Dual functional ionic liquids as antimicrobials and plasticisers for medical grade PVCs†
Seong Ying Choi‡
a,
Héctor Rodríguezbc,
H. Q. Nimal Gunaratneb,
Alberto V. Puga§
b,
Deirdre Gilpind,
Stephanie McGrathd,
Joseph S. Vylee,
Michael M. Tunneyd,
Robin D. Rogersf and
Tony McNally*g
aSchool of Mechanical and Aerospace Engineering, Queen's University Belfast, BT9 5AH, UK
bQUILL, School of Chemistry and Chemical Engineering, Queen's University Belfast, BT9 5AG, UK
cDepartment of Chemical Engineering, University of Santiago de Compostela, Santiago de Compostela, E-15782, Spain
dClinical and Practice Research Group, School of Pharmacy, Queen's University Belfast, BT9 7BL, UK
eSchool of Chemistry and Chemical Engineering, Queen's University Belfast, BT9 5AG, UK
fDepartment of Chemistry and Center for Green Manufacturing, The University of Alabama, Tuscaloosa, AL 35487, USA
gWMG, University of Warwick, Coventry, CV4 7AL, UK. E-mail: t.mcnally@warwick.ac.uk; Fax: +44 (0) 2476 574287; Tel: +44 (0) 2476573256
First published on 15th January 2014
Abstract
Two ionic liquids, 1-ethylpyridinium docusate (IL1) and tri-n-butyl(2-hydroxyethyl)phosphonium docusate (IL2), were designed and synthesized with the explicit intention of imparting a combination of plasticization and antimicrobial efficacy when incorporated into medical grade poly(vinyl chloride)s (PVCs). The glass transition (Tg) of PVC can be reduced by >20 °C on addition of 15 wt% IL2. Both IL1 and IL2 leached to varying extents from the base PVC resins rendering the surface of the PVCs hydrophilic. The antimicrobial activity of both ILs is related to the presence and concentration of both cationic and anionic component of the ILs leached from the PVC and inversely proportional to the extent of PVC gelation. Blends of the PVCs with IL1 displayed antibacterial activity against almost all Gram-positive bacteria tested, including coagulase-negative Staphylococci (CoNS) and methicillin-resistant Staphylococcus aureus (MRSA), but not with IL2 at low concentration in contrast to our previous study when high concentrations of IL2 were used. The more hydrophilic IL1 when added to PVC retards biofilm formation.
1. Introduction
Indwelling medical devices, such as catheters, are susceptible to infection by a range of pathogens, including Staphylococcus aureus, and coagulase-negative Staphylococci (CoNS) such as Staphylococcus epidermidis.1,2 Additionally, nosocomial infections caused by antibiotic-resistant pathogens including methicillin-resistant Staphylococcus aureus (MRSA), continue to be a major problem in hospitals worldwide3. With intravascular and urinary catheters being the two most commonly inserted medical devices in the United States of America, both can be a source of nosocomially-acquired bloodstream infection.4 One proposed mechanism to prevent catheter-related infection is to add antibacterial agents into polymeric materials during the manufacturing of the device.5–7Poly(vinyl chloride) or PVC, is a polymer widely used in medical devices due to its good mechanical properties combined with high flexibility and kink resistance after the addition of plasticizers. Without a plasticizer, PVC is inherently rigid and difficult to process into desired shapes. However, phthalates, the most commonly used plasticizers for PVC, have been put under rigorous investigation due to their potential reprotoxicity, particularly, di-n-ethylhexyl phthalate (DEHP), di-n-butyl phthalate (DBP), n-butylbenzyl phthalate (BBP), di-isobutyl phthalate (DIBP) and di-n-hexyl phthalate (DnHP).8,9 Most recently, DEHP and DBP have been shown to induce epigenetic trans-generational inheritance of obesity, reproductive disease and sperm epimutations in rats.10 Phthalates are known to leach into the surrounding environment11 and into the human body system, via inhalation of dust from building materials,12 ingestion of phthalates from consumer products or food packaging,13 and diffusion into the bloodstream from healthcare products,14 thus highlighting the necessity to reconsider the use of phthalates as plasticizers for PVC, particularly when used in medical devices.
Ionic liquids (ILs), generally known as salts which melt at less than 100 °C but with a special emphasis on salts which are liquid at room temperature, can be tailored to have negligible vapour pressure, high thermal and chemical stability. Due to the respective tunable chemical and physical properties of the cation and anion, ILs having multi-functionality can be achieved via different combinations of cation and anion. In a previous study, we demonstrated the dual functionality of two ILs, 1-ethylpyridinium docusate (IL1) and tributyl(2-hydroxyethyl)phosphonium docusate (IL2), both exhibited a plasticising and antimicrobial effect when incorporated into a rigid PVC. The PVC/IL composites were processed via melt extrusion, one of the most commonly used industrial processing methods for PVC and the process employed in the production of catheters and other medical tubing.15 These two ILs were designed specifically to meet the criteria required for an efficient plasticizer; (1) be liquid at room temperature (to facilitate polymer chain mobility and ease of mixing); (2) have molecular weight greater than 300 g mol−1 (critical minimum molar volume to occupy the available free volume in polymer matrix); (3) possess good thermal stability (>200 °C) at typical PVC melt processing temperature; and (4) possess polar functional groups on either cation or anion (for interaction with polar carbon–chlorine bonds in PVC and minimum toxicity).15 Nevertheless, it is important to ensure such multi-functional ILs meet both requirements, and not alter other factors that may affect their functionality, for example factors such as ease of processing, degree of PVC gelation, and the extent of plasticization in relation to anti-plasticization. PVC gelation or fusion, is a process related to melting of PVC primary particles that allows molecular entanglement at the primary particle boundary to form a three-dimensional network, upon cooling and recrystallization after processing (input of shear and heat).16,17 The degree of gelation strongly influences the mechanical properties of the final product, and fusion of the plasticizer-PVC system is a time/temperature dependent process. Apart from softening of the PVC particles, enhanced molecular motion and thus increased polymer free volume, plasticizers that reduce either the time or temperature of the fusion process are normally given more attention.17,18 However, anti-plasticization may occur when the amount of plasticizer added is lower than 15 parts per hundred (p.h.r), resulting in crystallite formation that leads to a stiffening effect, i.e. increased tensile strength/modulus instead of elongation at break.17–19 Furthermore, the degree of gelation can influence the antimicrobial properties of the PVC/IL blend. Our previous studies demonstrated that antimicrobial properties were related to the release of IL into the surrounding aqueous solution and the rate of release found to be directly proportional to the antimicrobial activity of the composites.15
With the exception of our previously reported study,15 no other studies characterized PVC/IL blends processed using hot-melt extrusion,15 indeed the limited studies published have focused on PVC/IL blends prepared using mostly environmentally contentious solvent casting methods.20,21 Additionally, neither the effects of the degree of PVC gelation, anti-plasticization, nor PVC resin molecular weight have been described previously. In this study, we report, for the first time, the melt mixing of two ILs, 1-ethylpyridinium docusate and tri-n-butyl(2-hydroxyethyl)phosphonium docusate with flexible medical grade PVC formulations. Furthermore, we have correlated antimicrobial activity, anti-biofilm forming properties and plasticizing efficiency of these PVC/IL blends as a function of PVC molecular weight.
2. Experimental
2.1 Materials
The PVC resins (K-60, K-64 and K-70) and additives were kindly supplied by Colorite Europe Ltd., with the formulations used shown in Table 1. The synthesis of both ILs, 1-ethylpyridinium docusate (IL1) and tri-n-butyl(2-hydroxyethyl) phosphonium docusate (IL2), has been described in detail previously.15 Dioctyl phthalate (DOP) (C24H33O4, 99% Eastman DOP, product number 525154), was purchased from Aldrich, Sigma-Aldrich Co. The following coding system was adopted to name the blends prepared; blends were abbreviated as K-value/wt% of IL or DOP, as in K-60/5% IL1 (seen in tables and captions) refers to PVC formulation containing K-60 PVC resin processed with 5 wt% IL1.| PVC resin and additives | Weight (g) |
|---|---|
| PVC resin (K-60, K-64 and K-70) | 100 |
| Impact modifier | 4 |
| Calcium–zinc stabilizer | 3 |
| Lubricant | 1.1 |
| Process aid | 0.8 |
2.2 Preparation of PVC/IL blends and test specimens
Mixtures of the respective PVC formulation (K-60, K-64 or K-70) with 5%, 10% and 15% by weight of each IL or DOP (total weight 65 g) were mixed using a Rondol Speedmixer (Rondol Technology Ltd.) at 2000 rpm for 2 min. The mixtures were then melt processed using a Thermo Haake PolyLab OS Rheomix 600 Mixer equipped with two counter rotating roller rotors. Mixtures containing K-60 and K-64 PVC resins were processed at 160 °C and rotor speed of 40 rpm for 150 s, while those with K-70 PVC resin were processed at 165 °C and rotor speed of 50 rpm for 150 s. Torque data, temperature and rotor speed were recorded using Polysoft OS software so as to observe gelation behaviour of the samples. Fused PVC compounds were compression moulded into a rectangular shape (140 mm × 110 mm × 1.4 mm) between aluminium sheets lined with PTFE (poly(tetrafluoroethylene)) sheets immediately after mixing (see ESI, Table S1† for processing conditions applied). This was followed by crash cooling to below room temperature at a pressure of 100 MPa for 60 s.To produce samples with desirable thickness (0.5–0.9 mm) for characterization, the samples were cut into smaller dimensions to be compressed again in a mould with dimension 90 mm × 50 mm × 0.50 mm under processing conditions detailed in Table S2† using only aluminum sheets, this time without PTFE sheets to ensure a consistent surface for all samples and minimize the possible role of surface roughness on IL leaching. The samples were cut or stamped into desired shapes and dimensions for characterization.
