Short synthesis of ordered silicas with very large mesopores†
Liang
Cao‡
ab and
Michal
Kruk
*ab
aDepartment of Chemistry, College of Staten Island, City University of New York, 2800 Victory Boulevard, Staten Island, NY 10314, USA. E-mail: Michal.Kruk@csi.cuny.edu; Fax: +1 718 982 3910; Tel: +1 718 982 4030
bGraduate Center, City University of New York, 365 Fifth Avenue, New York, New York 10016, USA
First published on 8th November 2013
Abstract
SBA-15 silicas with cylindrical mesopores of diameter from 10 to ∼30 nm were synthesized within hours (2.5–7.5 hours) instead of one or more days required in hitherto reported syntheses of large-pore SBA-15 with (100) interplanar spacing above 13 nm. The synthesis was carried out using Pluronic P123 surfactant, 1,3,5-triisopropylbenzene swelling agent and silica hydrolysis/condensation catalyst, NH4F, under low-temperature initial synthesis conditions (12–17 °C) similar to those used in our earlier report on the synthesis of SBA-15 with a wide range of pore diameters (10–26 nm). Herein, it is shown that the low temperature step at which the ordered material forms can be shortened to one hour and the mesoporous material can be recovered at this stage. However, the attainment of a larger pore size and/or better structural ordering requires a hydrothermal treatment, which can be as short as 1–6 hours, if appropriately high temperature (150–170 °C) is selected. Additionally, improved synthesis conditions are reported for ultra-large-pore SBA-15 with (100) interplanar spacing of 25–30 nm. Standard SBA-15 synthesis without a swelling agent was also streamlined. Moreover, the synthesis of large-pore FDU-12 silica with face-centered cubic structure of spherical mesopores using Pluronic F127 surfactant and 1,3,5-trimethylbenzene swelling agent can be shortened to ∼9 hours. The streamlined synthesis procedures can be followed by surfactant removal via calcination with high ramping rate and short dwell time. The synthesis approach based on a short self-assembly step and short high-temperature hydrothermal treatment provides a convenient way to produce large-pore ordered mesoporous silicas suitable for numerous applications.
Introduction
Over the last twenty one years, surfactant-micelle-templated ordered mesoporous materials (OMMs) have evolved from a breathtaking discovery1,2 to a widely known, vast family of well-defined materials that manifest the power of the bottom-up synthesis paradigm.3–6 Some of these materials, like MCM-41 (ref. 1 and 7) and SBA-15 (ref. 8–10) silicas, have become ubiquitous in fields of heterogeneous and enzymatic catalysis,11,12 separations,13 adsorption,12,14,15 nanostructure synthesis16,17 and so forth. Applications of surfactant-micelle-templated OMMs significantly rely on the development of cost-effective synthesis pathways to these remarkable materials. One of the underlying issues is the duration of the synthesis.18 A pathway from precursors to the final material involves the self-assembly of a framework precursor with a surfactant to form ordered framework-surfactant nanocomposite,19–23 followed by the recovery of the material (for instance by filtration and drying) and the removal of the surfactant.1,24,25 For the latter to be successful, the framework needs to be sufficiently condensed (cross-linked) to make it sustainable upon the surfactant removal.26 While it is known that in some cases the self-assembly and cross-linking may take seconds,27 typically used synthesis procedures take days,1,8 which ensures that the originally formed structure would not collapse or rearrange upon the surfactant removal28 and allows one to suppress structural shrinkage29 and/or to restructure the framework to reach the pore size and pore volume potentially achievable9,30 or to promote the desired pore connectivity.31,32 The above processes may take hours or days.29,30,32 It is important from the points of view of applications and basic research to understand the time scale of OMM formation23,33,34 and to develop optimized synthesis methods that will take fractions of normally used time and yet will inherit the benefits of reproducibility and custom-tailorability characteristic of the hitherto reported OMM syntheses.18It has been recognized that the synthesis time for SBA-15 silica with a typical (100) interplanar spacing, d100, of ∼10 nm can be reduced from one or more days8,9 to 2–8 hours using optimized procedures involving conventional heating35–37 or microwave heating.18 The recent work on a large-scale synthesis with fast mixing that affords an ordered mesoporous silica analogous to SBA-15 indicated that the synthesis time can be further reduced to minutes with an appropriate selection of a precursor and conditions.38 However, a limited progress has been achieved in the shortening of the synthesis of large-pore SBA-15 (LP-SBA-15) silicas30,39–44 with d100 of 13 nm or larger, even though these materials are desirable as catalyst supports,45–49 adsorbents,50 media for immobilization of biomolecules,51 supports for polymer brushes,49,52 and templates for nanostructures.53 In our earlier work,30 the synthesis involving 1,3,5-triisopropylbenzene as a swelling agent and NH4F as a hydrolysis/condensation catalyst was shortened to 9 hours, but the obtained d100 was only 13.5 nm. In another report, a similar synthesis involving heptane,54 which can potentially afford LP-SBA-15,43,54,55 was found amenable to the shortening to 9 hours, but the product had a pore diameter similar to that for a regular SBA-15.54 The reduction of the time needed for the synthesis of large-pore FDU-12 (LP-FDU-12) has been explored to an even smaller extent. The initially developed procedure takes four days, as it involves a low-temperature step (∼15 °C), a hydrothermal treatment and an acid treatment.56 Subsequent studies showed that either the hydrothermal treatment29 or the acid treatment57,58 or both58 can be skipped when preparing FDU-12 with large accessible mesopores, but the resulting modified synthesis still takes ∼2 days.29,57,58 Because of the fact that LP-FDU-12 is very useful as a catalyst support,57 template for nanostructures,53,56,59 proteomic reactor60 and adsorbent for biomolecules,61 the development of its streamlined synthesis is highly desirable.
