5-phenylisoxazol-3-yl)phenyl)methanone (1a) (208 mg, yield 64%). The identity and purity of the product was confirmed by spectroscopic analysis.(B) General experimental procedure for the preparation of 2-(5-phenylisoxazol-3-yl)phenyl acetate (1a′) from 3,5-diphenylisoxazole (1)
An oven-dried flask was charged with 3,5-diphenylisoxazole (1) (221 mg, 1.0 mmol), diacetoxyiodobenzene (DIB) (386 mg, 1.2 mmol), Pd(OAc)2 (22 mg, 0.10 mmol), and acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (2 mL in 3
toluene (2 mL in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). After that the reaction mixture was subjected to reflux in a preheated oil bath at 110 °C and stirring was maintained for the stipulated period of time. The progress of the reaction was monitored by TLC. After the completion of the reaction as judged from TLC, the reaction mixture was cooled and admixed with water (5 mL). The product was extracted with ethyl acetate (2 × 10 mL) and the combined organic layer was washed with saturated sodium bicarbonate (NaHCO3) solution. The organic layer was dried over anhydrous sodium sulphate (Na2SO4), and evaporated under reduced pressure. The crude product obtained here was further purified through silica gel column chromatography (10% EtOAc–hexane) to yield the pure 2-(5-phenylisoxazol-3-yl)phenyl acetate (1a′) (172 mg, yield 62%). The identity and purity of the product was confirmed by spectroscopic analysis.
1 ratio). After that the reaction mixture was subjected to reflux in a preheated oil bath at 110 °C and stirring was maintained for the stipulated period of time. The progress of the reaction was monitored by TLC. After the completion of the reaction as judged from TLC, the reaction mixture was cooled and admixed with water (5 mL). The product was extracted with ethyl acetate (2 × 10 mL) and the combined organic layer was washed with saturated sodium bicarbonate (NaHCO3) solution. The organic layer was dried over anhydrous sodium sulphate (Na2SO4), and evaporated under reduced pressure. The crude product obtained here was further purified through silica gel column chromatography (10% EtOAc–hexane) to yield the pure 2-(5-phenylisoxazol-3-yl)phenyl acetate (1a′) (172 mg, yield 62%). The identity and purity of the product was confirmed by spectroscopic analysis.
Acknowledgements
B. K. P. acknowledges the support of this research by the Department of Science and Technology (DST) (SR/S1/OC-79/2009), New Delhi, and CSIR (02(0096)/12/EMR-II). AB, SKS and SG thank CSIR for fellowships. Thanks are due to Central Instruments Facility (CIF) IIT Guwahati for NMR spectra.References
- (a) F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826 CrossRef CAS PubMed; (b) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (c) L.-C. Campeau and K. Fagnou, Chem. Soc. Rev., 2007, 36, 1058 RSC; (d) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (e) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (f) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (g) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (h) L.-M. Xu, B.-J. Li, Z. Yang and Z.-J. Shi, Chem. Soc. Rev., 2010, 39, 712 RSC; (i) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (j) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (k) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082 CrossRef CAS PubMed; (l) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef CAS PubMed; (m) G. Dyker, Handbook of C–H Transformations, Wiley-VCH, Weinheim, 2005 Search PubMed; (n) D. R. Stuart and K. Fagnou, Science, 2007, 316, 1172 CrossRef CAS PubMed; (o) A. N. Verdernikov, Acc. Chem. Res., 2012, 45, 803 CrossRef PubMed; (p) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS PubMed; (q) R. J. Phipps and M. J. Gaunt, Science, 2009, 323, 1593 CrossRef CAS PubMed; (r) D.-H. Wang, K. M. Engle, B.-F. Shi and J.-Q. Yu, Science, 2010, 327, 315 CrossRef CAS PubMed; (s) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (t) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (u) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (v) M. C. White, Science, 2012, 335, 807 CrossRef CAS PubMed; (w) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369 CrossRef CAS PubMed.
- (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) J. A. Ashenhurst, Chem. Soc. Rev., 2010, 39, 540 RSC; (c) C. J. Scheuermann, Chem.–Asian J., 2010, 5, 436 CrossRef CAS PubMed.
- (a) N. Chatani, Directed Metallation, Springer, Berlin, Germany, 2008 Search PubMed; (b) O. Daugulis, H. Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (c) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (d) N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236 CrossRef CAS PubMed.
- (a) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (b) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (c) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (d) J. D. Neukom, A. S. Aquino and J. P. Wolfe, Org. Lett., 2011, 13, 2196 CrossRef CAS PubMed; (e) D. Kim and S. Hong, Org. Lett., 2011, 13, 4466 CrossRef CAS PubMed; (f) S. R. Neufeldt and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936 CrossRef CAS PubMed; (g) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed.
