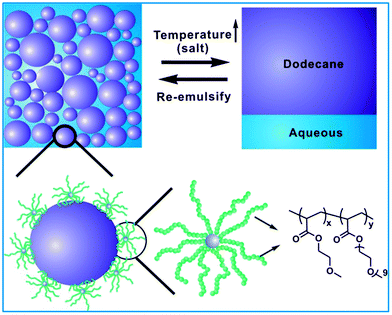
Core cross-linked star (CCS) polymers with temperature and salt dual responsiveness: synthesis, formation of high internal phase emulsions (HIPEs) and triggered demulsification†
Qijing
Chen
a,
Yuanyuan
Xu
a,
Xueteng
Cao
a,
Lianjie
Qin
b and
Zesheng
An
*a
aInstitute of Nanochemistry and Nanobiology, College of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, P. R. China. E-mail: an.zesheng@shu.edu.cn
bSchool of Environmental and Material Engineering, Yantai University, Yantai 264005, P. R. China
First published on 30th August 2013
Abstract
A series of five core cross-linked star (CCS) polymers has been synthesized by RAFT-mediated heterogeneous polymerization in aqueous media. These CCS polymers consist of poly(MEAx-co-PEGAy) (MEA is 2-methoxyethyl acrylate and PEGA is poly(ethylene glycol) acrylate) of different compositions. They exhibit responsiveness to both temperature and salt. Addition of kosmotropes reduces the cloud point, and addition of chaotropes raises the cloud point. These CCS polymers can stabilize dodecane-in-water high internal phase emulsions (HIPEs) in the absence or presence of salts. Addition of salts has negligible effect on the maximum oil fraction, the size of oil droplets or the stability of the HIPEs. HIPEs with large oil fractions (up to 92 vol%) at low concentrations of CCS polymers (≤1 wt%) can be routinely obtained. When the oil volume fraction is larger than 74 vol%, gelled HIPEs are formed with long-term stabilities (more than three months). The temperature and salt responsiveness of the CCS polymers is transferred to the CCS-stabilized HIPEs. Addition of kosmotropes can enhance demulsification efficiency, while addition of chaotropes can decrease demulsification efficiency or enhance the thermal stability of the CCS-stabilized HIPEs. The emulsification–demulsification cycle can be successfully repeated four times. The facile and aqueous synthesis of CCS polymers and the fast response of the CCS-stabilized HIPEs will open up new opportunities for the preparation and exploitation of a range of smart soft materials.
Introduction
Emulsions consist of at least two immiscible phases with one dispersed into the other, stabilized by surface active species such as small molecular and polymeric surfactants or particles.1 For small molecular and polymeric surfactants, it is essential that these emulsifiers have amphiphilicity when in action. Emulsions stabilized by particles, Pickering emulsions,2,3 require that the particles have suitable wettability for the two phases. Novel polymeric emulsifiers are intensively pursued on account of their versatile synthesis and easy functionalization.4–15 The majority of polymeric emulsifiers generally fall into two categories: they are either amphiphilic polymers when in action, or in the form of particles. A clear gap exists where new polymeric emulsifiers need to be developed to combine the flexibility of polymers and the nanosize of particles into the same emulsifier, such that the novel emulsifier may exhibit the favourable properties of both.Recently, much effort has been focused on responsive emulsions in order to generate “smart” soft materials by using stimuli-responsive emulsifiers.16–18 Responsive emulsions have been realized using small molecular surfactants,19–21 polymers4 and particles.9,10,12,22–24 One particularly studied area is on demand triggering of demulsification.9,12
High internal phase emulsions (HIPEs) with an internal phase volume fraction larger than 0.74 have attracted much recent attention due to their applications in various technological sectors.25,26 Traditionally, HIPEs are prepared with a large amount of surfactant (5–50 wt%).25 Several types of colloidal particles have been studied for the formation of HIPEs of both oil-in-water (o/w) and water-in-oil (w/o) types, including titania,27 silica,28,29 Fe3O4,30 polymer particles18,31,32 and microgels.33
Core cross-linked star (CCS) polymers have been actively studied for a wide range of applications.34–50 We have recently developed several CCS polymers and investigated their use as emulsifiers.51–53 These CCS polymers are prepared via reversible addition–fragmentation chain transfer (RAFT) cross-linking copolymerization in aqueous heterogeneous media, which is a novel strategy, in contrast to the prevailing synthesis in homogeneous solutions, for the efficient synthesis of well-defined CCS polymers.51–58 CCS polymers have many arms connected a central, highly cross-linked core that is smaller in size than the dimensions of the arms. These materials can be regarded as an intermediate between common soluble polymers and polymeric nanoparticles, in that CCS polymers have a high fraction of flexible arms and also possess a hydrodynamic diameter typically in the 10–50 nm range. The arm flexibility allows CCS polymers to adopt a favorable configuration at the interface, and the nanometre size provides sufficient energy (>kBT) to stabilize the interface. Importantly, the flexibility and nanometre size guarantee the fast responsiveness of the CCS polymers to external stimuli. These unique properties have led to their use as interfacial stabilizers, including dispersing agents for carbon nanotubes,38 stabilizers for nanoprecipitation process57 and emulsifiers in emulsions.51–53
We previously reported the first example of CCS-stabilized HIPEs that showed high stability at low concentrations of stabilizer and fast responsiveness to pH changes using a poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) CCS polymer.52 Despite the fact that the CCS polymer demonstrated high efficiency for the formation of HIPEs and endowed them with fast responsiveness, several important scientific questions still need to be addressed. For instance, are other CCS polymers also effective in stabilizing HIPEs? Can other types of responsiveness be incorporated into the CCS polymers and thus into the CCS-stabilized HIPEs? Constructive answers to these questions will significantly expand the scope and versatility of CCS-stabilized HIPEs and help to establish the importance of CCS-stabilized HIPEs in emulsion and emulsion-templating technologies. In this article, we attempt to address these questions by synthesizing a series of novel CCS polymers that are responsive to both temperature and salts, and investigating their effectiveness as stabilizers for HIPEs and triggered demulsification (Scheme 1). This study demonstrates the first example of temperature and salt dual responsive CCS-stabilized HIPEs.