Herein, it is shown that the time needed to synthesize the best-known large-pore ordered mesoporous silicas (LP-SBA-15 and LP-FDU-12) can be decreased 5–10 times (from two or more days to several hours) through the identification of the time spans needed for the self-assembly and the high-temperature hydrothermal treatment.62 It is demonstrated that in the case of LP-SBA-15 synthesis, the shortening of the procedure does not compromise the pore size adjustability and the degree of structural ordering.62
Experimental section
Synthesis
The synthesis mixture composition for LP-SBA-15 (ref. 62) followed that used in our earlier study,30 although the amount of the swelling agent was in some cases optimized to obtain more ordered ultra-large-pore materials. A typical synthesis of LP-SBA-15 was as follows. 2.4 g of Pluronic P123 surfactant (BASF, EO20PO70EO20) and 0.027 g of NH4F were dissolved using a mechanical stirrer in 84.0 mL of 1.30 M aqueous HCl solution at a selected initial temperature (12.25–17 °C). After 30 minutes, a mixture of 5.5 mL TEOS and a selected amount of TIPB (1.2 to 3.6 mL) was added and the resulting synthesis mixture was stirred for one hour at the same temperature in an open polypropene container. Afterwards, the mixture was moved into a closed Teflon-lined autoclave and heated in an oven set at 170 °C (or other temperature) for periods of time ranging from one to six hours. As-synthesized surfactant-templated silicas were isolated by filtering, dried and calcined under air at 400 °C for 2 h with a heating ramp 20 °C min−1. The obtained samples are denoted X g + YC/Y′H + ZC/Z′H, which means that a sample was synthesized with X grams of TIPB (per 2.4 g of Pluronic P123) at an initial synthesis temperature Y (°C) maintained for Y′ hours (from the time of addition of TEOS–TIPB mixture) and with the hydrothermal treatment at temperature Z (°C) for Z′ hours.The conditions of the formation of LP-FDU-12 (ref. 62) were similar to those reported by others,63 but modified to reduce the duration of the synthesis. The synthesis was as follows. 1.0 g of Pluronic F127 (BASF, EO106PO70EO106) copolymer and 5 g of KCl were dissolved using a mechanical stirrer in 61 mL of 2.0 M aqueous HCl solution at 15 °C. After 30 minutes, 1.4 mL of TMB was introduced. After 30 minutes of constant stirring, 4.5 mL of TEOS was added and the mixture was stirred for 3 hours in an open polypropene container. Afterwards, the product was moved into a closed Teflon-lined autoclave and heated at 170 °C for 4.5 hours. As-synthesized silica was isolated by filtering without washing and calcined under air at 500 °C for 2 h (heating ramp 20 °C min−1).
Characterization
Small-angle X-ray scattering (SAXS) patterns were recorded using a Bruker Nanostar U small-angle/wide-angle X-ray scattering instrument with a rotating anode X-ray source and Vantec-2000 two-dimensional detector. Transmission electron microscopy (TEM) images were acquired on a FEI Tecnai Spirit microscope operated at 120 kV. Before the imaging, the samples were sonicated in ethanol to disperse them and then the resulting suspension was deposited on a carbon-coated copper grid. Nitrogen adsorption isotherms at −196 °C were measured on a Micromeritics ASAP 2020 volumetric adsorption analyzer. The samples were outgassed under vacuum at 200 °C before the adsorption measurements.The specific surface area, SBET, was calculated from nitrogen adsorption data in the relative pressure range from 0.04 to 0.2 using the Brunauer–Emmett–Teller (BET) method.64 The total pore volume, Vt, was obtained on the basis of the amount adsorbed at a relative pressure of 0.99.64 The micropore volume, Vmi, was determined using the αs plot method64,65 in the standard reduced adsorption (αs) range from 0.9 to 1.2. The sum of the primary (ordered) mesopore volume, Vp, and the micropore volume, Vmi, was estimated using the αs plot method in the αs range selected within 2.0–2.55 interval.30 The reference adsorption isotherm for a macroporous silica was employed in the αs plot calculations.65 For SBA-15 samples with pore diameter above ∼18 nm, it was not a good approximation to assess Vp + Vmi using the αs plot method, because it would require calculations from adsorption data at pressures very close to the bulk condensation pressure (p/p0 > 0.95, where p is equilibrium vapor pressure and p0 is the saturation vapor pressure), for which fully reliable reference adsorption data are difficult to acquire. In addition, any possible contribution from the capillary condensation in secondary (interparticle) pores would have a detrimental effect on the accuracy of the evaluation of Vp + Vmi Therefore, Vp + Vmi (which after subtraction of Vmi yields Vp, the latter being used in eqn (1) to calculate the pore size, see below) was estimated as 0.783 × Vt, the constant 0.783 being determined on the basis of data for samples for which (Vp + Vmi)/Vt ratio was determined with acceptable confidence in our earlier study.30 Pore size distributions (PSDs) were determined from adsorption branches of isotherms66 using the Barrett–Joyner–Halenda (BJH) calculation procedure67 with the Kelvin equation for hemispherical meniscus and with Kruk–Jaroniec–Sayari (KJS) correction,66 and with the statistical film thickness curve for a macroporous silica gel.65 The BJH–KJS pore diameter, wKJS, is defined herein as a position of the maximum on PSD.