- (a) X. Jia, S. Zhang, W. Wang, F. Luo and J. Cheng, Org. Lett., 2009, 11, 3120 CrossRef CAS PubMed; (b) O. Baslé, J. Bidange, Q. Shuai and C.-J. Li, Adv. Synth. Catal., 2010, 352, 1145 CrossRef; (c) Y. Wu, B. Li, F. Mao, X. Li and F. Y. Kwong, Org. Lett., 2011, 13, 3258 CrossRef CAS PubMed; (d) C. Li, L. Wang, P. Li and W. Zhou, Chem.–Eur. J., 2011, 17, 10208 CrossRef CAS PubMed; (e) C.-W. Chan, Z. Zhou and W.-Y. Yu, Adv. Synth. Catal., 2011, 353, 2999 CrossRef CAS; (f) J. Park, E. Park, A. Kim, Y. Lee, K. W. Chi, J. H. Kwak, Y. H. Jung and I. S. Kim, Org. Lett., 2011, 13, 4390 CrossRef CAS PubMed; (g) C.-W. Chan, Z. Zhou, A. S. C. Chan and W.-Y. Yu, Org. Lett., 2010, 12, 3926 CrossRef CAS PubMed; (h) A. Banerjee, S. K. Santra, S. Guin, S. K. Rout and B. K. Patel, Eur. J. Org. Chem., 2013, 1367 CrossRef CAS.
- (a) F. Xiao, Q. Shuai, F. Zhao, O. Baslé, G. Deng and C.-J. Li, Org. Lett., 2011, 13, 1614 CrossRef CAS PubMed; (b) Y. Yuan, D. Chen and X. Wang, Adv. Synth. Catal., 2011, 353, 3373 CrossRef CAS.
- (a) S. Guin, S. K. Rout, A. Banerjee, S. Nandi and B. K. Patel, Org. Lett., 2012, 14, 5294 CrossRef CAS PubMed; (b) Y. Wu, P. Y. Choy, F. Mao and F. Y. Kwong, Chem. Commun., 2013, 49, 689 RSC; (c) Z. Yin and P. Sun, J. Org. Chem., 2012, 77, 11339 CrossRef CAS PubMed.
- (a) H. Wang, L.-N. Guo and X.-H. Duan, Org. Lett., 2012, 14, 4358 CrossRef CAS PubMed; (b) M. Kim, J. Park, S. Sharma, A. Kim, E. Park, J. H. Kwak, Y. H. Jung and I. S. Kim, Chem. Commun., 2013, 49, 925 RSC; (c) P. Fang, M. Li and H. Ge, J. Am. Chem. Soc., 2010, 132, 11898 CrossRef CAS PubMed; (d) J. Park, M. Kim, S. Sharma, E. Park, A. Kim, S. H. Lee, J. H. Kwak, Y. H. Jung and I. S. Kim, Chem. Commun., 2013, 49, 1654 RSC; (e) S. Sharma, A. Kim, E. Park, J. Park, M. Kim, J. H. Kwak, S. H. Lee, Y. H. Jung and I. S. Kim, Adv. Synth. Catal., 2013, 355, 667 CrossRef CAS; (f) C.-D. Pan, H.-M. Jin, X. Liu, Y.-X. Cheng and C.-J. Zhu, Chem. Commun., 2013, 49, 2933 RSC.
- (a) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (b) S. H. Kim, H. S. Lee, S. H. Kim and J. N. Kim, Tetrahedron Lett., 2008, 49, 5863 CrossRef CAS; (c) F.-R. Gou, X.-C. Wang, P.-F. Huo, H.-P. Bi, Z.-H. Guan and Y.-M. Liang, Org. Lett., 2009, 11, 5726 CrossRef CAS PubMed; (d) L. Wang, X.-D. Xia, W. Guo, J.-R. Chen and W.-J. Xiao, Org. Biomol. Chem., 2011, 9, 6895 RSC; (e) D. Kalyani and M. S. Sanford, Org. Lett., 2005, 7, 4149 CrossRef CAS PubMed; (f) A. Company and S. Enthaler, Chem. Soc. Rev., 2011, 40, 4912 RSC; (g) D. A. Alonso, C. Nájera, I. M. Pastor and M. Yus, Chem.–Eur. J., 2010, 16, 5274 CrossRef CAS PubMed; (h) X. Zheng, B. Song and B. Xu, Eur. J. Org. Chem., 2010, 2010, 4376 CrossRef; (i) X. Yang, G. Shan and Y. Rao, Org. Lett., 2013, 15, 2334 CrossRef CAS PubMed; (j) Y. Yan, P. Feng, Q.-Z. Zheng, Y.-F. Liang, J.-F. Lu, Y. Cui and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 5827 CrossRef CAS PubMed; (k) F. Mo, L. J. Trzepkowski and G. Dong, Angew. Chem., Int. Ed., 2012, 51, 13075 CrossRef CAS PubMed; (l) A. Banerjee, A. Bera, S. Guin, S. K. Rout and B. K. Patel, Tetrahedron, 2013, 69, 2175 CrossRef CAS.