2.3 Dynamic mechanical thermal analysis (DMTA)
Rectangular samples with width and length of 3–4 mm and 25 mm respectively, were used for determination of Tg, using a Triton thermoanalyzer, DMA Tritec 2000. Measurements were made in the tensile mode using a frequency of 1 Hz, clamp distance of 5.32 mm, displacement of 0.01 mm, and a heating rate of 2 K min−1 from room temperature (RT) to 100 °C.2.4 Static tensile testing
Five dumb-bell shape samples of each blend with dimensions complying to test specimen type 1BA in ISO 527-2 were stamped from the compression moulded sheet. Tensile testing was carried out using a Materials Testing Machine, Lloyd Instruments, Model LRX 2K5 equipped with a 500 N load cell and a pair of eccentric roller grips. Nexygen software (Batch version 4.1 Issue 28) attached to the load cell on the unit was used for data collection. The measurements were performed at RT, using a strain rate of 10 mm min−1 and gauge length of 30 mm. Stress–strain curves were generated and the ultimate tensile strength (U.T.S.), Young's modulus and elongation at break determined.2.5 Thermogravimetric analysis (TGA)
A Thermogravimetric analyzer TGA/SDTA 851e/LF/1600/1382 (Mettler-Toledo) was used to investigate the thermal stability of all samples (<8 mg) using a heating rate of 10 K min−1 in a nitrogen atmosphere over the temperature range 25 °C to 600 °C.2.6 Leaching experiments
A preliminary leaching experiment was carried out using rectangular DMTA specimens of all blends immersed in 15 mL Millipore water for 1 h, 1 day and 4 days. The Millipore water that remained after removal of test specimens was characterised using UV spectrophotometry (Lamda 950, Perkin-Elmer) to identify the presence of leached IL, by comparing with UV measurements taken on standard solutions prepared with known concentration of IL precursors dissolved in Millipore water (see ESI, Fig. S1†). PVC samples removed from Millipore water were left to dry in air until a constant weight was obtained. Weight loss of these samples was measured and plotted as percentage of final weight over initial weight as a function of time. Fourier Transform-Infrared spectra of the PVC samples were recorded using single-bounce universal attenuated total reflection (FTIR-UATR, Perkin-Elmer), at 128 accumulated scans in the wavelength range 4000–650 cm−1. Peak area indicating the presence of sulfonate (a functional group contained in the docusate anion) within the wavelength range 1070–990 cm−1 was calculated using Spectrum software (version 6.3.4), and calibration curves constructed using samples not immersed in Millipore water (ESI, Fig. S2†). Dynamic mechanical thermal analysis was also carried out on these samples, similar to that described in Section 2.3, to determine changes in Tg after a constant weight was achieved upon drying over 3 days or greater. The blend containing the resin that gave the least IL leached was selected (including the samples containing 5 wt%, 10 wt% and 15 wt% DOP), and the leaching process repeated with two replicates. All samples were immersed in 10 mL Millipore water for 1 h, 1 day, 3 days, 6 days, 10 days and 14 days.2.7 Sessile drop contact angle measurement
Sessile drop contact angle measurements were performed using an Easy Drop Standard drop shape analysis system (Krüss) equipped with DSA 1 v1.9 Drop Shape Analysis software, using Millipore water droplets. Images and videos were captured via a monochrome interline CCD (25/30 fps) camera and DSA 1 v1.9 software.2.8 Antibacterial activity
Disc diffusion tests were carried out on a range of different type strains and clinical isolates comprised of 16 Gram-positive bacteria and 8 Gram-negative bacteria (see Table 5), according to the British Society for Antimicrobial Chemotherapy (BSAC) guidelines.22 Logarithmic phase cultures were adjusted to an optical density of 0.15 at 550 nm (equivalent to a 0.5 MacFarland standard) and applied using sterile swabs onto Müller-Hinton agar plates. Three sample discs were placed onto the inoculated agars followed by overnight incubation at 37 °C. Three measurements on the diameter of the zone of inhibition formed were taken, and an image of each plate digitally captured using a GelDocII imaging system (Bio-Rad).2.9 Bacterial adherence and biofilm formation
Samples that generated the optimal antimicrobial effect against planktonically grown bacteria in the disc diffusion test were assessed for antimicrobial efficacy against bacterial isolates grown in bio-film. Two strains of CoNS, B10 and B11R, the latter antibiotic resistant, were cultured from continuous ambulatory peritoneal dialysis catheters. Bacterial suspensions (2 mL) of each isolate (prepared as described in Section 2.8) were pipetted into each well of 12-well plates, which contained sample discs of 4 mm diameter. The plates were then incubated overnight at 37 °C. The samples were removed from the bacterial suspension, rinsed gently in phosphate buffer saline (PBS), transferred to a fresh 12-well plate containing 1 mL PBS, followed by 5 min ultra-sonication to remove adherent bacteria. Following serial dilution, the number of colony forming units were counted and expressed relative to the surface area of the section (cfu cm−2), and percentage inhibition was calculated based on the ratio of the number of colony forming units of PVC/IL and PVC/DOP samples to that of PVC control samples.2.10 Scanning electron microscopy (SEM) of biofilms
Bacterial suspensions prepared as described in Section 2.8 were used to immerse 4 mm diameter disc samples of a PVC control and PVC/IL or PVC/DOP blends, overnight at 37 °C. The samples were then removed from bacterial suspensions and rinsed gently in PBS, before overnight immersion in 0.1 M sodium cacodylate (C2H8AsNaO2) containing 3% glutaraldehyde at 4 °C. This was followed by dehydration carried out at room temperature, by transferring the samples to a 96-well plate containing 50%, 70% and 90% ethanol for 30 min, respectively, and 100% ethanol for three consecutive 30 min periods. Samples were then transferred to a 96-well plate containing hexamethyldisilazane (HMDS) and left to dry overnight in a fume cupboard. Dried samples were mounted on discs, gold-coated, and observed using a JEOL JSM-840A scanning electron microscope (SEM) at magnification of ×3000 with an operating voltage of 15 kV.3. Results and discussion
Torque rheometer testing is a technique commonly used to measure both stability and lubricity of vinyl compounds, from which plots of torque values as a function of time can be generated. From these plots, gelation/fusion time, minimum torque, torque at fusion, equilibrium torque, and ultimate stability time at the onset of crosslinking can be determined.23 In this study, equilibrium torque and ultimate stability were not determined as these may be destructive to the samples. A general curve was observed, characterized by the softening and compaction of the PVC formulation at the very start of mixing, indicated by a sharp peak, followed by minimum torque values where the PVC grains are broken-down into primary particle flow units. Subsequently, formation of a plateau (or an increment in torque) results from entanglement of molecular chain ends, indicating gelation or fusion after the particles coated with plasticizer are subjected to heat and shear, may be observed. This allows for the determination of equilibrium torque and ultimate stability time, if the processing time is sufficiently long. A good plasticizer compatible with the PVC system should exhibit gelation at a shorter gelation/fusion time under the same processing conditions, indicating formation of a flowing homogenous composition of PVC particles and plasticizer.17 Fig. 1 illustrates the gelation behaviour of the PVC formulations containing K-60, K-64 and K-70 resin processed with and without addition of IL1, IL2 and DOP. Earlier gelation was observed for all PVCs with IL2 or DOP added, and gelation time decreased as IL2 or DOP content increased, compared to the unfilled PVC (control), regardless of the K-value of the resin. Increased torque values were not obvious at higher IL2 or DOP content (i.e. 15 wt%), which could be due to higher liquid content. Unlike PVC processed with IL2 or DOP, there was no predictable trend observed when PVC was processed with IL1. For example, for K-60 PVC with 10 wt% IL1, IL1 tended to delay gelation while the opposite was observed for K-64 PVC. As for the PVC formulations containing K-70 PVC resin, no gelation was observed irrespective of IL1 loading, as assessed from torque–time curves. This is represented by straight horizontal line (Fig. 1(c)) showing no change in torque. The sample also had a powdery appearance (not shown) after processing prior to compression moulding.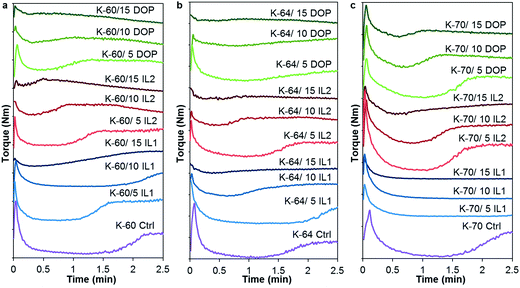 | ||
| Fig. 1 Plastograms (torque as a function of time) for IL and DOP filled non-rigid PVC formulations with PVC having K-values of (a) 60, (b) 64, and (c) 70. | ||
DMTA was used to determine the change in the glass transition temperature (Tg) of the blends over a wide temperature range, as a shift in Tg of the base PVC resin to lower temperatures on addition of ILs or DOP is indicative of plasticization. A decrease of Tg was obtained for the PVCs irrespective of K-value upon addition of DOP or either IL (Fig. 2). The maximum in the tan delta (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) peak at 1 Hz was used to estimate Tg for all PVC samples and the storage modulus (E′) at 37.5 °C recorded, see Table 2. Among IL1, IL2 and DOP, DOP had the highest plasticizing efficiency in all PVC systems, with a total reduction of Tg up to 33 °C, 29 °C, 32 °C for K-60, K-64 and K-70 PVC respectively, for a 15 wt% loading. This is followed by IL2 with a total reduction in Tg of 21 °C, 16 °C, 19 °C; and IL1 having the least plasticizing effect, with Tg reduction of 11 °C, 9 °C, 9 °C for K-60, K-64 and K-70 respectively, again at the 15 wt% level. A reduction in E′ was also observed compared to the unfilled PVC systems; addition of 15 wt% DOP resulted in largest reduction of E′, 1.31 GPa (55%), 0.93 GPa (38%), 1.65 GPa (57%) for K-60, K-64 and K-70 PVC, respectively. The second highest reduction of E′ was observed for addition of 15 wt% IL1, by 0.87 GPa (37%), 0.9 GPa (37%), 1.3 GPa (45%) for K-60, K-64 and K-70 PVC systems. Addition of 15 wt% IL2 led to the lowest reduction in E′, 0.57 GPa (24%), 0.48 GPa (20%), 1.07 GPa (37%) values lower than E′ of unfilled K-60, K-64 and K-70 PVC.