Experimental
Materials
2,2′-Azobis(2-methylpropionamidine) dihydrochloride (V-50, 97%), 2-methoxyethyl acrylate (MEA, 98%), poly(ethylene glycol) diacrylate (PEGDA, Mn = 258) and poly(ethylene glycol) methyl ether acrylate (PEGA, Mn = 480) were purchased from Sigma-Aldrich. 1,6-Hexanediol diacrylate (HDDA, 95%) was purchased from Aladdin Reagent. 2,2′-Azobis(2-methylpropionitrile) (AIBN, CP), n-butyl acrylate (BA, CP), tetrahydrofuran (THF, 99+%), petroleum ether (AR), N,N-dimethylformamide (DMF) (99.5+%), and potassium persulfate (KPS, AR) were purchased from Sinopharm Chemical Reagent Co. Ltd. Nile red (99%) was purchased from J&K Scientific. L-Ascorbic acid sodium salt (NaAs, 99%) and 1,3,5-trioxane (98%) were purchased from Alfa-Aesar. All liquid monomers were passed through a column of Al2O3 to remove the inhibitor before use.Characterization
Synthesis of poly(MEA-co-PEGA) macro-CTAs
Thermally responsive copolymers, P(MEA-co-PEGA)s, were synthesized by RAFT copolymerization of MEA and PEGA in DMF at 70 °C (Scheme S1†). The target number-average degree of polymerization (DP) was set to 100 and the molar ratio of MEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGA was varied to prepare copolymers with adjustable cloud points (CPs). Three molar ratios of MEA
PEGA was varied to prepare copolymers with adjustable cloud points (CPs). Three molar ratios of MEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGA (80
PEGA (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 86
20, 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, and 90
14, and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were used to synthesize the copolymers.
10) were used to synthesize the copolymers.
Synthesis of CCS polymers by RAFT heterogeneous polymerizations
The synthesis of CCS polymers was conducted either at 35 °C in water via emulsion polymerization (Scheme S2†) or at 70 °C in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water solution via dispersion polymerization (Scheme S3†). All the syntheses were conducted at an arm concentration of 10% w/v.
1 ethanol–water solution via dispersion polymerization (Scheme S3†). All the syntheses were conducted at an arm concentration of 10% w/v.
CCS polymer synthesis using P(MEA80-co-PEGA20) was conducted at 35 °C in water, using the redox initiator sodium ascorbate/potassium persulfate (NaAs/KPS), in the presence of the cross-linker PEGDA and a spacing monomer BA. The molar ratio of P(MEA80-co-PEGA20)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGDA
PEGDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BA
BA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaAs
NaAs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KPS was controlled at 1
KPS was controlled at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1.
0.1.
CCS polymer syntheses using P(MEA56-co-PEGA9) or P(MEA74-co-PEGA8) were conducted at 70 °C in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–ethanol using V-50 as the initiator, in the presence of the cross-linker HDDA. The molar ratio of (P(MEA56-co-PEGA9) or P(MEA74-co-PEGA8))
1 water–ethanol using V-50 as the initiator, in the presence of the cross-linker HDDA. The molar ratio of (P(MEA56-co-PEGA9) or P(MEA74-co-PEGA8))![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDDA
HDDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V-50 was controlled at 1
V-50 was controlled at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 or 20
10 or 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1.
0.1.
The yield of CCS polymer was calculated from GPC chromatograms as:
| CCS polymer yield = area of CCS polymer/(area of CCS polymer + area of low molecular weight polymers). |
The synthesized CCS polymers are designated as S(Dh, CP), where Dh and CP represent the hydrodynamic diameter at 25 °C and the CP of the CCS polymer measured at a concentration of 0.5 wt%.
For detailed information on the synthesis of the CCS polymers, please refer to the ESI.†
Procedure for purification of CCS polymers
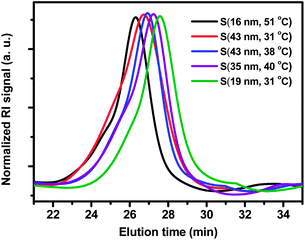
All the CCS polymers were purified via ultrafiltration to remove low-molecular-weight species prior to their use in emulsification. In detail, 3 mL of the as-synthesized CCS polymer solution was added into a 15 mL centrifugal filter tube (Amicon Ultra-15) with regenerated cellulose membrane (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO). Then 7 mL of water was added into the filer tube to dilute the solution. The solution was centrifuged at a speed of 6000 rpm for 30 min. The procedure was repeated and the purity was checked by GPC. The final purity of CCS polymers was determined by the area of the CCS polymers relative to the total area of both CCS polymers and low-molecular weight species. For all the samples, the purity of the CCS polymers was greater than 98% (Table 1).
000 MWCO). Then 7 mL of water was added into the filer tube to dilute the solution. The solution was centrifuged at a speed of 6000 rpm for 30 min. The procedure was repeated and the purity was checked by GPC. The final purity of CCS polymers was determined by the area of the CCS polymers relative to the total area of both CCS polymers and low-molecular weight species. For all the samples, the purity of the CCS polymers was greater than 98% (Table 1).
| CCS code | Arm polymer | Yielda (%) | Purityb (%) | M n (kg mol−1) | Đ | N arm | χ arm (%) | D h (nm) | PDIg | CPh (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
a CCS yield determined by GPC, CCS yield = ACCS/(ACCS + Alow molecular weight polymers).
b CCS purity after extensive ultrafiltration.
c Determined by triple-detection GPC.
d Determined by RI-GPC (PMMA standard).
e Average arm number per CCS polymer.
f Arm mass fraction.
g Determined by DLS at 25 °C.
h Determined by variable temperature DLS.
i Molar ratio of P(MEA80-co-PEGA20)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGDA PEGDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BA BA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (NaAs (NaAs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KPS) = 1 KPS) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (0.1 (0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), 35 °C, 6 h, water.
j Molar ratio of P(MEA56-co-PEGA9) or P(MEA74-co-PEGA8) 0.1), 35 °C, 6 h, water.
j Molar ratio of P(MEA56-co-PEGA9) or P(MEA74-co-PEGA8)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDDA HDDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V-50 = 1 V-50 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 or 20 10 or 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 70 °C, 3 h, water–ethanol (1 0.1, 70 °C, 3 h, water–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1).
|
||||||||||
| S(16 nm, 51 °C)i | P(MEA80-co-PEGA20) | 80 | 98.3 | 278.9 | 1.16 | 23 | 86 | 16 | 0.23 | 51 |
| S(35 nm, 40 °C)j | P(MEA56-co-PEGA9) | 93 | 98.4 | 193.5 | 1.19 | 28 | 83 | 35 | 0.17 | 40 |
| S(43 nm, 38 °C)j | P(MEA56-co-PEGA9) | 90 | 98.4 | 194.2 | 1.26 | 22 | 71 | 43 | 0.15 | 38 |
| S(19 nm, 31 °C)j | P(MEA74-co-PEGA8) | 83 | 98.2 | 149.6 | 1.17 | 17 | 84 | 19 | 0.14 | 31 |
| S(43 nm, 31 °C)j | P(MEA74-co-PEGA8) | 90 | 98.3 | 195.7 | 1.16 | 21 | 73 | 43 | 0.15 | 31 |
Preparation of CCS-stabilized emulsions
The total volume of all emulsions prepared was 1.5 mL. CCS-stabilized emulsions were prepared by homogenizing aqueous solutions of CCS polymers, in the absence or presence of the desired salt, with appropriate amounts of dodecane, using a Polytron PT 1200E homogenizer (7 mm head), operating first at the ¼ position of the scale for 30 s and then at the maximum speed (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 30 s.