The diameter of ordered mesopores, wd, of SBA-15 was determined using a geometrical equation for materials with 2-D hexagonal structure of cylindrical pores that are separated from one another by microporous walls:68
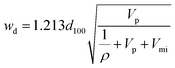 | (1) |
For FDU-12, the diameter of ordered mesopores, wd, was evaluated using a geometrical equation for materials with face-centered cubic structure of spherical mesopores that are separated from one another by microporous walls:69
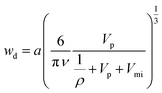 | (2) |
Results and discussion
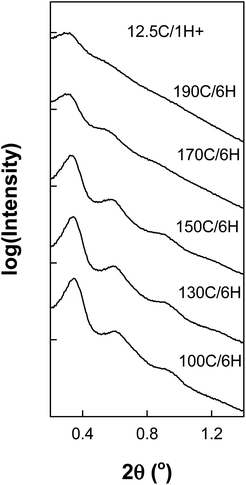
Large-pore SBA-15 silica was synthesized using triblock copolymer Pluronic P123 as a surfactant and 1,3,5-triisopropylbenzene (TIPB) as a micelle swelling agent in the presence of ammonia fluoride (NH4F) in a similar manner as we reported earlier,30 but with modifications that allowed us to decrease the synthesis duration by about an order of magnitude. A typical synthesis procedure was described in the Experimental section and particular synthesis conditions for different samples were listed in ESI Table S1.† The synthesis time from the beginning of the surfactant dissolution to the end of the hydrothermal treatment (except for cooling) was as little as 4.5–7.5 hours. Pluronic P123 block copolymer surfactant quickly dissolved in an acidic medium and formed a clear solution within 30 minutes under mechanical stirring. Afterwards, a mixture of TEOS and TIPB was added and the synthesis mixture was stirred for an hour. Then, a hydrothermal treatment was performed at temperatures up to 190 °C, typically for three to six hours. After cooling down the reaction mixture, the as-synthesized material was recovered by filtration, dried and calcined under air using a fast heating ramp (20 °C min−1) and short dwell time (2 h). The samples prepared in our rapid synthesis showed structures as highly ordered as those of samples prepared through the standard procedure that takes at least 2 days30 (see ESI Table S1 and Fig. S1†). Small-angle X-ray scattering (SAXS) patterns of the samples (Fig. 1) showed three or more obvious peaks indexable as (100), (110) and (210) reflections of the 2-D hexagonal structure, except for samples with d100 approaching 30 nm, whose SAXS patterns typically featured one peak and one shoulder. SAXS peaks positions continually shifted to lower 2θ values and corresponding (100) interplanar spacing (d100) increased to 29 nm with lowering of the initial synthesis temperature and increasing TIPB content in the synthesis mixture. This behavior follows the behavior observed in our earlier study.30 The ability to tune the (100) interplanar spacing by selecting the initial synthesis temperature provides a strong indication that the formation of the SBA-15 structure takes place within the first hour after the addition of the mixture of the silica precursor and the swelling agent. Indeed, a product can be recovered at this stage, even though its ordering may be inferior to that attained after the hydrothermal treatment. The formation of a sustainable structure within one hour is somewhat surprising, but not fully unexpected, given that ammonium fluoride present in the synthesis mixture is known as a catalyst for silica hydrolysis and condensation, which greatly facilitates the formation of SBA-15.70 Very low shrinkage (<3%) of all the samples achieved after the high-temperature hydrothermal treatment indicated facile thermal stability of the products (see ESI Table S1†).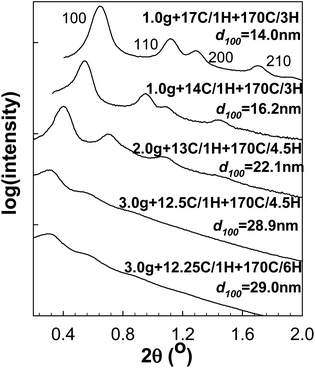 | ||
| Fig. 1 SAXS patterns of calcined SBA-15 samples prepared in a short synthesis procedure with optimized amounts of TIPB.62 The SAXS patterns were normalized and offset vertically to facilitate comparison. | ||
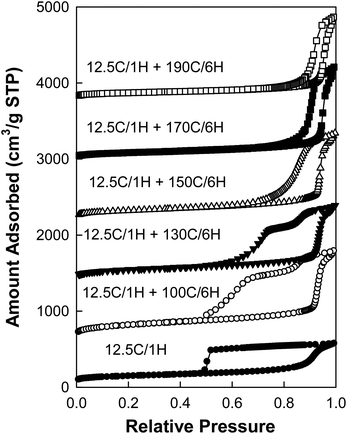
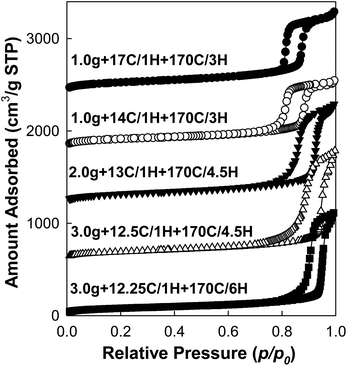
As far as nitrogen adsorption isotherms (Fig. 2) of the obtained materials are concerned, the capillary condensation steps gradually shifted to the relative pressure as high as ∼0.95 (for samples 3.0 g + 12.5C/1H + 170C/4.5H and 3.0 g + 12.25C/1H + 170C/6H), which to the best of our knowledge is the highest relative pressure of capillary condensation for well-documented SBA-15 (see additional characterization data below). The corresponding BJH pore diameters (Fig. 3) increased from ∼17 nm to ∼39 nm with retention of narrow pore size distributions. Because of the fact that the BJH method with the form of the Kelvin equation used herein is known to overestimate the diameter of large cylindrical mesopores (diameter > ∼12 nm),30,44 more reliable pore diameter values were calculated using the geometrical equation for a 2-D hexagonal structure of cylindrical pores separated by microporous walls (eqn (1)).30,44 The resulting values covered a range from 10 to 30 nm (see ESI Table S1†), which includes very large pore diameters and goes beyond the range reported in our earlier work (which was up to 26 nm).30
 | ||
| Fig. 2 Nitrogen adsorption isotherms of calcined SBA-15 samples prepared in a short synthesis procedure with optimized amounts of TIPB.62 The isotherm for samples 3.0 g + 12.5C/1H + 170C/4.5H, 2.0 g + 13C/1H + 170C/4.5H, 1.0 g + 14C/1H + 170C/3H and 1.0 g + 17C/1H + 170C/3H were shifted vertically 600, 1200, 1800 and 2400 cm3 STP g−1, respectively. | ||
 | ||
| Fig. 3 Pore size distributions of calcined SBA-15 samples prepared in a short synthesis procedure with optimized amounts of TIPB.62 | ||
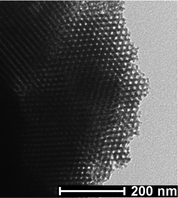
Transmission electron microscopy (TEM) images (Fig. 4) confirmed the 2-D hexagonal structures of the products, because honeycombs (images of the 2-D hexagonal structure viewed through the cylindrical mesopores) and parallel stripes (images of the structure with cylindrical mesopores perpendicular to the electron beam) were clearly observed.