δ) peak at 1 Hz was used to estimate Tg for all PVC samples and the storage modulus (E′) at 37.5 °C recorded, see Table 2. Among IL1, IL2 and DOP, DOP had the highest plasticizing efficiency in all PVC systems, with a total reduction of Tg up to 33 °C, 29 °C, 32 °C for K-60, K-64 and K-70 PVC respectively, for a 15 wt% loading. This is followed by IL2 with a total reduction in Tg of 21 °C, 16 °C, 19 °C; and IL1 having the least plasticizing effect, with Tg reduction of 11 °C, 9 °C, 9 °C for K-60, K-64 and K-70 respectively, again at the 15 wt% level. A reduction in E′ was also observed compared to the unfilled PVC systems; addition of 15 wt% DOP resulted in largest reduction of E′, 1.31 GPa (55%), 0.93 GPa (38%), 1.65 GPa (57%) for K-60, K-64 and K-70 PVC, respectively. The second highest reduction of E′ was observed for addition of 15 wt% IL1, by 0.87 GPa (37%), 0.9 GPa (37%), 1.3 GPa (45%) for K-60, K-64 and K-70 PVC systems. Addition of 15 wt% IL2 led to the lowest reduction in E′, 0.57 GPa (24%), 0.48 GPa (20%), 1.07 GPa (37%) values lower than E′ of unfilled K-60, K-64 and K-70 PVC.
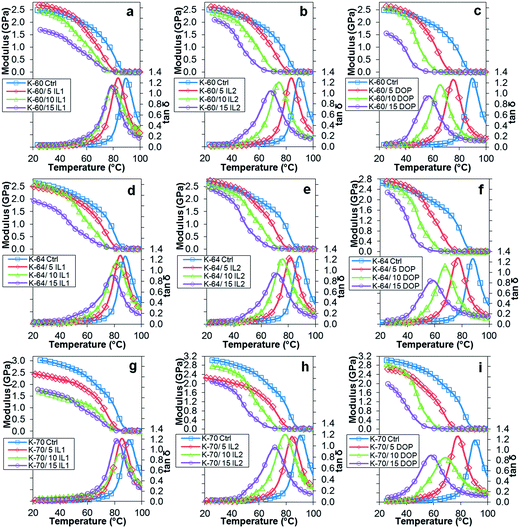 | ||
Fig. 2 Variation in storage modulus and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ with temperature for (a) K-60/IL1, (b) K-60/IL2, (c) K-60/DOP; (d) K-64/IL1, (e) K-64/IL2, (f) K-64/DOP, (g) K-70/IL1, (h) K-70/IL2 and (i) K-70/DOP. δ with temperature for (a) K-60/IL1, (b) K-60/IL2, (c) K-60/DOP; (d) K-64/IL1, (e) K-64/IL2, (f) K-64/DOP, (g) K-70/IL1, (h) K-70/IL2 and (i) K-70/DOP. | ||
| Sample | Wt% | Tg (°C) | E′ at 37.5 °C (GPa) | ||||
|---|---|---|---|---|---|---|---|
| K-60 | K-64 | K-70 | K-60 | K-64 | K-70 | ||
| PVC | 100 | 89.5 | 87.9 | 90.3 | 2.38 | 2.45 | 2.89 |
| IL1 | 5 | 83.6 | 84.7 | 85.6 | 2.54 | 2.31 | 2.25 |
| 10 | 79.3 | 82.4 | 84.7 | 2.24 | 2.37 | 1.48 | |
| 15 | 78.7 | 78.9 | 81.5 | 1.51 | 1.55 | 1.59 | |
| IL2 | 5 | 83.6 | 81.5 | 84.2 | 2.48 | 2.52 | 2.06 |
| 10 | 74.5 | 76.0 | 78.8 | 2.22 | 2.28 | 2.61 | |
| 15 | 68.6 | 71.9 | 71.6 | 1.81 | 1.97 | 1.82 | |
| DOP | 5 | 75.1 | 77.0 | 78.3 | 2.48 | 2.56 | 2.49 |
| 10 | 65.6 | 67.9 | 68.2 | 2.30 | 2.27 | 2.54 | |
| 15 | 56.9 | 58.8 | 57.9 | 1.07 | 1.53 | 1.24 | |
Comparing both ILs, IL2 was a more effective plasticizer, manifest by greater suppression of PVC Tg for equivalent amounts of IL1. This behavior can be explained using Moorhead's empirical approach and free volume theory (Marcilla, 2004),24 based on the difference in chemical structure of IL1 and IL2. Since both ILs shared the same anion (docusate), only differences in their cations are discussed. For IL1, the pyridinium cation has a highly polarized ring structure due to the presence of a quaternary nitrogen, which may form mutual points of attraction with the PVC polymer chain, but flexibility was reduced, as the ethyl group (non-polar group) present may not be sufficiently long in separating or shielding polymer dipoles. While for IL2, the phosphonium cation has more and longer alkyl groups (three butyl groups) attached to it, shielding the polymer dipoles formed around a polar hydroxyl group, therefore higher flexibility could be achieved.
