An environmentally friendly approach to functionalizing carbon nanotubes for fabricating a strong biocomposite Film†
Abstract
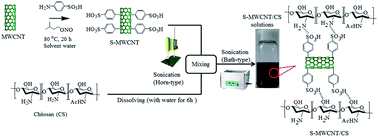
Water-soluble, highly functionalized multiwalled carbon nanotubes (MWNTs) were prepared via a facile, environmentally benign method. The effectiveness of the highly functionalized MWNTs as a reinforcing filler in a water-soluble mechanically weak chitosan biopolymer was evaluated. We observed a substantial improvement in the mechanical properties of the film; the stretchability of the film was maintained when 5 wt% MWNTs was added. In addition, the biocomposite film exhibited long-term antibacterial activity. The reinforcing effect can be explained by the homogeneous dispersion of the nanotubes in the polymer matrix through the strong hydrogen bonding between the sulfonic acid groups (–SO3H) on the sidewalls of the MWNTs and the amine (–NH2) and hydroxyl (–OH) groups on the chitosan backbone. The improvement in the mechanical properties of the MWNT–chitosan biocomposite may make it suitable for many environmental and clinical applications.

 Please wait while we load your content...
Please wait while we load your content...