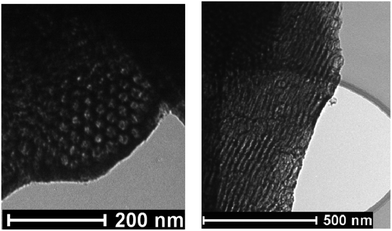 | ||
| Fig. 4 TEM images of calcined sample 3.0 g + 12.5C/1H + 170C/4.5H.62 | ||
The appearance of a precipitate is an indication of the initial formation of the silica framework and is observed early in the synthesis. Further solidification of the material can be accelerated by applying a high-temperature hydrothermal treatment. It is known that structural changes that take place during the hydrothermal treatment, including the pore size enlargement (due to the decrease in the framework shrinkage upon calcination and to the pore wall thinning)44 can be significantly accelerated if the hydrothermal treatment is performed at a temperature higher than 100 °C. For instance in the case of FDU-1 silica,71 the hydrothermal treatment at 140 °C for 1/2 day had a similar effect as the treatment at 100 °C for 24 days.32 To fully investigate the hydrothermal treatment conditions, a series of control experiments was done using 1.0 g TIPB and the initial synthesis step at 17 °C for 1 hour, but with hydrothermal treatment temperatures from 100 to 190 °C and durations from 1 to 3 hours. (100) interplanar spacing of the resulting calcined samples systematically increased with temperature and time (as seen from SAXS peaks positions that continually shifted to lower 2θ values; see ESI Fig. S2(a)†), until they reached (or became very close to) their values before the calcination (see ESI Table S1†). Concomitantly, the mesopore size increase was observed in nitrogen adsorption analysis as the pressures of capillary condensation (at mid-points of the steps) shifted from ∼0.79 to ∼0.87 p/p0 (ESI Fig. S2(b)†) as the hydrothermal treatment temperature was increased. Moreover, higher hydrothermal treatment temperatures (≥150 °C) and longer times (above 2 h) can effectively eliminate tails of the desorption branches of the isotherms, which were seen for lower hydrothermal treatment temperatures or shorter times of the treatment. These tails were indicative of the presence of caps at mesopore ends or/and constrictions existing inside the cylindrical mesopores.72 BJH pore diameters gradually increased with the hydrothermal treatment temperature and time, reaching 17.2 nm after the treatment for 3 h at 170 °C (ESI Fig. S2(c) and S2(d)†). However, it needs to be recognized that the substantial pore size increase apparently took place as the temperature increased from 150 to 170 °C, even though the unit-cell size and the mesopore volume did not increase and the microporosity decreased moderately (suggesting only minor pore wall thickness decrease). A similar behavior was observed before and attributed to the development of large gaps in the walls of the mesopores, resulting in their shape departing from cylindrical as the adjacent mesopores merge to some extent.44,73 Therefore, if one wants to retain the cylindrical mesopore shape, an optimum hydrothermal treatment temperature in an accelerated synthesis was 150 °C in this case, especially as Pluronic P123 may start to decompose above 180 °C, thus potentially exposing the silica framework to water and contributing to its degradation.74 TEM images for the resulting SBA-15 material clearly showed projections attributable to 2-D hexagonal structure (ESI Fig. S2(e) and (f)†).
As the unit-cell size of SBA-15 silica increases when the initial synthesis temperature is lowered, it may be beneficial to select somewhat higher hydrothermal treatment temperature (ca. 170 °C) or longer time (4.5–6 hours). As can be seen in Fig. 5, the sample prepared at 12.5 °C without a hydrothermal treatment had a very broad hysteresis loop with desorption at the lower limit of adsorption–desorption hysteresis, indicating that its large (∼20 nm) mesopores (see Fig. 6) were accessible through openings or constrictions of diameter below 5 nm.72,75 These relatively narrow openings are expected to be related to the presence of caps at the ends of the cylindrical mesopores72 and/or possibly porous “plugs” in the mesopores.72,76 When the hydrothermal treatments were carried out over 6 hours, the hysteresis loops became more narrow as the treatment temperature was increased and they became quite narrow (as expected for open cylindrical mesopores) at a temperature of 150 °C, and even more narrow at 170 °C. Therefore, the preparation of very large pore SBA-15 without narrow entrances to the mesopores required 6 h at 150 °C. It is notable that the positions of the desorption branches of the isotherms shifted to higher relative pressures as the hydrothermal treatment temperature was increased (or time was prolonged; data not shown) and thus the size of entrances to the cylindrical mesopores can be tuned by selecting the hydrothermal treatment temperature and/or time, in a manner analogous to that for silicas with spherical mesopores.32,77,78 It should be remarked that two-step capillary evaporation branches may have resulted from the presence of a fraction of open cylindrical mesopores together with a fraction of capped and/or plugged mesopores, the latter exhibiting capillary evaporation at much lower relative pressure.