- (a) L. V. Desai, K. J. Stowers and M. S. Sanford, J. Am. Chem. Soc., 2008, 130, 13285 CrossRef CAS PubMed; (b) T. W. Lyons, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 4455 CrossRef CAS PubMed.
- For recent examples of ketone directed C–H functionalisation: (a) E. M. Simmons and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 17092 CrossRef CAS PubMed; (b) F. Kakiuchi, T. Kochi, E. Mizushima and S. Murai, J. Am. Chem. Soc., 2010, 132, 17741 CrossRef CAS PubMed; (c) K. Muralirajan, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2011, 50, 4169 CrossRef CAS PubMed; (d) K. Padala and M. Jeganmoham, Org. Lett., 2011, 13, 6144 CrossRef CAS PubMed; (e) N. Schröder, J. Wencel-Delord and F. Glorius, J. Am. Chem. Soc., 2012, 134, 8298 CrossRef PubMed; (f) B. Xiao, T.-J. Gong, J. Xu, Z.-J. Liu and L. Liu, J. Am. Chem. Soc., 2011, 133, 1466 CrossRef CAS PubMed; (g) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064 CrossRef CAS PubMed; (h) For recent examples of acid directed C–H functionalisation: B.-F. Shi, Y.-H. Zhang, J. K. Lam, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 460 CrossRef CAS PubMed; (i) K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2010, 49, 6169 CrossRef CAS PubMed; (j) K. M. Engle, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 14137 CrossRef CAS PubMed; (k) R. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510 CrossRef CAS PubMed.
- For recent examples of ether/phenol directed C–H functionalisation: (a) G. Li, D. Leow, L. Wan and J.-Q. Yu, Angew. Chem., Int. Ed., 2013, 52, 1245 CrossRef CAS PubMed; (b) X. Wang, Y. Lu, H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 12203 CrossRef CAS PubMed; (c) Y. Lu, D.-H. Wang, K.-M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 5916 CrossRef CAS PubMed; (d) X. Wang, Y. Lu, H.-X. Dai and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 12203 CrossRef CAS PubMed; (e) Y. Li, D. Leow, X. Wang, K. M. Engel and J.-Q. Yu, Chem. Sci., 2011, 2, 967 RSC.
- J.-H. Chu, C.-C. Chen and M.-J. Wu, Organometallics, 2008, 27, 5173 CrossRef CAS.
- (a) J. J. Talley, D. L. Brown, J. S. Carter, M. J. Craneto, C. M. Koboldt, J. L. Masferrer, W. E. Perkins, R. S. Rogers, A. F. Shaffer, Y. Y. Zhang, B. S. Zweifel and K. Seibert, J. Med. Chem., 2000, 43, 775 CrossRef CAS PubMed; (b) M. P. Giovannoni, C. Vergelli, C. Ghelardini, N. Galeotti, A. Bartolini and V. D. Piaz, J. Med. Chem., 2003, 46, 1055 CrossRef CAS PubMed; (c) M. S. Malamas, E. S. Manas, R. E. McDevitt, I. Gunawan, Z. B. Xu, M. D. Collini, C. P. Miller, T. Dinh, R. A. Henderson, J. C. Keith Jr and H. A. Harris, J. Med. Chem., 2004, 47, 5021 CrossRef CAS PubMed; (d) M. B. Soellner, K. A. Rawls, C. Grundner, T. Alber and J. A. Ellman, J. Am. Chem. Soc., 2007, 129, 9613 CrossRef CAS PubMed.
- (a) M. Nitta and T. Kobayashi, J. Chem. Soc., Perkin Trans. 1, 1985, 1401 RSC; (b) J. W. Bode and E. M. Carreira, Org. Lett., 2001, 3, 1587 CrossRef CAS PubMed; (c) G. K. Tranmer and W. Tam, Org. Lett., 2002, 4, 4101 CrossRef CAS PubMed; (d) M. Nitta and T. Kobayashi, J. Chem. Soc., Chem. Commun., 1982, 877 RSC; (e) D. Donati, S. Ferrini, S. Fusi and F. Ponticelli, J. Heterocycl. Chem., 2004, 41, 761 CrossRef CAS; (f) C. Kashima, S. Tobe, N. Sugiyama and M. Yamamoto, Bull. Chem. Soc. Jpn., 1973, 46, 310 CrossRef CAS; (g) N. R. Natale, Tetrahedron Lett., 1982, 23, 5009 CrossRef CAS; (h) J. V. Greenhill, Chem. Soc. Rev., 1977, 6, 277 RSC.