Considering free volume theory, the phosphonium cation has a greater number of chain ends due to larger number of side chains (four on the IL2 cation compared to one for the IL1 cation), as well as higher molecular weight (247.38 g mol−1 for the IL2 cation compared to 108.16 g mol−1 for the IL1 cation). Thus, more free volume can be created by IL2 compared to IL1, although the presence of ester groups in the docusate anion could have contributed to any plasticization in both PVC/IL blends according to studies using sodium docusate surfactant as a plasticizer.25,26
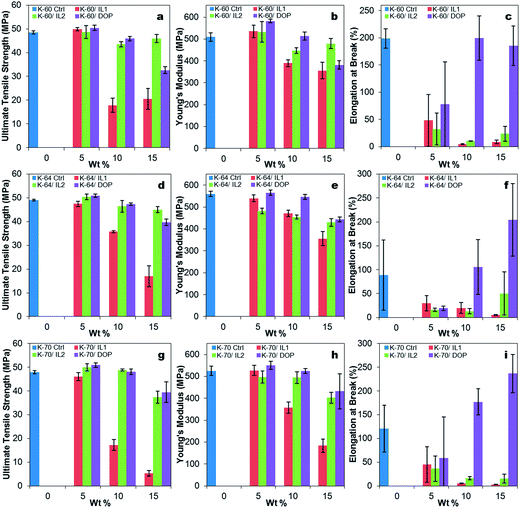
A general reduction of ultimate tensile strength (U.T.S.) and Young's modulus was observed for all samples with increasing addition of DOP and IL, see Fig. 3(a) and (b). The difference between brittle and flexible was revealed by the changes in elongation at break (related to toughness), see Fig. 3(c). On addition of DOP, an increase in elongation at break was observed, demonstrating the plasticizing effect of DOP for all three PVC systems. In contrast, the addition of both ILs resulted in a reduction in elongation at break of all PVC systems as IL loading was increased, showing some degree of embrittlement of the PVC compositions containing 5 wt%, 10 wt% and 15 wt% ILs. This suggested the possibility of anti-plasticization, where the ILs may have promoted crystallite formation, offset plasticization of the amorphous regions, and thus act as stress concentration points.19
Weight loss and derivative weight loss curves for all PVC samples were generated as a function of temperature (ESI, Fig. S3†). A typical two-step thermal degradation was observed in the derivative peaks of all the PVC samples, which comprises a first step dehydrochlorination process (between 230 and 310 °C), followed by second step of polymer backbone chain scission (450–470 °C). A slight decrease in thermal stability was observed in all PVC systems with DOP and ILs included, by comparing the temperature at 5% weight loss with neat PVC (ESI, Table S3†). The reduced thermal stability was reflected in the initiation of weight loss which occurred at lower temperatures, as the amount of IL or DOP added increased. In unfilled K-60, K-64 and K-70 PVC, 5% weight loss occurred at 256 °C, 261 °C and 260 °C, respectively. Following addition of 15 wt% IL1, 5% weight loss occurred at 215 °C, 218 °C and 219 °C for K-60/IL1, K-64/IL1 and K-70/IL1 samples. While for K-60, K-64 and K-70 PVC with 15 wt% DOP added, 5% weight loss occurred at temperatures of 215 °C, 217 °C and 212 °C. IL2 seemed to have the greatest effect in reducing thermal stability of the PVC formulations, as addition of 15 wt% of IL2 reduced the temperature at which 5% weight loss occurred to 207 °C, 205 °C and 209 °C in K-60, K-64 and K-70 PVC, respectively. The difference in thermal stability could be due to the differences in solvating power of ILs or DOP, i.e. IL2 possibly has the strongest solvating power, resulting in the parts of PVC that are solvated undergoing decomposition at lower temperatures.27
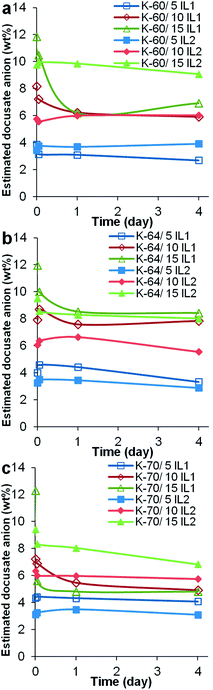
Due to a significant number of samples, a 4 day preliminary leaching test was completed on one sample from each sample set. The information obtained was used to select one particular batch of samples and extract the important experimental variables, whether higher or lower volume of solvents should be used or shorter or longer leaching test should be conducted. In general, it was observed that the K-70 PVC samples suffered the highest weight loss, followed by K-60 then K-64, the latter having the least weight loss, see Fig. 4. Comparing PVC compounds containing the same resin but different ILs, generally the greater weight loss was recorded for PVC/IL1 samples compared to PVC/IL2 samples. This was evident from the higher amount of IL1 detected in the Millipore water used to immerse the PVC compounds, from UV-Vis spectrophotometry (Fig. 5) and measurement of docusate anion in PVC compounds after immersion in Millipore water using FTIR-ATR (Fig. 7). Since the leaching medium (18 MΩ Millipore water) has a negligible concentration of charged species, the same rate of cation and anion leaching from the IL would be expected, in order to keep the charge balance. However, this is contrary to the experimental observations, as described below. We postulate that the different leaching rates of the IL cation and the IL anion are balanced, from the charge point of view, by leaching of charged species used as additives in the formulation of the medical PVCs used such as organic salts of calcium and zinc that can counterbalance the independent leaching of the IL ions. In fact, calculations from UV-Vis spectra showed that the cationic component of IL1 leached out prior to that of its anionic counterpart. This may somehow lead to a more drastic reduction of docusate anion in all PVC/IL1 samples regardless of PVC K-value, as estimated using FTIR-ATR (Fig. 7). However, due to the weak absorption of the IL2 cation and anion as seen in the absorbance values measured using UV-Vis spectrophotometry (see ESI, Fig. S4†), it is difficult to determine whether the cation or anion was released first based on UV-Vis spectra alone for the PVC/IL2 compounds. From Fig. 6, the amount of IL2 cation detected was very high at the beginning of preliminary leaching test for several samples. This could be a consequence of the limited solubility of IL2 in water, or the higher hydrophobicity than the cation of IL1. The concentration of docusate anion detected in Millipore water used to immerse PVC/IL2 compounds also gave very low absorbance values (<0.1 Abs a.u.), and the absorbance values were not directly proportional to the amount of IL2 added, indicating the possibility of noise due to very low amounts of docusate anion detected. To compensate the limitation of docusate anion measurements using UV-Vis spectrophotometry, FTIR-ATR measurements were completed on solid PVC/IL samples after the leaching tests were complete, see Fig. 6. The results indicated significant loss of the anionic component of IL1 from all PVC systems with 15 wt% IL1 added, particularly for the K-70 PVC samples. A smaller loss of anion was detected for PVC samples containing lower IL content. Comparing the different grades of PVC samples, the greatest reduction of anion content was observed for the K-70 PVC samples (up to 7.4 wt% loss from PVC sample K-70/15% IL1), followed by K-60 PVC samples (up to 4.9 wt% loss from PVC sample K-60/15% IL1 added) and K-64 PVC samples (up to 3.5 wt% loss from PVC sample K-64/15% IL1 added). Loss of anion from PVC/IL2 samples was also generally lower than that observed for PVC/IL1 samples, where for PVC K-70/15% IL2 a reduction of 2.6 wt% anion was determined, 1.5 wt% for PVC K-64/15% IL2 and 0.7 wt% for the K-60/15% IL2 sample.
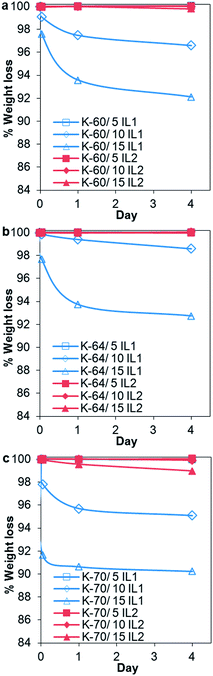 | ||
| Fig. 4 Percentage weight loss for (a) K-60 PVC, (b) K-64 PVC, and (c) K-70 PVC samples as a function of time after immersion in sterile Millipore water during preliminary leaching test. | ||
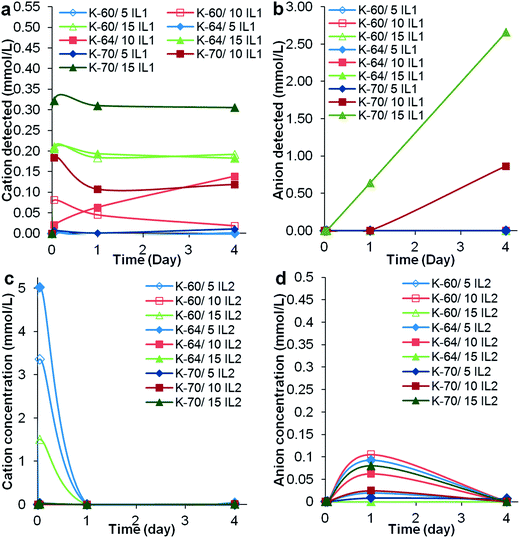 | ||
| Fig. 5 Concentration of (a) IL1 cation, (b) IL1 anion, (c) IL2 cation and (d) IL2 anion determined using UV-Vis spectrophotometry. | ||
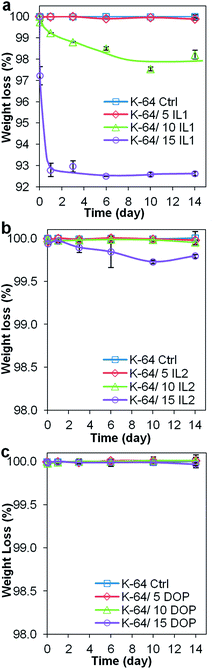 | ||
| Fig. 7 Percentage weight loss of PVC samples (a) K-64/-IL1, (b) K-64/IL2, (c) K-64/DOP throughout the 14 day leaching test as a function of immersion time in Millipore water. | ||
The preliminary leaching test indicated that K-70 PVC samples, especially K-70/10% IL1 and K-70/15% IL1 samples, had the greatest weight loss, with loss of both cation and anion determined from UV-Vis and FTIR-ATR spectra. This could be correlated with PVC gelation observed in Fig. 1, where no indication of gelation was observed in these two particular samples. Since gelation involves intercalation of PVC chain ends during processing, the high loss of IL1 can be explained as reduction in plasticizer (IL1) encapsulation within the PVC matrix as a result of less intercalation and concomitant lower gelation, and is a limitation of the mixing process.