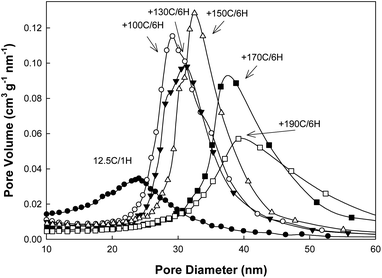 | ||
| Fig. 6 Pore size distributions of calcined samples prepared in a short synthesis procedure at the initial temperature of 12.5 °C with different hydrothermal treatment temperatures. | ||
Some of the materials discussed above are examples of ultra-large-pore SBA-15 (ULP-SBA-15) silicas with a high degree of structural ordering, as seen from SAXS (Fig. 7). Two strong peaks ((100) and (110) reflections) and one clear shoulder ((210) reflection) were seen and the positions of the main SAXS peaks corresponded to d100 = 25.6–26.8 nm for the samples hydrothermally treated at 100–150 °C. Similarly well-ordered material can be obtained with the hydrothermal treatment at 170 °C for as short as 3 hours (SAXS pattern, nitrogen adsorption isotherm and pore size distribution shown in ESI Fig. S3†). It was even possible to obtain an SBA-15 silica with strong (100) and (110) peaks reflections and one clear shoulder at a position expected of (210) reflection, and with d100 = 28 nm, which is exceptionally large (ESI Fig. S4†). In comparison to ULP-SBA-15 silicas reported in our earlier study,30 the samples discussed herein had strong (100) peaks that were well separated from the background at low angles. These SBA-15 silicas were obtained through the optimization of the relative amount of TIPB in the synthesis mixture. We have found earlier30 that for TIPB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P123 mass ratios of 1
P123 mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 or higher, the increase of the relative amount of TIPB may increase the quality of the product with minor changes in the pore size. Herein, it is reported for the first time that for the initial synthesis temperature of 12.25–12.5 °C, which leads to the formation of SBA-15 with the highest interplanar spacings achievable through our current synthesis, the optimal TIPB
2.4 or higher, the increase of the relative amount of TIPB may increase the quality of the product with minor changes in the pore size. Herein, it is reported for the first time that for the initial synthesis temperature of 12.25–12.5 °C, which leads to the formation of SBA-15 with the highest interplanar spacings achievable through our current synthesis, the optimal TIPB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P123 mass ratio was found to be 3
P123 mass ratio was found to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4, which is somewhat higher than 2
2.4, which is somewhat higher than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4–2.5
2.4–2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 ratios that we identified and reported earlier.30 It should be noted that lower TIPB/P123 mass ratios were found to result in less well ordered products (as seen from SAXS and TEM) or products containing some smaller-pore impurity,30 while, as reported herein, higher ratios typically led to the development of foams with spherical cells (silica mesocellular foams, MCFs39) or sometimes with cylindrical cells. As noted elsewhere,30 foams may be observed as a contamination of SBA-15 products of the synthesis involving TIPB and P123, but their content usually seems to be minor (based on TEM).
2.4 ratios that we identified and reported earlier.30 It should be noted that lower TIPB/P123 mass ratios were found to result in less well ordered products (as seen from SAXS and TEM) or products containing some smaller-pore impurity,30 while, as reported herein, higher ratios typically led to the development of foams with spherical cells (silica mesocellular foams, MCFs39) or sometimes with cylindrical cells. As noted elsewhere,30 foams may be observed as a contamination of SBA-15 products of the synthesis involving TIPB and P123, but their content usually seems to be minor (based on TEM).
Our streamlined calcination conditions (400 °C for 2 hours and heating ramp 20 °C min−1) were found as efficient as normal calcination conditions (500–550 °C for 5–6 hours; heating ramp for instance 2 °C min−1),9,29,30,44,63 because they led to a complete removal of the surfactant template. The comparisons of SAXS and nitrogen adsorption data further support that OMSs obtained using the streamlined calcination have similar d100 and pore diameters, specific surface areas and pore volumes as OMSs freed of surfactant under common calcination conditions (ESI Fig. S5†).
To extend the scope of the proposed rapid synthesis strategy, we extended our method to the standard SBA-15 preparation, which does not involve TIPB and NH4F and has an initial step under a higher temperature (35–40 °C).9 The control experiment was performed with the initial synthesis step at 40 °C for 2 hours and the hydrothermal treatment was carried out at 170 °C for 3 hours. The synthesized sample had a similar pore diameter (∼11 nm), specific surface area (850 m2 g−1) and total pore volume (1.26 cm3 g−1) as normal SBA-15,9 but the required synthesis time was reduced to 6 hours (ESI Fig. S6†). While similarly short synthesis times were reported earlier for normal SBA-15 synthesis (as discussed above), typically the obtained mesopore sizes were lower than those obtained using the synthesis with the high-temperature hydrothermal treatment employed herein.