000 rpm) for 30 s.
Induced demulsification of HIPEs
HIPEs were first formed using 80 vol% of dodecane and 20 vol% of aqueous solution of CCS (0.5 wt%), in the absence or presence of the desired salt, at ambient temperature. The HIPEs were then induced to demulsify by either heating at the designated temperature for a period of time or by adding salts while maintaining the temperature.Results and discussion
Synthesis and characterization of CCS polymers
A series of five novel CCS polymers (Scheme 1) was synthesized by RAFT cross-linking copolymerization in heterogeneous systems via either emulsion polymerization in water or dispersion polymerization in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) water–ethanol solution (please see ESI† for details). Poly(MEAx-co-PEGAy) (MEA is 2-methoxyethyl acrylate and PEGA is poly(ethylene glycol) acrylate) of different compositions were first synthesized by RAFT polymerization (Table S1, ESI†). The molar ratio of MEA and PEGA was systematically varied to adjust the cloud points (CPs) of the CCS polymers.54 A higher proportion of PEGA leads to a higher LCST of the CCS polymer. These poly(MEAx-co-PEGAy) were then used as macro-CTAs to prepare CCS polymers using either poly(ethylene glycol) diacrylate (PEGDA) or 1,6-hexanediol diacrylate (HDDA) as the cross-linker, with butyl acrylate as the optional spacing monomer. Synthetic conditions were also adjusted such that CCS polymers of different sizes were prepared using the same macro-CTA. The CCS polymers are designated as S(Dh, CP), where Dh is the hydrodynamic diameter of the CCS polymer measured in water at 25 °C, reflecting the size of the CCS polymer, and CP is the parameter reflecting the relative composition of the arm and thus the relative hydrophilicity of the CCS polymer.
1 (v/v) water–ethanol solution (please see ESI† for details). Poly(MEAx-co-PEGAy) (MEA is 2-methoxyethyl acrylate and PEGA is poly(ethylene glycol) acrylate) of different compositions were first synthesized by RAFT polymerization (Table S1, ESI†). The molar ratio of MEA and PEGA was systematically varied to adjust the cloud points (CPs) of the CCS polymers.54 A higher proportion of PEGA leads to a higher LCST of the CCS polymer. These poly(MEAx-co-PEGAy) were then used as macro-CTAs to prepare CCS polymers using either poly(ethylene glycol) diacrylate (PEGDA) or 1,6-hexanediol diacrylate (HDDA) as the cross-linker, with butyl acrylate as the optional spacing monomer. Synthetic conditions were also adjusted such that CCS polymers of different sizes were prepared using the same macro-CTA. The CCS polymers are designated as S(Dh, CP), where Dh is the hydrodynamic diameter of the CCS polymer measured in water at 25 °C, reflecting the size of the CCS polymer, and CP is the parameter reflecting the relative composition of the arm and thus the relative hydrophilicity of the CCS polymer.
The CCS polymers were efficiently synthesized with various star yields (≥80%), which were purposely controlled, along with other synthetic parameters, as a means to adjust the final molecular weights and thus the sizes of the CCS polymers. In order to remove the low-molecular-weight species, the CCS polymers were purified by extensive ultrafiltration, and the purity of all the CCS polymers was higher than 98% as determined by GPC (Fig. 1). The purified CCS polymers were characterized by NMR, GPC and dynamic light scattering (DLS) (Table 1). The molecular weight (Mn) of the CCS polymers was in the range of 150–279 kg mol−1 and each CCS polymer had a low dispersity Đ (≤1.26). The number of arms per CCS polymer was in the range of 17–28. The hydrodynamic diameter (Dh) determined by DLS at 25 °C was between 16 nm and 43 nm. S(16 nm, 51 °C) has the largest Mn but the smallest Dh, which may seem contradictory. However, these results were fully reproducible from repeated measurements. It should be noted that the arms in S(16 nm, 51 °C) are composed of P(MEA80-co-PEGA20) and have the highest proportion of PEGA grafts. As a result, the arms in S(16 nm, 51 °C) have a higher molecular weight and more compact structure than those in other CCS polymers. In addition, both the core and the arms contribute to the size and molecular weight of the CCS polymers.
Temperature and salt responsiveness of CCS polymers
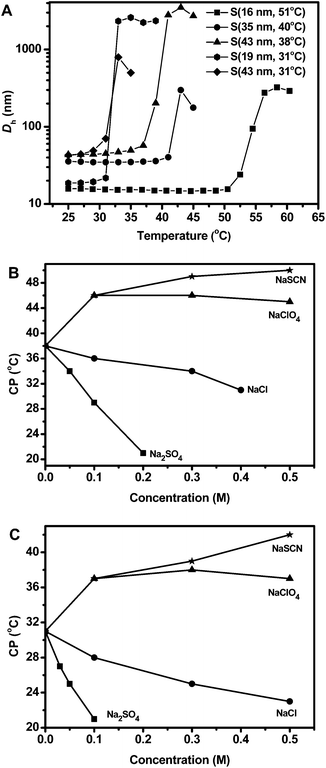
The CCS polymers displayed LCST-type thermally responsive properties, which is expected because the CCS polymers consist of thermosensitive arms of poly(MEAx-co-PEGAy). The solubility of polymers or the LCST of thermally responsive polymers has been shown to be affected by the concentration and type of inorganic salts, especially those of anions, according to the Hofmeister series.59–61 LCST or CP is lowered by kosmotropes but increased by chaotropes. Therefore, we also studied the effect of salts on the CPs of these CCS polymers in order to provide a second means for tuning the CPs and thus the responsiveness of the CCS-stabilized emulsions. NaSCN and NaClO4 were chosen as the chaotropes, while NaCl and Na2SO4 were selected as the kosmotropes.The CPs of the CCS polymers, in the absence or presence of different salts, were measured by DLS. Fig. 2A illustrates the thermal response of the CCS polymers in water. Each CCS polymer exhibits a sharp response to temperature change upon heating, i.e., the observed Dh is significantly increased due to the aggregation of the CCS polymers, which become more hydrophobic upon heating through the CP. The CP value of the CCS polymers ranges from 31 °C to 51 °C and increases upon increasing the molar ratio of the more hydrophilic PEGA in the arm polymer.