The effect of IL/DOP leaching on Tg of these PVC compounds was investigated using DMTA and the results listed in Table 3. With the exception of PVC samples containing 5 wt% IL1, K-60/10% IL1, K-70/10% IL1 and K-60/5% IL2, the other samples showed increased Tg at the end of the 4 day leaching test. The main reason for the increased Tg was expected to be due to migration of ILs from the base PVC resin, in which the ILs had acted as plasticizer.20 Another possibility could be cross-linking due to a ‘post-curing effect’ after prolonged immersion in water at slightly elevated temperature,28 especially in samples which did not show significant weight loss, see Fig. 4. In the cases where reduction of Tg was observed, plasticization may be due to the presence of water,28 lower molecular weight polymer as a result of degradation,29 and porosity (micro-void formation after leaching of plasticizer).30 Possibly the lack of uniformity in the processing conditions applied during compression moulding, this may have resulted in different degrees of gelation that affected Tg changes after cation/anion leaching. Comparing only PVCs with 15 wt% IL1 added, PVC K-64/IL1 had the least weight loss. Therefore, PVC compounds using K-64 resins were selected to proceed to a longer, more complete leaching study, lasting up to 14 days due to the slow release of IL2 observed in this study.
| Samples | Tg measured with DMTA after time (°C) | |||
|---|---|---|---|---|
| 0 | 1 h | 1 day | 4 day | |
| K-60 ctrl | 89.5 | — | — | — |
| K-60/5% IL1 | 83.6 | 82.3 | 82.8 | 82 |
| K-60/10% IL1 | 79.3 | 80.8 | 81.2 | 81.4 |
| K-60/15% IL1 | 78.7 | 80.5 | 81.9 | 82 |
| K-60/5% IL2 | 83.6 | 82.9 | 81.6 | 82.9 |
| K-60/10% IL2 | 74.5 | 78.3 | 77.9 | 78.2 |
| K-60/15% IL2 | 68.6 | 71.5 | 75.0 | 74.1 |
| K-64 ctrl | 87.9 | — | — | — |
| K-64/5% IL1 | 84.7 | 83.3 | 82.5 | 82.5 |
| K-64/10% IL1 | 82.4 | 80.2 | 80.4 | 80.7 |
| K-64/15% IL1 | 78.9 | 80.5 | 82.8 | 82.1 |
| K-64/5% IL2 | 81.5 | 84.6 | 84.0 | 84.7 |
| K-64/10% IL2 | 76.0 | 79.1 | 79.6 | 77.7 |
| K-64/15% IL2 | 71.9 | 73.9 | 73.7 | 73.3 |
| K-70 ctrl | 90.3 | — | — | — |
| K-70/5% IL1 | 85.6 | 84.8 | 85.2 | 84.7 |
| K-70/10% IL1 | 84.7 | 84.7 | 84.4 | 84.3 |
| K-70/15% IL1 | 81.5 | 86.5 | 86.0 | 84.9 |
| K-70/5% IL2 | 84.2 | 85.5 | 84.7 | 85.4 |
| K-70/10% IL2 | 78.8 | 78.3 | 79.8 | 79.7 |
| K-70/15% IL2 | 71.6 | 75.1 | 74.2 | 78.3 |
As was the case for the preliminary test, K-64 PVC/IL1 samples suffered weight loss (Fig. 7(a)) greater than the weight loss of K-64 PVC/IL2 samples (Fig. 7(b)). K-64 PVC/DOP samples showed the least weight loss (Fig. 7(c)). The amount of plasticizer leached was directly proportional to the amount of plasticizer added. Only K-64/15% IL1, K-64/10% IL1 and K-64/15% IL2 had weight loss, of 7.5%, 2.0% and 0.25%, respectively over the 14 days immersion in Millipore water. Negligible weight loss was observed for the other samples, including K-64/5% IL1, K-64/5% IL2, K-64/10% IL2 samples and all K-64 PVC/DOP samples (<0.1%).
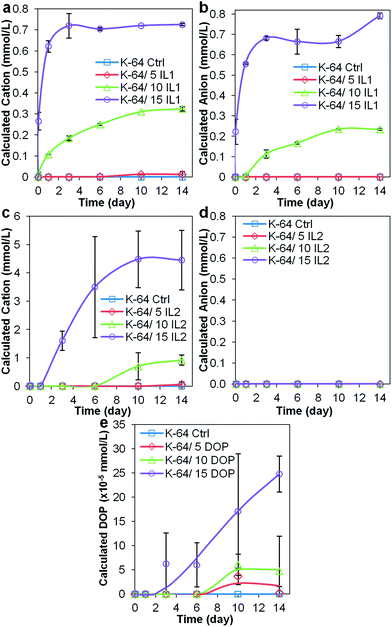 | ||
| Fig. 8 Concentration of (a) IL1 cation, (b) IL1 anion, (c) IL2 cation, (d) IL2 anion, and (e) DOP, determined using UV-Vis spectrophotometry. | ||
UV-Vis spectra of Millipore water samples obtained after immersion of the K-64 PVC/IL1 compounds (see Fig. 8), showed results similar to that for the preliminary test for K-64 PVC/IL1 samples, that is, detection of cation in the first hour of immersion in Millipore water. Due to the difference in the amount of Millipore water used to immerse the samples (15 mL in preliminary test, 10 mL in leaching test for the selected samples), the concentration of IL1 cation detected in the leaching test for K-64 batch of samples was higher. This highlighted that the amount of Millipore water used was crucial for the detection of the docusate anion using UV-Vis spectrophotometry. The slightly lower amount of anion detected may suggest cation leaching before that of anion, which was envisaged based on our previous studies,15 since the cation has a smaller molar volume than the anion. The higher concentrations of IL1 cations detected compared to those found in the preliminary test may have promoted the leaching of anions from the K-64/IL1 PVC samples. In contrast, following immersion of K-64/IL2 samples in water, the concentration of anionic component of IL2 detected using UV-Vis spectrophotometry was negligible and only the cationic component of was detected. Similar results were obtained in our previous study.15 As anticipated, low concentrations of DOP in the leachate were detected by spectroscopic means – the maximum concentration (approximately 25 × 10−5 mmol L−1 to 0.097 mg L−1) corresponded to the solubility limit of DOP in water.31
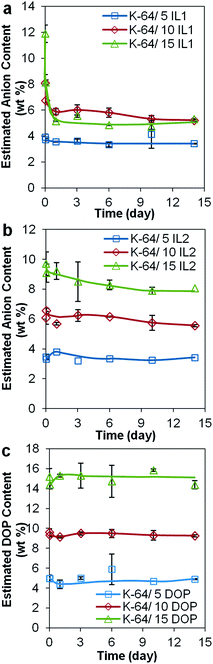 | ||
| Fig. 9 Estimated amount (wt%) of anionic component of (a) IL1 in K-64/IL1 and (b) IL2 in K-64/IL2, and (c) DOP in K-64/DOP, after 14 day leaching test, as detected by FTIR-ATR. | ||
The concentrations of anions from IL1, IL2 and DOP that leached from K-64 PVC were determined using FTIR-ATR spectroscopy (Fig. 9). The concentration of docusate anion that leached from K-64/IL1 samples was found to be the highest among the three types of ‘plasticizer’, indicating the amount of IL1 that could be encapsulated by this particular non-rigid PVC formulation was limited to approximately 5% by weight. The loss of docusate anion could be as high as 6.6 wt% (from K-64/15% IL1) but with reduced IL1 concentrations or with the phosphonium counter ion in IL2 attenuated leaching rates were observed (e.g., 1.7 wt% from K-64/15% IL2) and insignificant anion loss (0.07–0.5 wt%) from formulations with low IL content. The amount of solution-phase DOP detected showed no significant change, ranging from approximately 0.08–0.8 wt%, as expected due to its low solubility in water.31
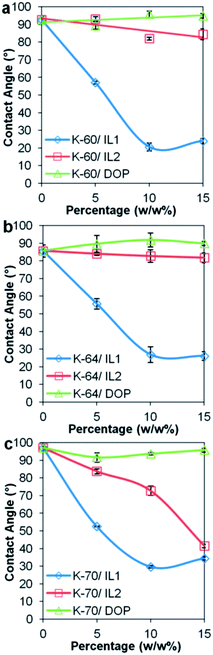 | ||
| Fig. 10 Change in contact angle for (a) K-60 PVC, (b) K-64 PVC and (c) K-70 PVC, as a function of IL1, IL2 and DOP content. | ||
Table 4 lists the changes in Tg for the PVC samples as measured using DMTA after the 14 day leaching test. All K-64 PVC samples showed increased Tg at the end of the leaching test, with fluctuating values for Tg observed in samples that had been immersed between 1 h and 14 days. This could be the result of a combination of various factors discussed earlier.28–30 The fact that K-64 PVC control samples also showed fluctuation of Tg during the leaching test, suggested the possibility of a ‘post-curing effect’, and mild plasticization by presence of water in PVC samples without addition of plasticizer.28
| K-64 Samples | Tg measured with DMTA after time (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 h | 1 day | 3 day | 6 day | 10 day | 14 day | |
| Ctrl | 87.9 | 89.8 | 90.1 | 91.2 | 90.0 | 88.5 | 90.6 |
| 5% IL1 | 84.7 | 85.1 | 84.3 | 85.6 | 85.3 | 84.8 | 84.9 |
| 10% IL1 | 82.4 | 82.3 | 84.9 | 84.1 | 82.8 | 83.0 | 83.2 |
| 15% IL1 | 78.9 | 82.1 | 84.8 | 85.4 | 83.4 | 83.4 | 84.1 |
| 5% IL2 | 81.5 | 83.4 | 82.7 | 84.1 | 84.2 | 83.2 | 82.8 |
| 10% IL2 | 76.0 | 77.0 | 78.3 | 79.0 | 78.9 | 77.0 | 78.8 |
| 15% IL2 | 71.0 | 73.9 | 73.3 | 75.8 | 74.7 | 74.1 | 73.0 |
| 5% DOP | 77.0 | 78.8 | 78.6 | 79.5 | 78.2 | 78.5 | 77.8 |
| 10% DOP | 67.9 | 70.3 | 69.6 | 69.3 | 68.9 | 69.2 | 68.6 |
| 15% DOP | 58.8 | 58.7 | 61.4 | 61.6 | 62.7 | 64.3 | 64.1 |
Changes of contact angle in sessile drop contact angle measurements plotted as a function of IL or DOP content are shown in Fig. 10. A comparison of all three PVCs with 10 wt% IL or DOP added is also shown in ESI, (Fig. S4†). Addition of ILs generally increased the hydrophilicity of the PVC surface to varying magnitude, while addition of DOP increased slightly the hydrophobicity of the PVC surface. A significant reduction in contact angle was observed for all PVC/IL1 formulations with 15 wt% IL1 added, in which the reductions in contact angle were 68.7°, 59.6° and 62.5° for PVC K-60, K-64 and K-70, respectively. While for PVC/IL2 compounds, the reductions in contact angle were less significant. For a 15 wt% IL2 addition to K-60, K-64 and K-70 PVC, the contact angles measured were 8.2°, 3.8° and 58.6° lower than corresponding PVC control samples. The least change in contact angle was observed for PVC/DOP samples, where addition of 15 wt% DOP to K-60 and K-64 only showed a contact angle increment of 2.3°and 4.2° respectively. For K-70 PVC with 15 wt% DOP, a reduction in contact angle as low as 1.7° was recorded. It is noteworthy to point out that for K-70/IL2, unlike the K-60/IL2 and K-64/IL2 samples, a significant increase in hydrophilicity of the polymer surface was observed. Although there were no significant leaching of IL2 observed from K-70/IL2 samples (see Fig. 5 and 6), it is possible that higher amounts of the anionic component of IL2 leached from K-70/IL2 compared to K-60/IL2 and K-64/IL2 samples, altered the properties of Millipore water and further ‘accentuated’ the hydrophilic tendency of the PVC/IL2 sample surface.