Large-pore FDU-12 (LP-FDU-12) with 3-D cubic structure was also synthesized using a streamlined procedure. Its synthesis involved triblock copolymer EO106PO70EO106 (Pluronic F127, BASF) as a template, TMB as a swelling agent and TEOS as a silica precursor. Both as-synthesized and calcined samples exhibited SAXS peaks indexable as (111), (220) and (311) reflections of the face-centered cubic structure (Fm3m) (ESI Fig. S7†). The (111) interplanar spacing, d111, was ∼19.0 nm and the corresponding unit-cell parameter was 32.9 nm for the calcined material. Nitrogen adsorption (Fig. 8) showed that calcined LP-FDU-12 had the pore volume of 0.87 cm3 g−1, the specific surface area of 426 m2 g−1, and connected spherical mesopores of diameter ∼18 nm (estimated using the BJH–KJS method for cylindrical mesopores, which is known to underestimate the size of spherical mesopores29) and the entrance size on the order of 10 nm (estimated on the basis of the capillary evaporation pressure). All of these parameters are close to those of LP-FDU-12 from standard synthesis that lasts 2–4 days,29,56 although the standard procedure may afford a little larger unit-cell size. TEM images (see Fig. 9) confirmed an ordered mesoporous structure.
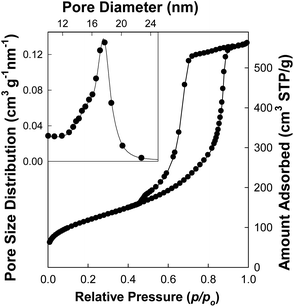 | ||
| Fig. 8 Nitrogen adsorption isotherm with pore diameter distribution (inset) for calcined LP-FDU-12 silica from the rapid synthesis. | ||
SBA-15 samples prepared using the streamlined synthesis can be stored in closed containers for four of more years in calcined form without any appreciable degradation (see ESI Fig. S8 and S9†), even if the low-temperature step (lasting an hour) was not followed by the hydrothermal treatment. The resolution of SAXS patterns and the unit-cell size remained essentially unchanged, while the BET surface area decreased by 27–30% and the total pore volume decreased 15–19% for the two samples whose stability was studied. These findings are consistent with an earlier report on block-copolymer-templated silicas synthesized using a typical (long) procedure.75 In the case of the sample prepared without the hydrothermal treatment, the storage appeared to lead to a change of relative intensities of SAXS peaks that suggested the pore wall thinning44 (consolidation), which is consistent with the 50% decrease in microporosity that was inferred from nitrogen adsorption. It is not surprising that one-hour synthesis may result in walls that have somewhat lower degree of condensation and which may slightly restructure over time.
Conclusions
The low-temperature synthesis of large-pore SBA-15 in the presence of 1,3,5-triisopropylbenzene as a swelling agent and NH4F as a silica hydrolysis/condensation catalyst can be completed in several hours. The structure forms during the initial period at low temperature, which takes one hour or less. The hydrothermal treatment decreases the shrinkage of the material upon the surfactant removal and it may be needed to obtain a well-ordered material if one wants to synthesize SBA-15 with the highest d100 attainable using our synthesis (that is, close to 30 nm). The hydrothermal treatment period can be shortened to 3–6 hours if sufficiently high temperature is selected (150–170 °C). Overall, the time from the dissolution of the surfactant to the recovery of the product by filtration can be reduced by almost an order of magnitude. The degree of structural ordering of ultra-large-pore SBA-15 with d100 ≥ 25 nm can be significantly improved through the adjustment of the relative amount of the swelling agent used, which is fully compatible with the streamlined synthesis procedure. For ultra-large-pore SBA-15, the size of entrances to the cylindrical mesopores can be tuned by selecting the hydrothermal treatment temperature and/or time, in a manner analogous to that known earlier for silicas with spherical mesopores. The synthesis of large-pore FDU-12 with 1,3,5-trimethylbenzene as a swelling agent can be shortened to about eight hours. These results suggest that the swelling-agent-based syntheses of highly ordered large-pore mesoporous materials may be carried out expeditiously, which is expected the facilitate applications of these remarkable materials.Acknowledgements
Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for partial support of this research (Award PRF #49093-DNI5). NSF is acknowledged for funding the SAXS/WAXS system through award CHE-0723028. Some SAXS measurements were performed at the Cornell High Energy Synchrotron Source (CHESS, Cornell University), which is supported by the National Science Foundation under award DMR-0225180. Dr Detlef M. Smilgies (CHESS, Cornell University) is gratefully acknowledged for assistance in the SAXS measurements at CHESS. Ms Tiffany Man (College of Staten Island and Graduate Center, City University of New York) is gratefully acknowledged for help with several SAXS and nitrogen adsorption measurements. BASF is gratefully acknowledged for providing Pluronic surfactants.Notes and references
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- S. Inagaki, Y. Fukushima and K. Kuroda, J. Chem. Soc., Chem. Commun., 1993, 680–682 RSC.
- F. Schueth, Chem. Mater., 2001, 13, 3184–3195 CrossRef CAS.
- J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56–77 CrossRef CAS.
- Y. Wan, Y. Shi and D. Zhao, Chem. Commun., 2007, 897–926 RSC.
- Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS PubMed.
- Q. Huo, D. I. Margolese and G. D. Stucky, Chem. Mater., 1996, 8, 1147–1160 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Frederickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- R. Ryoo, C. H. Ko, M. Kruk, V. Antochshuk and M. Jaroniec, J. Phys. Chem. B, 2000, 104, 11465–11471 CrossRef CAS.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1–45 CrossRef CAS PubMed.
- M. Hartmann, Chem. Mater., 2005, 17, 4577–4593 CrossRef CAS.