In order to evaluate the effect of added salts on the CPs of the CCS polymers, we selected S(43 nm, 38 °C) and S(43 nm, 31 °C) as representative CCS polymers, because these two CCS polymers have the same hydrodynamic diameter but different CPs, which is convenient for comparing the effect of CP in triggered demulsification by added salts (vide infra). From Fig. 2B and C, we can see that addition of salts can significantly change the CPs, depending on the type and concentration of the added salts. For both CCS polymers, kosmotropes (Na2SO4 and NaCl) lower the CPs while chaotropes (NaClO4 and NaSCN) increase the CPs, and the magnitude of change in CP agrees well with the Hofmeister series.59
HIPEs stabilized by CCS polymers in the absence of salts
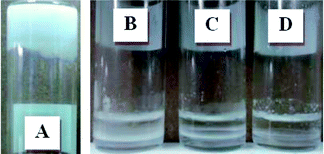
Emulsions using dodecane as the oil phase and aqueous solutions of CCS polymers were prepared over a wide range of oil/water volumetric ratios. In order to eliminate the interference of low-molecular-weight polymers, purified CCS polymers were used in emulsification. For all the emulsion studies, the concentration of CCS polymers refers to their concentration in water. All the CCS polymers were effective as emulsifiers to stabilize o/w emulsions and, interestingly, all of them were capable of stabilizing HIPEs. Fig. 3 shows a representative photograph of emulsions with different volumetric fractions of dodecane, stabilized by S(43 nm, 31 °C) at 1.0 wt%. At low oil fractions (30 vol% and 50 vol%), conventional o/w emulsions are formed, which cream on the top of the mixture due to the lower density of the emulsion. Upon further increasing the oil volumetric fraction to 70 vol%, the emulsion becomes very viscous and the creaming rate is much reduced. Significantly, for emulsions with oil fractions from 75 vol% to 90 vol%, flow is completely inhibited and gelled HIPEs are formed (inverted vials). Long term stability tests showed that all the HIPEs prepared using the five different CCS polymers were stable for more than 1 month, and some HIPEs were even stable for 3 months. Photographs of emulsions containing HIPEs stabilized by other CCS polymers can be found in Fig. S3–S7 (ESI†). In order to illustrate the importance of the CCS architecture for emulsification, control experiments with the macro-CTAs that formed the arms in the CCS polymers were conducted (Fig. S8, ESI†). Although transient emulsions were also formed at low oil fractions using the macro-CTAs, attempted emulsification at oil fractions larger than 70 vol% only resulted in clear oil/water separation. These results indicate that the architecture/nanosize of the CCS polymers is important for the CCS polymers to be effective as emulsifiers. Polymer nanoparticles have been demonstrated to form w/o HIPEs.31,32 Microgels have been reported to form o/w HIPEs,33 but the Dh of the microgels is about one order of magnitude larger than that of the CCS polymers used herein. | ||
| Fig. 3 Photograph of emulsions stabilized by S(43 nm, 31 °C) (1.0 wt% in water) with indicated dodecane volume fractions. | ||
As shown in Table 2, the maximum oil fraction in stable HIPEs is as high as 92 vol% and the lowest CCS polymer concentration required for the formation of stable HIPEs containing 80 vol% of oil is as low as 0.3 wt%. While most CCS polymers show similar emulsifying efficiency, S(16 nm, 51 °C), possessing both the smallest Dh and the highest CP, both of which are unfavourable for stabilization, is less effective in forming HIPEs than the other CCS polymers as it requires a higher CCS polymer concentration to stabilize 80 vol% HIPE.
| CCS polymer | CCS polymer concentration (wt%) | Oil fraction (vol%) | Lowest CCS concentrationb | |
|---|---|---|---|---|
| a The total volume of emulsion is 1.5 mL, dodecane is the oil phase, and CCS polymers are in the aqueous phase. b Lowest CCS concentration required to form a HIPE of 80 vol% oil. | ||||
| 1 | S(16 nm, 51 °C) | 1.0 | 88 | 1.0 |
| 2 | 2.0 | 90 | ||
| 3 | S(35 nm, 40 °C) | 0.3 | 87 | 0.3 |
| 4 | 0.4 | 91 | ||
| 5 | 0.5 | 90 | ||
| 6 | 1.0 | 89 | ||
| 7 | S(43 nm, 38 °C) | 0.3 | 86 | 0.3 |
| 8 | 0.5 | 92 | ||
| 9 | 1.0 | 88 | ||
| 10 | S(19 nm, 31 °C) | 0.4 | 90 | 0.4 |
| 11 | 0.5 | 88 | ||
| 12 | 1.0 | 88 | ||
| 13 | S(43 nm, 31 °C) | 0.3 | 90 | 0.3 |
| 14 | 0.5 | 92 | ||
| 15 | 1.0 | 89 | ||
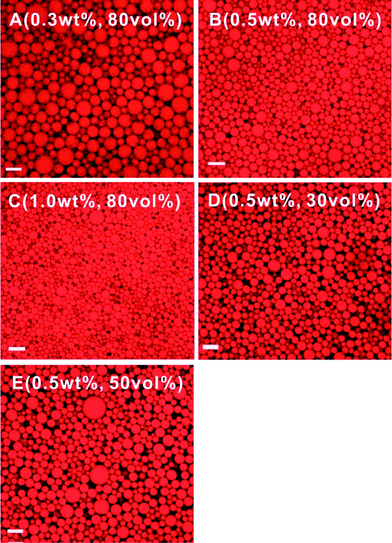
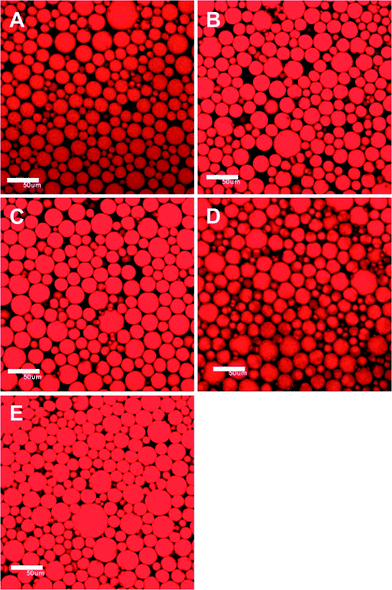
The confocal laser scanning microscopy (CLSM) images shown in Fig. 4 confirm that the formed emulsions are oil-in-water (o/w) emulsions. The emulsions consist of highly dispersed oil droplets that are characteristic of common emulsions. Upon increasing the CCS polymer concentration from 0.3 wt% (Fig. 4A) to 0.5 wt% (Fig. 4B) to 1.0 wt% (Fig. 4C) for HIPEs containing 80 vol% of oil, the average diameter of the oil droplets in the HIPEs drops from 35 μm to 20 μm to 12 μm, suggesting that a higher concentration of CCS polymers can reduce the size of the oil droplets and thus create more interfacial areas. However, the size of the oil droplets is shown to be insensitive to the oil fraction when the CCS polymer concentration is maintained at 0.5 wt% but the oil volumetric fraction is increased from 30 vol% (Fig. 4D) to 50 vol% (Fig. 4E) to 80 vol% (Fig. 4B); the average diameter of the oil droplets remains essentially unchanged (about 20 μm), except that the oil droplets pack more tightly in the HIPE (80 vol%). These results suggest that when the CCS polymer concentration is kept constant the droplet size is mainly determined by the input energy of the homogenizing process, which is the same for all these samples.