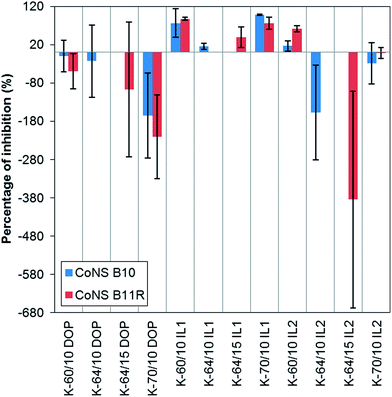 | ||
| Fig. 11 Percentage of inhibition to two clinical CoNS isolates, B10 and B11R, from non-rigid PVC samples containing 10 wt% and 15 wt% IL1, IL2 or DOP. | ||
Both ILs were shown to be bactericidal in a rate-of-kill assay reported in a previous study.15 In the present study, the disc diffusion assay (Table 5) showed that only the PVC systems containing 10 wt% and 15 wt% IL1 inhibited growth of Gram-positive bacteria with no activity apparent against Gram-negative bacteria. The antimicrobial activity of PVC/IL1 samples was expected due to the significant leaching of IL1, while the lack of inhibition from PVC/IL2 samples was due to limited leaching of IL2 from PVC.15 DOP has been proven to be antimicrobial inactive,21 although DOP did not leach from the PVCs. Among the PVC/IL1 samples, K-70/IL1 samples gave the best overall antimicrobial activity, based on the largest inhibition zones observed when tested against Gram-positive organisms. K-64 PVC/IL1 samples demonstrated least inhibitory activity. Relating this to the degree of gelation and preliminary leaching test results, K-70/10% IL1 and K-70/15% IL1 did not show any indication of gelation (Fig. 1) and had the highest level of IL1 leaching. Therefore, very high antimicrobial activity was observed. K-64/IL1 samples demonstrated less antimicrobial activity compared to K-60 and K-70/IL1 samples, in agreement with the results of the preliminary leaching test. This suggests that the antimicrobial activity is directly proportional to the extent of IL leaching and is inversely proportional to the degree of gelation.
| Organism | Zone of inhibition (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K-60/IL1 (wt%) | K-64/IL1 (wt%) | K-70/IL1 (wt%) | |||||||
| 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | |
| a *Type strains; **clinical isolates; R resistant to 3 or more antibiotics ‘—’ no inhibition; ‘Insig.’ insignificant inhibition; ‘+’ inhibition observed. Samples of K-60, K-64 and K-70 PVC processed with DOP and IL2 (not listed) did not give any inhibition at all concentration. | |||||||||
| Staphylococcus aureus NCTC 29213* | — | 7.88 ± 0.19 | 9.11 ± 0.19 | — | 5.22 ± 0.69 | 8.33 ± 0.33 | — | 7.55 ± 0.51 | 9.77 ± 0.38 |
| Staphylococcus epidermidis RP62A** | — | 8.78 ± 0.38 | 9.11 ± 0.84 | — | 4.44 ± 0.51 | 9.00 ± 0.33 | — | 7.33 ± 0.58 | 9.44 ± 0.51 |
| Staphylococcus E-MRSA 16** | — | 8.11 ± 0.19 | 8.55 ± 0.51 | — | 4.89 ± 1.02 | 9.78 ± 0.51 | — | 9.22 ± 0.19 | 9.78 ± 0.38 |
| Staphylococcus aureus AH-7** | — | 5.22 ± 0.38 | 6.33 ± 0.58 | — | 4.50 ± 0.00 | 6.00 ± 0.00 | — | 6.22 ± 0.19 | 6.56 ± 0.19 |
| CoNS B5** | — | 5.33 ± 0.33 | 6.67 ± 0.33 | — | 4.17 ± 0.29 | 6.89 ± 0.51 | — | 4.67 ± 0.29 | 6.67 ± 0.00 |
| CoNS B7R**,R | — | 5.89 ± 0.19 | 7.00 ± 0.00 | — | 4.22 ± 0.38 | 7.00 ± 0.00 | — | 5.44 ± 0.69 | 7.22 ± 0.38 |
| CoNS B8R**,R | — | 6.67 ± 0.33 | 9.00 ± 0.67 | — | — | 8.33 ± 0.69 | — | 6.56 ± 0.51 | 9.11 ± 0.69 |
| CoNS B10** | — | 8.67 ± 0.33 | 9.67 ± 0.58 | — | 5.56 ± 0.77 | 8.89 ± 0.51 | — | 9.00 ± 0.00 | 9.89 ± 0.19 |
| CoNS B11R**,R | — | 6.89 ± 0.19 | 7.11 ± 0.19 | — | 5.11 ± 1.02 | 7.11 ± 0.19 | — | 7.00 ± 0.00 | 8.11 ± 0.19 |
| CoNS B12** | — | 10.44 ± 0.51 | 11.33 ± 0.33 | — | 4.56 ± 0.69 | 11.22 ± 1.07 | — | 11.67 ± 0.67 | 13.00 ± 0.33 |
| MRSA CFP16** | — | 7.78 ± 1.58 | 8.00 ± 0.33 | — | 4.67 ± 0.58 | 7.67 ± 0.33 | — | 6.33 ± 0.58 | 7.33 ± 0.33 |
| MRSA CFP17** | — | 9.00 ± 1.00 | 9.89 ± 0.19 | — | 6.44 ± 0.77 | 9.00 ± 0.00 | — | 6.78 ± 1.07 | 9.78 ± 0.19 |
| MRSA CFP18** | — | 7.89 ± 0.38 | 8.33 ± 0.33 | — | 5.39 ± 1.11 | 8.67 ± 0.67 | — | 8.22 ± 0.38 | 9.78 ± 0.19 |
| MRSA CFP19** | — | 7.11 ± 0.19 | 8.33 ± 0.33 | — | 4.67 ± 0.29 | 8.89 ± 0.19 | — | 8.89 ± 0.19 | 9.33 ± 0.33 |
| MRSA 4D** | — | 8.44 ± 1.26 | 8.89 ± 0.19 | — | 5.22 ± 0.69 | 8.11 ± 0.19 | — | 7.78 ± 0.69 | 9.11 ± 0.51 |
| Enterococcus faecalis NCTC 12697* | — | 5.17 ± 0.29 | 6.00 ± 0.00 | — | 4.50 ± 0.73 | 6.78 ± 0.19 | — | 6.00 ± 0.00 | 7.33 ± 0.33 |
| Pseudomonas aeruginosa NCTC 12934* | — | Insig. | Insig. | — | Insig. | Insig. | — | Insig. | Insig. |
| Pseudomonas aeruginosa B1** | — | Insig. | Insig. | — | Insig. | Insig. | — | Insig. | Insig. |
| Pseudomonas aeruginosa C1** | — | — | — | — | — | — | — | — | — |
| Burkholderia cepacia complex BB1** | — | — | Insig. | — | — | Insig. | — | Insig. | Insig. |
| Burkholderia cepacia complex BB2** | — | — | — | — | — | — | — | — | — |
| Proteus mirabilis NCTC 11938* | — | Insig. | Insig. | — | Insig. | Insig. | — | Insig. | Insig. |
| Klebsiella aerogenes NCTC 10896* | — | Insig. | Insig. | — | Insig. | Insig. | — | Insig. | Insig. |
| Escherichia coli NCTC 10418* | — | — | — | — | — | — | — | — | — |
As sodium docusate (a sodium salt containing the anionic component of the ILs, and used in their synthesis by metathesis – see Experimental section) has been commonly used as a surfactant,32,33 the mechanism of antimicrobial activity of these ILs may resemble that of surface-active agents (or surfactants), achieved via damaging the bacterial cytoplasmic membrane and induce leakage of intracellular constituents. Cationic agents, such as quaternary ammonium compounds (i.e. the cationic component of IL1), induce leakage of intracellular constituents by interacting with phospholipid components in the cytoplasmic membrane. As a result, the membrane is distorted and the cell lysed under osmotic stress. Anionic agents such as sodium lauryl sulphate, induce leakage by disrupting permeability barriers via interaction with the protein components of cytoplasmic membranes.34 Another possible antimicrobial mechanism of ILs is membrane disruption via dissipation of proton-motive force (pmf). Proton-motive force is the potential gradient across the cytoplasmic membrane, and is generated by the electrochemical potential difference between the positively-charged cell interior within the bacteria and negatively-charged exterior. Membrane disruption is caused by protons or electrons carried or attracted across the membrane towards the direction opposite to the requirement of the cell.34 Since the ILs contain both charged cationic and anionic components, the imbalance in release of either component could have caused cell rupture via this mechanism. The lack of significant difference in inhibition zone formed around PVC/IL1 samples among both antibiotic-resistant and antibiotic-susceptible CoNS colonies suggests that the mechanism of action of the ILs under investigation is different from that of antibiotics. Therefore, it is reasonable to conclude that the antimicrobial activity of ILs relates to the presence and concentration of both cationic and anionic component of the ILs leached from the PVC. This is supported by the anion loss from the PVC compounds as measured by FTIR-ATR (Fig. 6). The anion loss from K-70/15% IL2 was comparable (approximately 2.6 wt%) to that of K-60/10% IL1 and K-70/10% IL1 (2.2–2.3 wt%), but no significant inhibition was observed for K-70/15% IL2. If the antimicrobial effect originates solely from the IL anionic component, these PVC samples should show a similar magnitude of inhibition, based on the assumption of similar differences of anion loss from samples into Millipore water in the leaching tests and on the Müller-Hinton agar in the disc diffusion test.
