Barium-triggered β-sheet formation and hydrogelation of a short peptide derivative†
Abstract
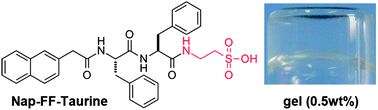
We reported on a short peptide–taurine conjugate that could adopt β-sheet conformation and form hydrogels triggered by Ba2+, which might be applied for the removal of Ba+ from water with high Ba2+ content.

 Please wait while we load your content...
Please wait while we load your content...