We have previously shown that CCS polymers adsorb onto the oil/water interface to stabilize the oil droplets. The adsorption of CCS polymers at the interface is expected to reduce the interfacial energy between dodecane and water. We performed interfacial tension measurements using the pendant drop method to assess the ability of the CCS polymers to reduce the interfacial energy. Table 3 shows the interfacial tensions for all five CCS polymers at 0.5 wt% CCS concentration and 25 °C. The interfacial tension is significantly reduced from 53.4 mN m−1 for dodecane/water to values of 8–12 mN m−1 in the presence of CCS polymers. Within experimental error, two extremes can be identified, with S(16 nm, 51 °C) showing the highest interfacial tension of 11.9 mN m−1 and S(43 nm, 31 °C) showing the lowest interfacial tension of 8.0 mN m−1. The interfacial tension seemed to be insensitive to the CCS polymer concentration; increasing the concentration of S(19 nm, 31 °C) from 0.3 wt% to 0.5 wt% to 1.0 wt% produced rather similar interfacial tensions of 9.5, 8.7 and 9.3 mN m−1, respectively. The insensitivity of interfacial tension to the CCS polymer concentration seems to be inconsistent with the CLSM results that show reduced droplet size with increasing CCS polymer concentration. Previously, Li and co-workers also observed that interfacial tension could not be directly correlated with the emulsifying behavior using other CCS polymers.62 Both our results and literature results suggest that CCS polymers play multiple roles rather than just lowering the interfacial tension. The viscoelasticity of CCS polymers at the interface that accounts for the coalescence stability of droplets should also be considered.
| CCS | S(16 nm, 51 °C) | S(35 nm, 40 °C) | S(43 nm, 38 °C) | S(19 nm, 31 °C) | S(43 nm, 31 °C) |
|---|---|---|---|---|---|
| a Measured by pendant drop method for aqueous solutions of 0.5 wt% CCS polymers and dodecane. | |||||
| Interfacial tension (mN m−1) | 11.9 | 9.6 | 8.8 | 8.7 | 8.0 |
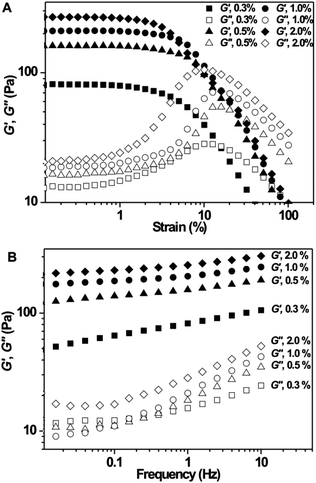
Having shown by vial inversion that the HIPEs were stable against creaming and coalescence, the properties of the HIPEs were further quantitatively investigated by rheology. Fig. 5A shows the changes in storage modulus (G′) and loss modulus (G′′) during strain sweeps at a fixed frequency of 1.0 Hz and 25 °C for HIPEs (80 vol% oil) stabilized by S(43 nm, 38 °C) at different concentrations. A linear regime for G′ is observed for all the HIPEs at low strains (<1%), and G′ increases monotonously with increasing CCS polymer concentration. In this linear regime, G′ is always significantly higher than G′′, indicating the solid-like properties of these HIPEs under the applied conditions. All the HIPEs show a yield point (crossover of G′ and G′′) at which the gels turn into fluidic emulsion droplets. The yield point first increases when the CCS polymer concentration is increased from 0.3 wt% to 0.5 wt%, and then decreases when the CCS polymer concentration is further increased to 2.0 wt%, suggesting that a higher storage modulus is achieved at the expense of reduced elasticity of the HIPEs when the concentration of CCS polymers is increased beyond a critical value (0.5 wt%). Fig. 5B shows the changes in storage modulus, G′, and loss modulus, G′′, during frequency sweeps at a fixed strain of 1.0% and 25 °C for these samples. G′ is always higher than the corresponding G′′, and G′ is almost independent of the frequency applied, confirming again the solid-like properties of these HIPEs.