Testing for biofilm formation was carried out using two clinical CoNS isolates, B11R and B10. Samples that gave the best inhibition to B10 and B11R in the disc diffusion test with lowest amount of IL1 were used in this test (ESI, Fig. S5†). All PVC samples containing 10 wt% IL or DOP were selected to test against CoNS B10, while K-60 and K-70 PVC processed with 10 wt% IL or DOP and K-64 PVC with 15 wt% IL or DOP were selected to test against CoNS B11R, see Fig. 11. Positive inhibition against both tested CoNS were obtained for all PVC/IL1 samples and the K-60/10% IL2 sample. The anti-biofilm forming activity of PVC/IL1 samples was probably due to leaching of IL1, as discussed in the previous section. With regard the antimicrobial and anti-biofilm activity of K-60/10% IL2 samples, a discrepancy was observed, in that this sample did not show inhibition in the disc diffusion test. This could be related to the difference in the medium in which the biofilm formation test was completed. The sample was immersed in the bacterial suspension (liquid medium) while only one side of the disc sample was in contact with the bacteria culture on an agar surface (semi-solid medium) in the disc diffusion test. As the percentage of inhibition was calculated based on comparison with control samples, negative percentage inhibition indicated the presence of more biofilm forming CoNS on the sample surface compared to that of the control samples. This was observed for all K-64/IL2 and K-70/DOP samples towards both CoNS isolates, and K-60/DOP samples towards CoNS B11R. Some ambiguity in the percentage inhibition was also observed, such as for K-60/10 wt% DOP, K-64/10 wt% DOP, K-70/10% IL2 towards CoNS B10, and K-64/15 wt% DOP and K-70/10% IL2 towards CoNS B11R. This was a result of mixed positive and negative inhibition in the replicate samples, probably caused by differences in degree of gelation, release rate of IL components due to the different processing conditions employed during the compression moulding process to shape the sample into the required thickness.
Overall, the results of biofilm formation testing and contact angle measurements indicated that these CoNS isolates had the tendency to grow better on hydrophobic surfaces. This result is in agreement with the published literature in that hydrophobic materials have been reported to have a higher tendency for more rapid biofilm development.35–37 However, with the exception of K-60/10% IL2 which was hydrophobic but showed anti-biofilm activity, it is suggested that factors other than surface hydrophobicity/hydrophilicity affects growth of biofilm.
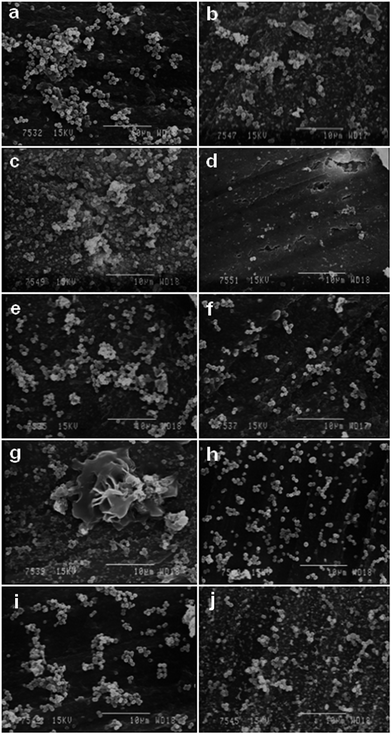
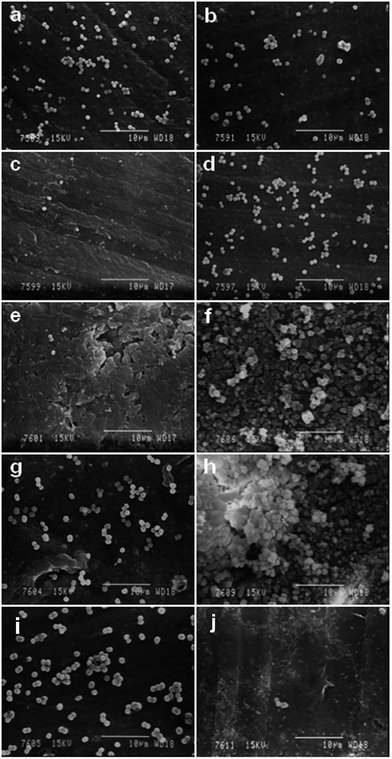
Biofilm growth on all the PVC samples was examined using SEM. Representative SEM images of CoNS B8R biofilm formation on K-64 PVC samples processed with ILs or DOP are shown in Fig. 12. Only the K-64/15% IL1 sample showed less biofilm adherence, in agreement with disc diffusion test results. Nevertheless, the presence of a few Staphylococcus bacteria adhering on this sample indicated the possibility of biofilm growth if incubation continued for more than 24 h. The same SEM image also showed adherence of Staphylococcus bacteria to surface irregularities (upper right corner of Fig. 12(d)), as reported in the literature.35–37 While the adherence of CoNS B8R onto samples K-64/5% IL1 and K-64/10% IL1 exceeded that of neat K-64 PVC sample, suggesting that the presence of IL1 below a minimum inhibition concentration could have promoted CoNS B8R adherence. The mechanism was possibly via altering of the electrostatic force on the PVC/IL1 sample surface due to the ionic nature of ILs,4,38 or surface charge density,39 in addition to the possible role of surface cracks and irregularities.35–37 The pattern of bacterial adherence on K-64/IL2 and K-64/DOP samples was found to be similar, as can be seen from Fig. 12. Compared to K-64/5% IL1, the pattern of adherence on these samples was more dispersed and less dense, probably due to less surface irregularities on these samples. Due to the similarity of the level of bacterial adhesion between PVC/IL2 and PVC/DOP samples (Fig. 12), K-64/IL1 and K-64/DOP samples cultured with CoNS B10 and B11R were selected for comparison, see Fig. 13. Significantly less CoNS B10 bacterial adherence was evident for K-64/10% IL1 and K-64/15% IL1 PVC sample, and less CoNS B11R bacterial adherence on K-64/15% IL1 sample. More bacterial adherence was observed on K-64/10% IL1 sample cultured with B11R CoNS. The pattern of bacterial adherence for CoNS B10 and B11R was also similar to CoNS B8R cultured on K-64/DOP samples.
4. Conclusions
IL1 and IL2 exhibited a plasticizing effect for medical grade PVCs, evident from a gradual reduction in Tg inversely proportional to IL content. Torque measurements during processing revealed the importance of gelation in controlling the release of ILs from the PVC matrix, which in turn affects antimicrobial activity. Furthermore, measurements of porosity and particle size distribution of the PVCs used should also be completed. Inclusion of ILs also altered the hydrophobicity/hydrophilicity of the PVC surface. This may also affect the electrostatic nature or surface charge density of the PVC surface, resulting in biofilm growth when the concentration of IL leached was insufficient to cause effective inhibition. However, it is possible to increase the IL content, which in turn will increase plasticizing efficacy by eliminating potential anti-plasticization, as well as increasing antimicrobial activity.15 Although the presence of ILs seemed to have reduced the thermal stability of PVC via slight acceleration of the degradation process, this should have minimum impact on the PVC/IL compounds for the intended medical device application, for instance catheters have a working temperature (37.5 °C) much lower than this degradation temperature. IL1 and IL2 are effective antimicrobial agents against a range of Gram-positive bacteria and can plasticize PVC comparable to DOP, although both properties are concentration dependent. DOP has no antimicrobial activity.Acknowledgements
S. Y. Choi thanks QUB for funding her studentship. We acknowledge the technical assistance of Gareth Graham, Drs Bronagh Millar, Paula Douglas, Saloni Shukla, Lan Wei, and Colorite Europe Ltd. for supplying the PVC formulations. We thank Dr David Timson (QUB) for helpful discussions.References
- M. T. McCann, B. F. Gilmore and S. P. Gorman, Staphylococcus epidermidis device-related infections: pathogenesis and clinical management, J. Pharm. Pharmacol., 2008, 60, 1551–1571 CAS.
