Facile synthesis of graphene-supported mesoporous Mn3O4 nanosheets with a high-performance in Li-ion batteries†
Chao Chen‡
,
Hong Jian‡,
Xinxin Fu,
Zhimin Ren,
Mi Yan,
Guodong Qian and
Zhiyu Wang*
State Key Laboratory of Silicon Materials, Department of Materials Science & Engineering, Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: wangzhiyu@zju.edu.cn; Fax: +86-571-879-52334; Tel: +86-571-52334
First published on 7th November 2013
Abstract
We report a room-temperature, facile and scalable strategy for the synthesis of Mn3O4 nanosheet/graphene nanocomposites. An important characteristic of these Mn3O4 nanosheets is their mesoporous nature and they have mesopores which are ∼4 nm in size. Such nanocomposites exhibit a high performance in lithium-ion batteries by virtue of this advantageous structural feature.
Lithium-ion batteries (LIBs) are considered to be the leading candidate as a power source for consumer electronics and electric vehicles.1 Researchers have devoted significant attention to the development of electrode materials with high energy density, a long lifespan and environmental benignity.2 Among the available alternative anode materials, manganese oxide (Mn3O4) is a very promising candidate as it has a much higher capacity (theoretical specific capacity is about 937 mA h g−1) than conventional graphite (372 mA h g−1), and it is nontoxic.3 Nevertheless, the high intrinsic resistance limits the performance of Mn3O4 based electrodes, especially at high current densities. Additionally, the volume change associated with alloying and dealloying processes always generates a large internal stress, leading to the disintegration of the electrode materials.4 This in turn leads to quick capacity fading over extended charge–discharge cycling.5 So far, an enormous effort has been made to circumvent the above issues by designing Mn3O4 based nanostructures with an optimized particle size, shape and component composition.6 However, the fabrication of truly durable Mn3O4 electrodes with satisfactory high-rate capabilities and highly specific capacities is still a great challenge. The use of hybrid nanostructures, where the nano-sized active materials are assembled onto or embedded into the conductive matrix via chemical bonding or noncovalent forces, has been regarded as one of the most effective approaches. Due to their superior electrical conductivity, large surface area and excellent structural flexibility, graphene and carbon nanotubes are popular materials utilized for the enhancement of anode materials.7
In this contribution, we report a room-temperature, facile and scalable strategy for the synthesis of Mn3O4 nanosheet nanocomposites supported by graphene (Mn3O4/G), as an advanced anode material for high-performance LIBs. The mesopores have a size distribution of 2–4 nm in the Mn3O4 nanosheets and are another important feature of the nanocomposite. In theory, such nanocomposites are expected to manifest greatly improved electrochemical performances due to the integration of two advantageous structural features. Specifically, the highly conductive and flexible graphene can provide a three-dimensional electronic network that facilitates the charge transfer. Furthermore, the mesoporous Mn3O4 nanosheets provide a void space for buffering the structural alterations which accompany Li insertion and extraction. Benefiting from the enhanced structural stability and kinetics for lithium transportation, the Mn3O4/G nanocomposite exhibits a high performance in LIBs.
An illustration of the synthetic route to Mn3O4/G nanocomposites is shown in Fig. 1. Details for the growth of the Mn3O4 nanosheets onto graphene are presented in the ESI;†the whole synthesis process was operated at room temperature. Graphene oxide (GO) was synthesized via a modified Hummers' method as described elsewhere.8 Fig. S1† presents the X-ray diffraction (XRD) patterns of the as-prepared nanocomposite. The peaks are in good agreement with hausmannite (Mn3O4) (JCPDS card: 24-0734). No observable XRD peaks corresponding to graphite are found in the XRD pattern, which may be ascribed to its low proportion within the nanocomposite. The graphene content was determined by thermogravimetric analysis (TGA) (Fig. S2†). A large weight loss can be observed at ∼400 °C, and the total amount of graphene present in the composite is measured to be about 5.5 wt%, which is quite consistent with the predicted 5 wt% in the recipe of the synthesis.
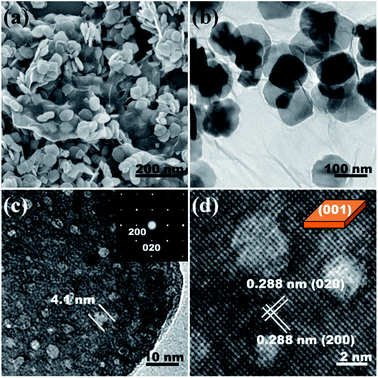
Fig. 2 shows the morphology of the as-prepared Mn3O4/G composite. It can be seen from Fig. 2a that uniform Mn3O4 NSs are supported by the graphene. The Mn3O4 NSs have a side length of about 100 nm, and a thickness of 15 nm. Most of the NSs are uniformly assembled on the graphene, providing enough surface contact between the graphene and Mn3O4 for charge transport to occur. The TEM image in Fig. 2b confirms the morphology of the sheets. It is interesting that from the high magnification TEM (Fig. 2c) of a single nanosheet, it can be seen that the inner microstructure consists of mesopores with a size distribution of 2–4 nm. We consider that the mesopores were formed during the transformation process from Mn(OH)2 to Mn3O4. The reaction in the solution was triggered by the injection of hydrazine hydrate and immediately after that, a white Mn(OH)2 floccule was formed. The Mn2+ of the Mn(OH)2 in aqueous solution was partially oxidized to Mn3+ and formed a brown Mn3O4 precipitate. From the viewpoint of mass transfer, the transformation from Mn(OH)2 to Mn3O4 can be regarded as the release of H and O atoms from the inside of the nanocrystal to the outside. This process may be the origin of the micropores. The selected area electron diffraction (SAED) of a single nanosheet was collected along the major zone axes of [001], and the clear diffraction spots suggest a single-crystalline nature of the nanosheet. The corresponding HRTEM in Fig. 2d confirms the high crystallinity and mesopores in the nanosheets. The lattice fringes with a spacing of 0.288 nm are assigned to the planes of (020) and (200). From this, we conclude that the top and bottom facets of the nanosheets are the (001) and (00![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) planes respectively.