HIPEs stabilized by CCS polymers in the presence of salts
Two CCS polymers, S(43 nm, 38 °C) and S(43 nm, 31 °C), were selected as representative cases for the investigation of HIPE properties in the presence of salts. Regardless of the difference in the type and concentration of the added salts, the properties of the HIPEs in the presence of salts were essentially unaffected for both CCS polymers when compared with those of the HIPEs in the absence of salts. From Table 4, we can see that the maximum oil fraction that can be emulsified into HIPEs remains nearly the same (∼90%) for both CCS polymers. The HIPEs formed in the presence of salts are also gelled and very stable under ambient temperature (<20 °C) (Fig. 6 and 7). In addition, CLSM micrographs show that the size of the oil droplets is insensitive to the presence of different concentrations and types of salts (Fig. 8). These results together suggest that the CCS polymers can retain their emulsifying capability even in the presence of salts, though the addition of salts can alter their CPs and thus the responsive temperature of the HIPEs (vide infra).| Water | Na2SO4 | NaCl | NaClO4 | NaSCN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a CPs were measured for 0.5 wt% CCS polymer solutions. HIPEs were formed using 0.5 wt% CCS polymer solutions in the absence or presence of salts. | ||||||||||||||
| S(43 nm, 38 °C) | Concentration (mol L−1) | 0.05 | 0.1 | 0.2 | 0.1 | 0.3 | 0.4 | 0.1 | 0.3 | 0.5 | 0.1 | 0.3 | 0.5 | |
| CP (°C) | 38 | 34 | 29 | 21 | 36 | 34 | 31 | 46 | 46 | 45 | 46 | 49 | 50 | |
| Oil fraction (%) | 90 | 92 | 89 | 89 | 91 | 89 | 90 | 91 | 91 | 91 | 91 | 89 | 90 | |
| S(43 nm, 31 °C) | Concentration (mol L−1) | 0.03 | 0.05 | 0.1 | 0.1 | 0.3 | 0.5 | 0.1 | 0.3 | 0.5 | 0.1 | 0.3 | 0.5 | |
| CP (°C) | 31 | 27 | 25 | 21 | 28 | 25 | 23 | 37 | 38 | 37 | 37 | 39 | 42 | |
| Oil fraction (%) | 90 | 89 | 90 | 89 | 90 | 90 | 90 | 90 | 91 | 90 | 91 | 90 | 91 | |
Temperature-responsive demulsification of HIPEs
The CCS polymers used herein for HIPE formation are thermally responsive with varying CPs, and the addition of salts can further tune the CPs. We therefore investigated the possibility of temperature and salt induced demulsification of HIPEs.In the absence of salts, the HIPEs were heated at temperatures higher than their CPs such that the CCS polymers dramatically changed their hydrophilicity, leading to destabilization of the HIPEs. Representative photographs of the demulsification of HIPEs (80 vol% oil) stabilized by S(19 nm, 31 °C) (0.5 wt%) are shown in Fig. 9. Significant demulsification occurs upon heating the HIPE for 11 min at 30 °C (Fig. 9B), which is a temperature near the CP. Heating the HIPE at temperatures higher than the CP leads to faster demulsification (Fig. 9C). Complete demulsification is rapidly reached within 3 min at 50 °C (Fig. 9D), which is 19 °C higher than the CP. Immediately after demulsification, the CCS polymer was seen as flocculate at the oil/water interface, but they became soluble in the aqueous phase again when cooled to room temperature. The fast and effective demulsification is attributed to the high degree of flexibility of the thermally responsive poly(MEAx-co-PEGAy) arm polymers, which are attached to the small core of the CCS polymer at one end, leaving the distal end highly mobile.
Photographs of demulsification of HIPEs stabilized by other CCS polymers can be found in the ESI.† The results for the demulsification of HIPEs stabilized by different CCS polymers are listed in Table 5. The efficiency of demulsification is correlated with the size and CP of the CCS polymers. For HIPEs stabilized by CCS polymers with a smaller size and lower CP, it is easier to induce demulsification. Except for S(16 nm, 51 °C), which has the highest CP, complete demulsification can be readily realized within 6 min under mild heating conditions. In principle, the emulsification–demulsification process can be infinitely repeated because temperature is used as the external trigger and no additives are necessary which can otherwise accumulate in the solution. To illustrate this point, we successfully performed four cycles of emulsification–demulsification and there was no detrimental effect on the formation of HIPEs (Fig. S13, ESI†).
| CCS polymer | Demulsification conditions |
|---|---|
| a The total volume of emulsion is 1.5 mL, the dodecane fraction is 80 vol%, and the CCS polymer concentration is 0.5 wt%. | |
| S(16 nm, 51 °C) | 80 °C, 6 min, >90% demulsification |
| S(35 nm, 40 °C) | 60 °C, 5 min, complete demulsification |
| S(43 nm, 38 °C) | 60 °C, 5 min, complete demulsification |
| S(19 nm, 31 °C) | 50 °C, 3 min, complete demulsification |
| S(43 nm, 31 °C) | 60 °C, 6 min, complete demulsification |
In Fig. 10, rheological test results show that the storage modulus G′ of the HIPE first increases with increasing temperature due to the thermal expansion of the oil droplets, and then drops significantly when the temperature is well above the CP. After demulsification, both G′ and G′′ are smaller than 1 Pa, indicating that a highly fluidic liquid is formed after demulsification.
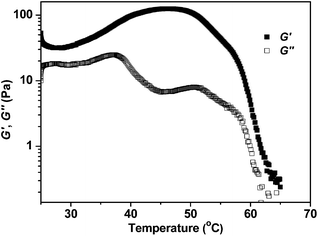 | ||
| Fig. 10 Temperature dependence of rheology tests of a HIPE (80 vol% oil) stabilized by S(19 nm, 31 °C) (0.5 wt%). Strain = 1%, frequency = 1 Hz. | ||
In order to elucidate the possible mechanisms of thermally triggered demulsification, interfacial tensions of water/hexadecane in the presence of CCS polymers were measured during the temperature ramping process. Surprisingly, the interfacial tensions (8–10 mN m−1) remained essentially unchanged at all temperatures, both below and above the CPs. It is evident from DLS measurements that the arms of the CCS polymers undergo a hydrophilic-to-hydrophobic transition when being heated past their CPs, leading to the formation of aggregates. The hydrophilic-to-hydrophobic transition should be equally expected for the CCS polymers in the interfacial tension measurements: the hydrophilic-to-hydrophobic transition should result in changes in wettability of the CCS polymers with respect to both phases, and the formation of aggregates should reduce the available concentration of the CCS polymers for interfacial stabilization. While the exact reason for this phenomenon is unclear at the moment, we tentatively suggest that this may be due to the difference in the samples for HIPEs and the interfacial tension measurements. In HIPEs, oil droplets are dispersed into a continuous aqueous phase containing CCS polymers, leading to the generation of a high interfacial area. In the interfacial tension measurements, only one drop of CCS polymer aqueous solution is formed in the oil phase using the pendant drop method. As such, the interfacial area created in the interfacial tension measurements is rather small, and can be sufficiently stabilized by a tiny amount of CCS polymer adsorbed onto the interface.