- A. Galarneau, J. Iapichella, D. Brunel, F. Fajula, Z. Bayram-Hahn, K. Unger, G. Puy, C. Demesmay and J. L. Rocca, J. Sep. Sci., 2006, 29, 844–855 CrossRef CAS.
- A. Katiyar, S. Yadav, P. G. Smirniotis and N. G. Pinto, J. Chromatogr., A, 2006, 1122, 13–20 CrossRef CAS PubMed.
- X. Feng, G. E. Fryxell, L. Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923–926 CrossRef CAS.
- S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 10712–10713 CrossRef CAS.
- S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169–172 CrossRef CAS PubMed.
- E. B. Celer and M. Jaroniec, J. Am. Chem. Soc., 2006, 128, 14408–14414 CrossRef CAS PubMed.
- A. Monnier, F. Schuth, Q. Huo, D. Kumar, D. Margolese, R. S. Maxwell, G. D. Stucky, M. Krishnamurty, P. Petroff, A. Firouzi, M. Janicke and B. F. Chmelka, Science, 1993, 261, 1299–1303 CAS.
- Y. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. Gong, Y. Guo, H. Soyez, B. Dunn, M. H. Huang and J. I. Zink, Nature, 1997, 389, 364–368 CrossRef CAS PubMed.
- K. Flodstrom, H. Wennerstrom and V. Alfredsson, Langmuir, 2004, 20, 680–688 CrossRef.
- S. Ruthstein, V. Frydman, S. Kababya, M. Landau and D. Goldfarb, J. Phys. Chem. B, 2003, 107, 1739–1748 CrossRef CAS.
- T. Kjellman and V. Alfredsson, Chem. Soc. Rev., 2013, 42, 3777–3791 RSC.
- S. Hitz and R. Prins, J. Catal., 1997, 168, 194–206 CrossRef CAS.
- B. Tian, X. Liu, C. Yu, F. Gao, Q. Luo, S. Xie, B. Tu and D. Zhao, Chem. Commun., 2002, 1186–1187 RSC.
- S. H. Tolbert, A. Firouzi, G. D. Stucky and B. F. Chmelka, Science, 1997, 278, 264–268 CrossRef CAS.
- H. Fan, F. Van Swol, Y. Lu and C. J. Brinker, J. Non-Cryst. Solids, 2001, 285, 71–78 CrossRef CAS.
- M.-C. Liu, H.-S. Sheu and S. Cheng, Chem. Commun., 2002, 2854–2855 RSC.
- M. Kruk and C. M. Hui, Microporous Mesoporous Mater., 2008, 114, 64–73 CrossRef CAS PubMed.
- L. Cao, T. Man and M. Kruk, Chem. Mater., 2009, 21, 1144–1153 CrossRef CAS.
- M. Kruk, V. Antochshuk, J. R. Matos, L. P. Mercuri and M. Jaroniec, J. Am. Chem. Soc., 2002, 124, 768–769 CrossRef CAS PubMed.
- J. R. Matos, M. Kruk, L. P. Mercuri, M. Jaroniec, L. Zhao, T. Kamiyama, O. Terasaki, T. J. Pinnavaia and Y. Liu, J. Am. Chem. Soc., 2003, 125, 821–829 CrossRef CAS PubMed.
- P. Linton, A. R. Rennie, M. Zackrisson and V. Alfredsson, Langmuir, 2009, 25, 4685–4691 CrossRef CAS PubMed.
- T. Kjellman, N. V. Reichhardt, M. Sakeye, J.-H. Smått, M. Lindén and V. Alfredsson, Chem. Mater., 2013, 25, 1989–1997 CrossRef CAS.
- P. F. Fulvio, S. Pikus and M. Jaroniec, J. Mater. Chem., 2005, 15, 5049–5053 RSC.
- S. Shen, P. S. Chow, S. Kim, K. Zhu and R. B. H. Tan, J. Colloid Interface Sci., 2008, 321, 365–372 CrossRef CAS PubMed.
- X. Liu, L. Li, Y. Du, Z. Guo, T. T. Ong, Y. Chen, S. C. Ng and Y. Yang, J. Chromatogr., A, 2009, 1216, 7767–7773 CrossRef CAS PubMed.
- J. A. G. Jammaer, T. S. van Erp, A. Aerts, C. E. A. Kirschhock and J. A. Martens, J. Am. Chem. Soc., 2011, 133, 13737–13745 CrossRef CAS PubMed.
- J. S. Lettow, Y. J. Han, P. Schmidt-Winkel, P. Yang, D. Zhao, G. D. Stucky and J. Y. Ying, Langmuir, 2000, 16, 8291–8295 CrossRef CAS.
- P. Feng, X. Bu and D. J. Pine, Langmuir, 2000, 16, 5304–5310 CrossRef CAS.
- Y. T. Chan, H. P. Lin, C. Y. Mou and S. T. Liu, Stud. Surf. Sci. Catal., 2003, 146, 113–116 CrossRef CAS.
- B. Li, S. Inagaki, C. Miyazaki and H. Takahashi, Chem. Res. Chin. Univ., 2002, 18, 200–205 CAS.
- J. Sun, H. Zhang, D. Ma, Y. Chen, X. Bao, A. Klein-Hoffmann, N. Pfaender and D. S. Su, Chem. Commun., 2005, 5343–5345 RSC.
- M. Kruk and L. Cao, Langmuir, 2007, 23, 7247–7254 CrossRef CAS PubMed.
- E. L. Roux, Y. Liang, M. P. Storz and R. Anwander, J. Am. Chem. Soc., 2010, 132, 16368–16371 CrossRef PubMed.
- C. K. Mouli, K. Soni, A. Dalai and J. Adjaye, Appl. Catal., A, 2011, 404, 21–29 CrossRef PubMed.