) planes respectively.
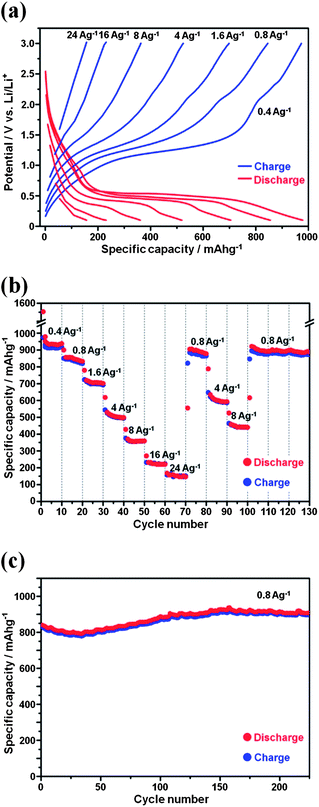
The electrochemical performance of the Mn3O4/G electrode was assessed in a half-cell configuration using lithium metal as the reference and counter electrode. We have examined the Li+ ion storage capacity of the electrode and the capacity within a cut-off voltage window of 0.1–3.0 V. Fig. S4† shows the charge and discharge curves of the Mn3O4/G anode for the first ten cycles at a current density of 0.4 A g−1. The initial discharge and charge capacities are found to be 1553 and 973 mA h g−1 respectively. The irreversible capacity loss of 37% may be mainly attributed to an irreversible process such as the inevitable formation of an inorganic solid electrolyte interface (SEI) film and electrolyte decomposition. The reaction between Li and Mn3O4 is based on a conversion (redox) reaction:
| Mn3O4 + 8Li+ + 8e− → 3Mn(0) + 8Li2O. |
Fig. 3a shows the representative charge and discharge voltage profiles of the Mn3O4/G anode at various current densities. With the increase in current density, the discharge potential of the anode decreased and the charge potential increased. The flat voltage plateaus at ∼0.4 V and reflects the Li+ discharge reaction. The charge curve shows a sloping plateau at ∼1.2 V due to the reverse reaction.
Benefiting from the high electrical conductivity of graphene, our Mn3O4/G nanocomposite exhibits an excellent cycling response to a continuously varying current rate although the Mn3O4 electrodes are generally observed to suffer from sluggish kinetics. Fig. 3b presents the rate capability of this anode material tested from 0.4 A g−1 to 24 A g−1 with identical discharge and charge current densities and with ten discharge–charge cycles at each rate. The cell was first cycled at a low current density of 0.4 A g−1 for ten cycles, where a stable specific capacity of about 920 mA h g−1 was obtained, based on the mass of the Mn3O4/G composite. The Mn3O4/G nanocomposite keeps a reversible capacity of about 710 mA h g−1 after ten cycles at a current density of 1.6 A g−1. When cycled at a higher rate of 4–8 A g−1, a decent capacity of 360–510 mA h g−1 can still be maintained. Moreover, after deep cycling at 16 A g−1 and 24 A g−1, a constant capacity of ∼880 mA h g−1 can be restored when returned to the current density of 0.8 A g−1. What is interesting is that this capacity is even higher than the previous capacity (cycles from 10th to 20th, at a current density of 0.8 A g−1). Similar increasing trends have also been observed in the following cycling at a density of 4, 8, and 0.8 A g−1. This phenomenon is attributed to the reversible growth of a polymeric gel-like film resulting from kinetically activated electrolyte degradation, which has been well-documented in other literature.9 To our knowledge, such a remarkable high-rate performance is superior to that of most Mn3O4 based electrodes.
