A detailed micrometer scale investigation of the solvent bonding process for microfluidic chip fabrication†
Abstract
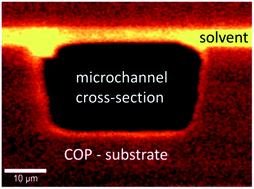
This paper focuses on the effect of polymer–solvent interactions and associated changes in the physical properties induced by the presence of solvent after vapour activation. The motivation was to understand the unbondability of activated parts during microfluidic chip fabrication at industrial scales after storage. In this context, missing knowledge due to still existing assumptions in literature concerning solvent bonding needs to be provided by experimental data finally to clarify the situation. A vapour infusion process using cyclohexane is demonstrated to successfully reproduce a defined solvent effect on injected moulded cyclo-olefin-polymer (COP) surfaces, characterized in this study at micro- and nanometer scales. Confocal Raman microscopy not only allows detection and visualization of remaining solvent in the top micrometer region, but also derivation of its distribution profile within the swollen surface layer. Atomic Force Microscopy (AFM) as a highly sensitive surface analysis technique enables a precise characterization of the top nanometer region of polymer surfaces by thermo-mechanical measurements. It further provides complementary results which are combined to create a complete picture about solvent effects on polymer surfaces. Understanding the influence of different parameters like activation time, time of bonding and post-activation treatments provides important knowledge for chip fabrication. The possibility to assess micrometer scale dimensions further enables imaging of specific areas of interest regarding chemical and thermal properties with local resolution at the nanometer scale. New aspects about solvent bonded interfaces can be graphically illustrated for the first time. A detailed characterization of final chips makes it possible to point out that the general assumption of complete solvent evaporation during bonding is insufficient regarding micrometer scale observations which is of great importance as microfluidic structures are of the same dimension. As long as physical interactions support solvent bonding, the conceptual knowledge derived in this study can be transferred to any polymer–solvent system.

 Please wait while we load your content...
Please wait while we load your content...