- (a) A. P. Tulloch, Chemistry of Waxes of Higher Plants, in Chemistry and Biochemistry of Natural Waxes, ed. P.E. Kolattukkudy, Elsevier, Amsterdam, 1976, p. 235 Search PubMed; (b) B. Ramaroson-Raonizafinimanana, E. M. Gaydou and I. Bombarda, J. Agric. Food Chem., 2000, 48, 4739 CrossRef CAS PubMed.
- Crystallographic description of 1d: crystal dimension (mm): 0.38 × 0.28 × 0.24. C23H17NO3, Mr = 355.38. Triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; a = 8.8993 (11) Å, b = 10.4759 (13) Å, c = 11.0032 (14) Å; α = 70.634(7)°, β = 72.001 (5)°, γ = 73.148 (7)°, V = 900.14 (19)
Å3; Z = 2; ρcal = 1.311 mg m−3; μ (mm−1) = 0.087; F(000) = 372.0; reflection collected/unique = 2964/2188; refinement method = full-matrix least-squares on F2; final R indices [I > 2σl ] R1 = 0.1770, wR2 = 0.4251, R indices (all data) R1 = 0.1980, wR2 = 0.4315; goodness of fit = 1.131 (see ESI.†).
; a = 8.8993 (11) Å, b = 10.4759 (13) Å, c = 11.0032 (14) Å; α = 70.634(7)°, β = 72.001 (5)°, γ = 73.148 (7)°, V = 900.14 (19)
Å3; Z = 2; ρcal = 1.311 mg m−3; μ (mm−1) = 0.087; F(000) = 372.0; reflection collected/unique = 2964/2188; refinement method = full-matrix least-squares on F2; final R indices [I > 2σl ] R1 = 0.1770, wR2 = 0.4251, R indices (all data) R1 = 0.1980, wR2 = 0.4315; goodness of fit = 1.131 (see ESI.†). - Crystallographic description of 1g: crystal dimension (mm): 0.54 × 0.42 × 0.30. C24H17NO4, Mr = 241.31. Triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; a = 6.2208 (3) Å, b = 12.7931 (5) Å, c = 13.3551 (5) Å; α = 113.491(2)°, β = 91.551 (2)°, γ = 97.433 (2)°, V = 962.95 (7) Å3; Z = 2; ρcal = 1.322 mg m−3; μ (mm−1) = 0.091; F(000) = 400.0; reflection collected/unique = 1645/1333; refinement method = full-matrix least-squares on F2; final R indices [I > 2σl] R1 = 0.0338, wR2 = 0.0825, R indices (all data) R1 = 0.0441, wR2 = 0.0893; goodness of fit = 1.070 (see ESI.†).
; a = 6.2208 (3) Å, b = 12.7931 (5) Å, c = 13.3551 (5) Å; α = 113.491(2)°, β = 91.551 (2)°, γ = 97.433 (2)°, V = 962.95 (7) Å3; Z = 2; ρcal = 1.322 mg m−3; μ (mm−1) = 0.091; F(000) = 400.0; reflection collected/unique = 1645/1333; refinement method = full-matrix least-squares on F2; final R indices [I > 2σl] R1 = 0.0338, wR2 = 0.0825, R indices (all data) R1 = 0.0441, wR2 = 0.0893; goodness of fit = 1.070 (see ESI.†). - (a) N. R. Deprez and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 11234 CrossRef CAS PubMed; (b) J. M. Racowski, A. R. Dick and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 10974 CrossRef CAS PubMed; (c) P. A. Sibbald, C. F. Rosewall, R. D. Swartz and F. E. Michael, J. Am. Chem. Soc., 2009, 131, 15945 CrossRef CAS PubMed; (d) S. R. Whitfield and M. S. Sanford, J. Am. Chem. Soc., 2007, 129, 15142 CrossRef CAS PubMed.
- (a) D. C. Powers, M. A. L. Geibel, J. E. M. N. Klein and T. Ritter, J. Am. Chem. Soc., 2009, 131, 17050 CrossRef CAS PubMed; (b) D. C. Powers and T. Ritter, Nat. Chem., 2009, 1, 302 CrossRef CAS PubMed.
- SMART V 4.043 Software for the CCD Detector System, Siemens Analytical Instruments Division, Madison, WI, 1995 Search PubMed.
- SAINT V 4.035 Software for the CCD Detector System, Siemens Analytical Instruments Division, Madison, WI, 1995 Search PubMed.
- G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen (Germany), 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra and HRMS spectra. CCDC reference numbers are 945394 and 945395. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra45403g |
| This journal is © The Royal Society of Chemistry 2014 |