Temperature- and salt-responsive demulsification of HIPEs
The addition of salts hardly affected the stability of the CCS-stabilized HIPEs, but did change the CPs of the CCS polymers and thus provided HIPEs with temperature and salt dual responsiveness. The demulsification of HIPEs was studied by two different methods. In Method I, both CCS polymers and salts were dissolved in the aqueous continuous phase to form HIPEs, which were then heated at temperatures above the CPs to induce demulsification. In Method II, HIPEs were first formed without addition of salts. Salts were then added to the HIPEs, which were maintained at a desired temperature, to observe the demulsification. For these experiments, the HIPEs were composed of 80 vol% oil and 0.5 wt% CCS polymer aqueous solution.For HIPEs stabilized by S(43 nm, 38 °C) and prepared by Method I, addition of kosmotropes such as Na2SO4 led to more efficient demulsification, while addition of chaotropes such as NaSCN resulted in the opposite effect. For example, in the presence of 0.1 M Na2SO4, which lowered the CP from 38 °C to 29 °C, the time required for complete demulsification by heating at 60 °C was reduced from 5 min to under 2 min. On the other hand, in the presence of 0.5 M NaSCN, which raised the CP from 38 °C to 50 °C, the time required for complete demulsification by heating at 60 °C was prolonged from 5 min to 25 min. Similar effects were also observed for HIPEs stabilized by S(43, 31 °C) in the presence of either kosmotropes or chaotropes. In the presence of 0.05 M Na2SO4, which lowered the CP from 31 °C to 25 °C, the time required for complete demulsification was reduced even at a lower temperature, from 8 min at 60 °C to 2 min at 50 °C. In the presence of 0.5 M NaSCN, which raised the CP from 31 °C to 42 °C, the time required for complete demulsification by heating at 60 °C was prolonged from 8 min to 12 min.
Salts were also added after formation of HIPEs to trigger demulsification at a specific temperature via Method II. In order to avoid dramatic alteration of the relative oil/water volume in a HIPE, the volume of salt solution added to a HIPE accounted for only 2% of the total volume of liquid in the HIPE. For instance, addition of Na2SO4 solution (to reach a final concentration of 0.1 M) to a HIPE stabilized by S(43 nm, 38 °C) resulted in a reduction of the CP from 38 °C to 29 °C, and maintaining the temperature at 30 °C for 5 min resulted in complete demulsification. Addition of Na2SO4 solution (to reach a final concentration of 0.05 M) to a HIPE stabilized by S(43 nm, 31 °C) resulted in a similar effect, albeit demulsification was achieved after 4 min at 25 °C.
Conclusions
We have demonstrated that CCS polymers containing poly(MEAx-co-PEGAy) arms respond to both temperature and salts. The CPs of the CCS polymers can be reduced by kosmotropes or raised by chaotropes. These CCS polymers can stabilize HIPEs with oil fractions up to 92 vol% at relatively low concentrations of CCS polymer. The addition of salts, either kosmotropes or chaotropes, hardly affects the stability and droplet size of the HIPEs. The HIPEs can be thermally triggered to completely demulsify at mild temperatures within a short time. While the addition of kosmotropes can enhance the demulsification efficiency, the addition of chaotropes can reduce the demulsification efficiency or enhance the thermal stability of the HIPEs. The high freedom of the arm polymers and the nanosize of the CCS polymers are critical to achieve both stability of the HIPEs and fast demulsification upon mild heating. The results reported herein further demonstrate that CCS polymers are a unique type of effective emulsifiers and have the diversity for incorporating other types of responsiveness into CCS-stabilized HIPEs.Acknowledgements
We are grateful for financial support from the Ministry of Science and Technology of China (2009CB930200), the National Natural Science Foundation of China (21274084), Shanghai Municipal Education Commission (11YZ05), Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, and Shanghai Leading Academic Disciplines (S30109). We also acknowledge the use of facilities at the Instrumental Analysis and Research Center, Shanghai University.Notes and references
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21–41 CrossRef CAS.
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CrossRef CAS.
- S. U. Pickering, J. Chem. Soc. Trans., 1907, 91, 2001–2021 RSC.
- S. Shahalom, T. Tong, S. Emmett and B. R. Saunders, Langmuir, 2006, 22, 8311–8317 CrossRef CAS PubMed.
- C. J. Hawker, K. L. Wooley and J. M. J. Fréchet, J. Chem. Soc., Perkin Trans. 1, 1993, 1287–1297 RSC.
- Z. S. Bo, J. P. Rabe and A. D. Schluter, Angew. Chem., Int. Ed., 1999, 38, 2370–2372 CrossRef CAS.
- J. V. M. Weaver, S. P. Rannard and A. I. Cooper, Angew. Chem., Int. Ed., 2009, 48, 2131–2134 CrossRef CAS PubMed.
- Y. Li, J. Zou, B. P. Das, M. Tsianou and C. Cheng, Macromolecules, 2012, 45, 4623–4629 CrossRef CAS.
- S. Fujii, E. S. Read, B. P. Binks and S. P. Armes, Adv. Mater., 2005, 17, 1014–1018 CrossRef CAS.
- B. P. Binks, R. Murakami, S. P. Armes and S. Fujii, Angew. Chem., Int. Ed., 2005, 44, 4795–4798 CrossRef CAS PubMed.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS PubMed.
- S. Fujii, Y. Cai, J. V. M. Weaver and S. P. Armes, J. Am. Chem. Soc., 2005, 127, 7304–7305 CrossRef CAS PubMed.
- K. L. Thompson, P. Chambon, R. Verber and S. P. Armes, J. Am. Chem. Soc., 2012, 134, 12450–12453 CrossRef CAS PubMed.
- M. Klapper, S. Nenov, R. Haschick, K. Müller and K. Müllen, Acc. Chem. Res., 2008, 41, 1190–1201 CrossRef CAS PubMed.
- A. Walther, M. Hoffmann and A. H. E. Müller, Angew. Chem., Int. Ed., 2008, 47, 711–714 CrossRef CAS PubMed.
- M. E. Helgeson, S. E. Moran, H. Z. An and P. S. Doyle, Nat. Mater., 2012, 11, 344–352 CrossRef CAS PubMed.
- B. P. Binks and J. A. Rodrigues, Angew. Chem., Int. Ed., 2005, 44, 441–444 CrossRef CAS PubMed.
- G. Sun, Z. Li and T. Ngai, Angew. Chem., Int. Ed., 2010, 49, 2163–2166 CrossRef CAS PubMed.
- P. Brown, C. P. Butts, J. Cheng, J. Eastoe, C. A. Russell and G. N. Smith, Soft Matter, 2012, 8, 7545–7546 RSC.
- Y. Shen, Y. Zhang, D. Kuehner, G. Yang, F. Yuan and L. Niu, ChemPhysChem, 2008, 9, 2198–2202 CrossRef CAS PubMed.
- J. Zhang, B. Han, C. Zhang, W. Li and X. Feng, Angew. Chem., Int. Ed., 2008, 47, 3012–3015 CrossRef CAS PubMed.
