In situ hydrogenation of molybdenum oxide nanowires for enhanced supercapacitors
Abstract
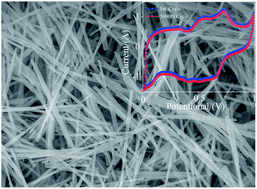
In situ hydrogenation of orthorhombic molybdenum trioxide (α-MoO3) nanowires has been achieved on a large scale by introducing alcohol during the hydrothermal synthesis for electrochemical energy storage supercapacitor devices. The hydrogenated molybdenum trioxide (HxMoO3) nanowires yield a specific capacitance of 168 F g−1 at 0.5 A g−1 and maintain 108 F g−1 at 10 A g−1, which is 36-fold higher than the capacitance obtained from pristine MoO3 nanowires at the same conditions. The electrochemical devices made with HxMoO3 nanowires exhibit excellent cycling stability by retaining 97% of their capacitance after 3000 cycles due to an enhanced electronic conductivity and increased density of hydroxyl groups on the surface of the MoO3 nanowires.

 Please wait while we load your content...
Please wait while we load your content...