- J. P. Dacquin, A. F. Lee, C. Pirez and K. Wilson, Chem. Commun., 2012, 48, 212–214 RSC.
- S. Zhao, D. Su, J. Che, B. Jiang and A. Orlov, Mater. Lett., 2011, 65, 3354–3357 CrossRef CAS PubMed.
- A. Martin, G. Morales, F. Martinez, R. van Grieken, L. Cao and M. Kruk, J. Mater. Chem., 2010, 20, 8026–8035 RSC.
- R. Sanz, G. Calleja, A. Arencibia and E. S. Sanz-Perez, J. Mater. Chem. A, 2013, 1, 1956–1962 CAS.
- H. Ikemoto, S. Tubasum, T. Pullerits, J. Ulstrup and Q. Chi, J. Phys. Chem. C, 2013, 117, 2868–2878 CAS.
- L. Cao, T. Man, J. Zhuang and M. Kruk, J. Mater. Chem., 2012, 22, 6939–6946 RSC.
- L. Cao and M. Kruk, Adsorption, 2010, 16, 465–472 CrossRef CAS.
- E. M. Johansson, M. A. Ballem, J. M. Córdoba and M. Odén, Langmuir, 2011, 27, 4994–4999 CrossRef CAS PubMed.
- H. Zhang, J. Sun, D. Ma, G. Weinberg, D. S. Su and X. Bao, J. Phys. Chem. B, 2006, 110, 25908–25915 CrossRef CAS PubMed.
- J. Fan, C. Yu, J. Lei, Q. Zhang, T. Li, B. Tu, W. Zhou and D. Zhao, J. Am. Chem. Soc., 2005, 127, 10794–10795 CrossRef CAS PubMed.
- G. Ma, X. Yan, Y. Li, L. Xiao, Z. Huang, Y. Lu and J. Fan, J. Am. Chem. Soc., 2010, 132, 9596–9597 CrossRef CAS PubMed.
- L. Huang, X. Yan and M. Kruk, Langmuir, 2010, 26, 14871–14878 CrossRef CAS PubMed.
- P. Mohanty, B. Kokoszka, C. Liu, M. Weinberger, M. Mandal, V. Stagno, Y. Fei and K. Landskron, Microporous Mesoporous Mater., 2012, 152, 214–218 CrossRef CAS PubMed.
- W. Shui, J. Fan, P. Yang, C. Liu, J. Zhai, J. Lei, Y. Yan, D. Zhao and X. Chen, Anal. Chem., 2006, 78, 4811–4819 CrossRef CAS PubMed.
- S. B. Hartono, S. Z. Qiao, K. Jack, B. P. Ladewig, Z. Hao and G. Q. M. Lu, Langmuir, 2009, 25, 6413–6424 CrossRef CAS PubMed.
- L. Cao, Ph.D. Dissertation, City University of New York, New York, 2010 Search PubMed.
- T. Yu, H. Zhang, X. Yan, Z. Chen, X. Zou, P. Oleynikov and D. Zhao, J. Phys. Chem. B, 2006, 110, 21467–21472 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- M. Jaroniec, M. Kruk and J. P. Olivier, Langmuir, 1999, 15, 5410–5413 CrossRef CAS.
- M. Kruk, M. Jaroniec and A. Sayari, Langmuir, 1997, 13, 6267–6273 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS.
- M. Kruk, M. Jaroniec and A. Sayari, Chem. Mater., 1999, 11, 492–500 CrossRef CAS.
- P. I. Ravikovitch and A. V. Neimark, Langmuir, 2002, 18, 1550–1560 CrossRef CAS.
- J. M. Kim, Y.-J. Han, G. D. Stucky and B. F. Chmelka, Chem. Commun., 2000, 2437–2438 RSC.
- C. Yu, Y. Yu and D. Zhao, Chem. Commun., 2000, 575–576 RSC.
- M. Mandal and M. Kruk, Chem. Mater., 2012, 24, 149–154 CrossRef CAS.
- P. Yuan, L. Tan, D. Pan, Y. Guo, L. Zhou, J. Yang, J. Zou and C. Yu, New J. Chem., 2011, 35, 2456–2461 RSC.
- N. Xiao, L. Wang, S. Liu, Y. Zou, C. Wang, Y. Ji, J. Song, F. Li, X. Meng and F.-S. Xiao, J. Mater. Chem., 2009, 19, 661–665 RSC.
- M. Kruk and M. Jaroniec, Chem. Mater., 2003, 15, 2942–2949 CrossRef CAS.
- P. Van Der Voort, P. I. Ravikovitch, K. P. De Jong, M. Benjelloun, E. Van Bavel, A. H. Janssen, A. V. Neimark, B. M. Weckhuysen and E. F. Vansant, J. Phys. Chem. B, 2002, 106, 5873–5877 CrossRef CAS.
- J. Fan, C. Yu, F. Gao, J. Lei, B. Tian, L. Wang, Q. Luo, B. Tu, W. Zhou and D. Zhao, Angew. Chem., Int. Ed., 2003, 42, 3146–3150 CrossRef CAS PubMed.
- T. W. Kim, R. Ryoo, M. Kruk, K. P. Gierszal, M. Jaroniec, S. Kamiya and O. Terasaki, J. Phys. Chem. B, 2004, 108, 11480–11489 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Figures with experimental SAXS, adsorption and pore size distribution data and TEM images. Table with structural parameters. See DOI: 10.1039/c3ra44203a |
| ‡ Current address: HGST, a Western Digital Company, 5601 Great Oaks Parkway, San Jose, CA 95119, USA. |
| This journal is © The Royal Society of Chemistry 2014 |