- C. R. Arciola, D. Campoccia, P. Speziale, L. Montanaro and J. W. Costerton, Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials, Biomaterials, 2012, 33, 5967–5982 CrossRef CAS PubMed.
- R. M. Klevens, M. A. Morrison, J. Nadle, S. Petit, K. Gershman and S. Ray, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States, JAMA, J. Am. Med. Assoc., 2007, 298, 1763–1771 CrossRef CAS PubMed.
- I. Raad, H. Hanna and M. Dennis, Intravascular catheter-related infections: advances in diagnosis, prevention and management, Lancet Infect. Dis., 2007, 7, 645–657 CrossRef.
- G. Donelli and I. Francolini, Efficacy of antiadhesive, antibiotic and antiseptic coatings in preventing catheter-related infections: Review, J. Chemother., 2001, 13, 595–606 CrossRef CAS PubMed.
- S. P. Gorman, J. G. McGovern, A. D. Woolfson, C. G. Adair and D. S. Jones, The concomitant development of poly(vinyl chloride)-related biofilm and antimicrobial resistance in relation to ventilator-associated pneumonia, Biomaterials, 2001, 33, 2741–2747 CrossRef.
- M. C. McBride, R. K. Malcolm, A. D. Woolfson and S. P. Gorman, Persistence of antimicrobial activity through sustained release of triclosan from pegylated silicone elastomers, Biomaterials, 2009, 30, 6739–6747 CrossRef CAS PubMed.
- S. G. Patrick, PVC Compounds and Processing, Rapra Rev. Rep., 2004, 15, 10 Search PubMed.
- M. A. Kamrin, Phthalate risks, phthalate regulation, and public health: A review, J. Toxicol. Environ. Health, Part B, 2009, 12, 157–174 CAS.
- M. Manikkam, R. Tracey, C. Guerrero-Bosagna and M. K. Skinner, Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations, PLoS One, 2013, 8, e55387 CAS.
- F. L. Mayer, D. L. Stalling and J. L. Johnson, Phthalate esters as environmental contaminants, Nature, 1972, 238, 411–413 CrossRef CAS.
- J. J. Jakkola and T. L. Knight, The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis, Environ. Health Perspect., 2008, 116, 845–853 CrossRef PubMed.
- K. M. Shea, Pediatric exposure and potential toxicity of phthalate plasticisers, Pediatrics, 2003, 111, 1467–1474 CrossRef.
- M. A. Faouzi, T. Dine, B. Gressier, K. Kambia, M. Luyckx and D. Pagniez, et al. Exposure of hemodialysis patients to di-2-ethylhexyl phthalate, Int. J. Pharm., 1999, 180, 113–121 CrossRef CAS.
- S. Y. Choi, H. Rodríguez, A. Mirjafari, D. F. Gilpin, S. McGrath and K. R. Malcolm, et al. Dual functional ionic liquids as plasticisers and antimicrobial agents for medical polymers, Green Chem., 2011, 13, 1527–1535 RSC.
- J. W. Summers, Introduction, in PVC Handbook, ed. C. E. Wilkes, J. W. Summers and C. A. Daniels, Munich, Carl Hanser Verlag, 2005, pp. 3–4 Search PubMed.
- B. L. Wadey, Plasticizers, in Encyclopedia of Physical Science and Technology, ed. R. A. Meyers, vol 12, 3rd edn New York, Academic Press; 2002, pp. 441–456 Search PubMed.
- L. G. Krauskopf and A. Godwin, Plasticizers, in PVC Handbook, ed. C. E. Wilkes, J. W. Summers and C. A. Daniels, Munich, Carl Hanser Verlag, 2005, pp. 174–175 Search PubMed.
- W. Coaker, Flexible PVC, in PVC Handbook, ed. C. E. Wilkes, J. W. Summers and C. A. Daniels, Munich, Carl Hanser Verlag, 2005, p. 320 Search PubMed.
- M. Rahman and C. S. Brazel, Ionic liquids: New generation stable plasticizers for poly(vinyl chloride), Polym. Degrad. Stab., 2006, 91, 3371–3382 CrossRef CAS PubMed.
- H. Katano and T. Tsukatani, Viscoelasticity, leachability, and antimicrobial activity of poly(vinyl chloride) blended with benzyldimethyltetradecylammonium bis(2-ethylhexyl) sulfonatosuccinate, Bull. Chem. Soc. Jpn., 2010, 83, 190–194 CrossRef CAS.
- BSAC Methods for Antimicrobial Susceptibility Testing, Version 8: British Society for Antimicrobial Chemotherapy (BSAC); 2009.
- T. C. Jennings and W. H. Starnes Jr PVC Stabilizers and Lubricants, in, PVC Handbook, ed. C. E. Wilkes CE, J. W. Summers and C. A. Daniels, Carl Hanser Verlag, Munich, 2005. pp. 142–143 Search PubMed.
- A. Marcilla and M. Beltrán, Mechanisms of Plasticizers action, in, Handbook of Plasticizers, ed. G. Wypych, ChemTec Publishing, Toronto, 2004. pp. 107–119 Search PubMed.
- A. N. Ghebremeskel, C. Vemavarapu and M. Lodaya, Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Stability testing of selected solid dispersions, Pharm. Res., 2006, 23, 1928–1936 CrossRef CAS PubMed.
- A. N. Ghebremeskel, C. Vemavarapu and M. Lodaya, Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer-surfactant combinations using solubility parameters and testing the processability, Int. J. Pharm., 2007, 328, 29–119 CrossRef PubMed.
- A. Marcilla, J. C. Garcia and M. Beltrán, Plasticization Steps, in, Handbook of Plasticizers, ed. G. Wypych, Toronto: ChemTec Publishing, 2004, pp. 92–179 Search PubMed.
- J. S. Earl and R. A. Shenoi, Hygrothermal ageing effects on FRP laminate and structural foam materials, Composites, Part A, 2004, 35, 1237–1247 Search PubMed.
- Y. Haba and M. Narkis, Development and Characterization of Reactively Extruded PVC/Polystyrene Blends, Polym. Eng. Sci., 2004, 44, 1473–1483 CAS.
- S. Kasapis, S. S. Sablani, M. S. Rahman, I. M. Al-Marhoobi and I. S. Al-Amri, Porosity and the Effect of Structural Changes on the Mechanical Glass Transition Temperature, J. Agric. Food Chem., 2007, 55, 2459–2466 CrossRef CAS PubMed.
- C. A. Staples, D. R. Peterson, T. F. Parkerton and W. J. Adams, The environmental fate of phthalate esters: A literature review, Chemosphere, 1997, 35, 667–749 CrossRef CAS.
- Y. Gong, A. Wen, D. Cheung, M. Wong and S. L. Sacks, Preclinical evaluation of docusate as protective agent from herpes simplex viruses, Antiviral Res., 2001, 52, 25–32 CrossRef CAS.
- R. Rosal, I. Rodea-Palomares, K. Boltes, F. Fernández-Piñas and F. Leganés, et al. Ecotoxicological assessment of surfactants in the aquatic environment: Combined toxicity of docusate sodium with chlorinated pollutants, Chemosphere, 2010, 81, 288–293 CrossRef CAS PubMed.
- A. D. Russell and I. Chopra. Chapter 3 Antiseptics, disinfectants and preservatives: their properties, mechanisms of action and uptake into bacteria. in, Understanding Antibacterial Action and Resistance, Great Britain, E. Horwood 1996, pp. 96–101, 107–149 Search PubMed.
- G. Donelli and I. Francolini, Efficacy of Antiadhesive, Antibiotic and Antiseptic Coatings in Preventing Catheter-Related Infections: Review, J. Chemother., 2001, 13, 595–606 CrossRef CAS PubMed.
- R. M. Donlan, Biofilm Formation: A Clinically Relevant Microbiological Process, Clin. Infect. Dis., 2001, 33, 13387–13392 CrossRef PubMed.
- M. Katsikogianni and Y. F. Missirlis, Concise review of mechanisms of bacterial adhesion to biomaterials of techniques used in estimating bacteria-material interactions, Eur. Cells Mater., 2004, 8, 37–57 CAS.
- A. M. A. Dias, S. Marceneiro, M. E. M. Braga, J. F. J. Coelhi and A. G. M. Ferreira, et al. Phosphonium-based ionic liquids as modifiers for biomedical grade poly(vinyl chloride), Acta Biomater., 2012, 8, 1366–1379 CrossRef CAS PubMed.
- H. Murata, R. R. Koepsel, K. Matyjaszewski and A. J. Russell, Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells, Biomaterials, 2007, 28, 4870–4879 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46425c |
| ‡ Present Address: School of Mechanical and Materials Engineering, University College Dublin, Dublin 4, Republic of Ireland. |
| § Present Address: Instituto de Tecnología Química (UPV-CSIC), Universidad Politécnica de Valencia, Avenida de los Naranjos s/n, 46022, Valencia, Spain. |
| This journal is © The Royal Society of Chemistry 2014 |