Ethanol adsorption on rutile TiO2(110)
Abstract
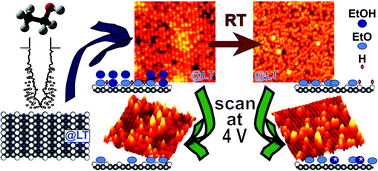
We investigated ethanol adsorption on TiO2(110) surfaces with scanning tunneling microscopy (STM) at 78 K. The ethanol was deposited by pulse injection. The STM images show that the ethanol molecules adsorbed above Ti4+ sites formed rows in the [001] direction even in the case of multilayer adsorption. Ethanol desorbed from the surface when the sample was annealed to room temperature, and the multilayer was reduced to a monolayer. Mainly ethoxy groups remained attached to the surface, and the majority of them were bound to the Ti rows. Furthermore, electron-stimulated desorption experiments revealed that only a few molecules desorbed from the surface when the scans were conducted with a sample bias ≥+3 V. In contrast, when scans were conducted at ≥+4 V, the majority of the hydrogen and covalent bonds between the adsorbates and the rutile were broken and only a small amount of species remained adsorbed on the surface. In the case of the cold dose without further annealing, the remaining ethoxy groups preferentially adsorbed on the Ti rows, whereas in the case of the annealed surface, many of the ethoxys shifted to oxygen bridging sites. This change in the adsorption behavior can be explained by the different oxidation states of the surface, which play an important role in the adsorption-desorption process.

 Please wait while we load your content...
Please wait while we load your content...