A nanocomposite of silver and thermo-associating polymer by a green route: a potential soft–hard material for controlled drug release†
Abstract
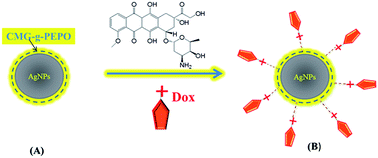
Major research efforts are continuously being made to look for alternative, environment friendly green chemicals for the synthesis of nanoparticles in place of conventional and hazardous reducing agents such as sodium borohydride and hydrazine. We report here on the synthesis and characterization of AgNPs using a thermo-associating polymer namely, carboxymethyl guar grafted poly(ethylene oxide-co-propylene oxide) [CMG-g-PEPO]. The polymer acts as both reducing agent as well as stabilizing/capping agent. The formation of AgNPs with polymer was confirmed by UV/Vis spectroscopy and the TEM images indicated the size of nanoparticles to be in the range of 10–20 nm. We also demonstrated the use of these nanoparticles in the controlled release of doxorubicin hydrochloride (Dox), an anticancer drug. The binding of Dox onto the polymer and AgNPs was investigated by XPS and Raman spectroscopy which indicates that a charge-transfer mechanism is operative between the Dox and polymer holding both the entities together. The first synthesis of AgNPs using non-toxic thermo-associating polymer and subsequent release of Dox with body temperature (37 °C) as a trigger is the highlight of the present work.

 Please wait while we load your content...
Please wait while we load your content...