- B. Brugger and W. Richtering, Langmuir, 2008, 24, 7769–7777 CrossRef CAS PubMed.
- B. Brugger, B. A. Rosen and W. Richtering, Langmuir, 2008, 24, 12202–12208 CrossRef CAS PubMed.
- Z. Li and T. Ngai, Colloid Polym. Sci., 2011, 289, 489–496 CAS.
- N. R. Cameron, Polymer, 2005, 46, 1439–1449 CrossRef CAS PubMed.
- H. F. Zhang and A. I. Cooper, Soft Matter, 2005, 1, 107–113 RSC.
- A. Menner, V. Ikem, M. Salgueiro, M. S. P. Shaffer and A. Bismarck, Chem. Commun., 2007, 4274–4276 RSC.
- V. O. Ikem, A. Menner and A. Bismarck, Angew. Chem., Int. Ed., 2008, 47, 8277–8279 CrossRef CAS PubMed.
- I. Gurevitch and M. S. Silverstein, Macromolecules, 2011, 44, 3398–3409 CrossRef CAS.
- I. Akartuna, A. R. Studart, E. Tervoort and L. J. Gauckler, Adv. Mater., 2008, 20, 4714–4718 CrossRef CAS.
- S. Zhang and J. Chen, Chem. Commun., 2009, 2217–2219 RSC.
- Y. Chen, N. Ballard, F. Gayet and S. A. F. Bon, Chem. Commun., 2012, 48, 1117–1119 RSC.
- Z. Li, T. Ming, J. Wang and T. Ngai, Angew. Chem., Int. Ed., 2009, 48, 8490–8493 CrossRef CAS PubMed.
- A. P. Bapat, D. Roy, J. G. Ray, D. A. Savin and B. S. Sumerlin, J. Am. Chem. Soc., 2011, 133, 19832–19838 CrossRef CAS PubMed.
- A. P. Bapat, J. G. Ray, D. A. Savin, E. A. Hoff, D. L. Patton and B. S. Sumerlin, Polym. Chem., 2012, 3, 3112–3120 RSC.
- C. T. Adkins, J. N. Dobish, C. S. Brown, B. Mayrsohn, S. K. Hamilton, F. Udoji, K. Radford, T. E. Yankeelov, J. C. Gore and E. Harth, Polym. Chem., 2012, 3, 390–398 RSC.
- Z. Iatridi, Y. Roiter, N. Stavrouli, S. Minko and C. Tsitsilianis, Polym. Chem., 2011, 2, 2037–2044 RSC.
- Z. Iatridi and C. Tsitsilianis, Soft Matter, 2013, 9, 185–193 RSC.
- A. W. Jackson, C. Stakes and D. A. Fulton, Polym. Chem., 2011, 2, 2500–2511 RSC.
- C. Boyer, J. Teo, P. Phillips, R. B. Erlich, S. Sagnella, G. Sharbeen, T. Dwarte, H. T. T. Duong, D. Goldstein, T. P. Davis, M. Kavallaris and J. McCarroll, Mol. Pharmaceutics, 2013, 10, 2435–2444 CrossRef CAS PubMed.
- J. Ferreira, J. Syrett, M. Whittaker, D. Haddleton, T. P. Davis and C. Boyer, Polym. Chem., 2011, 2, 1671–1677 RSC.
- A. Sulistio, J. Lowenthal, A. Blencowe, M. N. Bongiovanni, L. Ong, S. L. Gras, X. Zhang and G. G. Qiao, Biomacromolecules, 2011, 12, 3469–3477 CrossRef CAS PubMed.
- A. Sulistio, A. Widjaya, A. Blencowe, X. Zhang and G. Qiao, Chem. Commun., 2011, 47, 1151–1153 RSC.
- A. Sulistio, A. Blencowe, A. Widjaya, X. Zhang and G. Qiao, Polym. Chem., 2012, 3, 224–234 RSC.
- A. Blencowe, J. F. Tan, T. K. Goh and G. G. Qiao, Polymer, 2009, 50, 5–32 CrossRef CAS PubMed.
- H. Gao, Macromol. Rapid Commun., 2012, 33, 722–734 CrossRef CAS PubMed.
- W. Li and K. Matyjaszewski, J. Am. Chem. Soc., 2009, 131, 10378–10379 CrossRef CAS PubMed.
- J. Kamada, K. Koynov, C. Corten, A. Juhari, J. A. Yoon, M. W. Urban, A. C. Balazs and K. Matyjaszewski, Macromolecules, 2010, 43, 4133–4139 CrossRef CAS.
- K. Wang, H. Peng, K. J. Thurecht, S. Puttick and A. K. Whittaker, Polym. Chem., 2013, 4, 4480–4489 RSC.
- C. T. Adkins and E. Harth, Macromolecules, 2008, 41, 3472–3480 CrossRef CAS.
- Q. Qiu, G. Liu and Z. An, Chem. Commun., 2011, 47, 12685–12687 RSC.
- Q. Chen, X. Cao, H. Liu, W. Zhou, L. Qin and Z. An, Polym. Chem., 2013, 4, 4092–4102 RSC.
- X. Shi, M. Miao and Z. An, Polym. Chem., 2013, 4, 1950–1959 RSC.
- G. Liu, Q. Qiu and Z. An, Polym. Chem., 2012, 3, 504–513 RSC.
- X. Shi, W. Zhou, Q. Qiu and Z. An, Chem. Commun., 2012, 48, 7389–7391 RSC.
- C. Zhang, M. Miao, X. Cao and Z. An, Polym. Chem., 2012, 3, 2656–2664 RSC.
- M. Miao, Q. Chen, C. Zhang, X. Cao, W. Zhou, Q. Qiu and Z. An, Macromol. Chem. Phys., 2013, 214, 1158–1164 CrossRef CAS.
- W. Zhou, W. Yu and Z. An, Polym. Chem., 2013, 4, 1921–1931 RSC.
- Y. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- J. P. Magnusson, A. Khan, G. Pasparakis, A. O. Saeed, W. Wang and C. Alexander, J. Am. Chem. Soc., 2008, 130, 10852–10853 CrossRef CAS PubMed.
- L. Li, J.-H. Ryu and S. Thayumanavan, Langmuir, 2013, 29, 50–55 CrossRef CAS PubMed.
- W. Li, Y. Yu, M. Lamson, M. S. Silverstein, R. D. Tilton and K. Matyjaszewski, Macromolecules, 2012, 45, 9419–9426 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization of polymers, photographs of emulsion and demulsification. See DOI: 10.1039/c3py00942d |
| This journal is © The Royal Society of Chemistry 2014 |