Another important property of the electrode material is its cycling stability. Generally speaking, the charge and discharge processes always induce a large localized strain because of the concentration of polarization and the induction of a lower specific capacity. However, our Mn3O4/G electrode shows a superior cycling performance. As shown in Fig. 3c, the capacity of the Mn3O4/G electrode increased from ∼840 mA h g−1 to ∼910 mA h g−1 at a current density of 0.8 A g−1 after 230 discharge–charge cycles. We believe that its unique mesoporous structure may be attributed to its excellent cycling performance. The local void space could partially accommodate the volume change during cycling, thus delaying the capacity fading.10 The bare Mn3O4 nanosheets with mesopores also show a good cycling performance at a current rate of 80 mA g−1 (Fig. S4†). What's more, the relatively small size of the Mn3O4 NSs in the [001] direction and the graphene can provide particularly short Li+ diffusion paths, and can therefore accommodate the strain.
Conclusions
In summary, an advanced graphene-supported mesoporous Mn3O4 nanosheet nanocomposite has been constructed by a room-temperature, facile and scalable strategy. In virtue of its greatly enhanced kinetics for electrical conductivity and electrode stability, this unique nanocomposite exhibits an exceptionally high-rate capabilities, at high current densities, and an excellent cyclic performance. In addition, our research provides a very simple strategy to fabricate the graphene-supported mesoporous Mn3O4 nanosheets at room-temperature. Thus, this facile synthetic method of producing this high-performance nanocomposite will trigger their application in the next-generation of LIBs.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (no. 51229201), and the Doctoral Fund of the Ministry of Education of China (no. 20100101110039) for their financial support.Notes and references
- M. Thackeray, Nat. Mater., 2002, 1, 81 CrossRef CAS PubMed; T. Sasaki, Y. Ukyo and P. Novák, Nat. Mater., 2013, 12, 569 CrossRef PubMed; M. Gu, I. Belharouak, A. Genc, Z. Wang, D. Wang, K. Amine, F. Gao, G. Zhou, S. Thevuthasan, D. R. Baer, J. G. Zhang, N. D. Browning, J. Liu and C. Wang, Nano Lett., 2012, 12, 5186 CrossRef PubMed.
- R. Marom, S. F. Amalraj, N. Leifer, D. Jacob and D. Aurbach, J. Mater. Chem., 2011, 21, 9938 RSC; M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364 CrossRef CAS PubMed; A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed.
- H. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978 CrossRef CAS PubMed.
- J. Y. Huang, L. Zhong, C. M. Wang, J. P. Sullivan, W. Xu, L. Q. Zhang, S. X. Mao, N. S. Hudak, X. H. Liu, A. Subramanian, H. Fan, L. Qi, A. Kushima and J. Li, Science, 2010, 330, 1515 CrossRef CAS PubMed; M. Gu, Z. Wang, J. G. Connell, D. E. Perea, L. J. Lauhon, F. Gao and C. Wang, ACS Nano, 2013, 7, 6303 CrossRef PubMed; M. Gu, Y. Li, X. Li, S. Hu, X. Zhang, W. Xu, S. Thevuthasan, D. R. Baer, J. G. Zhang, J. Liu and C. Wang, ACS Nano, 2012, 6, 8439 CrossRef PubMed.
- Z. Wang, D. Luan, S. Madhavi, Y. Hu and X. W. Lou, Energy Environ. Sci., 2012, 5, 5252 Search PubMed; X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- L. Li, Z. Guo, A. Du and H. Liu, J. Mater. Chem., 2012, 22, 3600 RSC; J. Gao, M. A. Lowe and H. D. Abruna, Chem. Mater., 2011, 23, 3223 CrossRef CAS; Z. Li, N. Liu, X. Wang, C. Wang, Y. Qi and L. Yin, J. Mater. Chem., 2012, 22, 16640 RSC; C. Wang, L. Yin, D. Xiang and Y. Qi, ACS Appl. Mater. Interfaces, 2012, 4, 1636 Search PubMed.
- X. Xin, X. Zhou, J. Wu, X. Yao and Z. Liu, ACS Nano, 2012, 6, 11035 CrossRef CAS PubMed; D. Wang, Y. Li, Q. Wang and T. Wang, Eur. J. Inorg. Chem., 2012, 628 CrossRef; Z. S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 6, 3187 CrossRef PubMed.
- W. S. Hummers, Jr and R. E. Offema, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS; Y. Liang, H. Wang, H. S. Casalongue, Z. Chen and H. Dai, Nano Res., 2010, 3, 701 CrossRef PubMed.
- K. T. Nam, D. W. Kim, P. J. Yoo, C. Y. Chiang, N. Meethong, P. T. Hammond, Y. M. Chiang and A. M. Belcher, Science, 2006, 312, 885 CrossRef CAS PubMed; R. Wang, C. Xu, J. Sun, Y. Liu, L. Gao and C. Lin, Nanoscale, 2013, 5, 6960 RSC; Y. M. Lin, P. R. Abel, A. Heller and C. B. Mullins, J. Phys. Chem. Lett., 2011, 2, 2885 CrossRef.
- W. Wei, Z. Wang, Z. Liu, Y. Liu, L. He, D. Chen, A. Umar, L. Guo and J. Li, J. Power Sources, 2013, 238, 376 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and additional experimental results are available, including X-ray diffraction (XRD), thermogravimetric analysis (TGA) and galvanostatic discharge–charge voltage profiles. See DOI: 10.1039/c3ra45976d |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |