Chlorin e6 fused with a cobalt-bis(dicarbollide) nanoparticle provides efficient boron delivery and photoinduced cytotoxicity in cancer cells
Anastasija V.
Efremenko
ab,
Anastasija A.
Ignatova
ab,
Mikhail A.
Grin
c,
Igor B.
Sivaev
d,
Andrey F.
Mironov
c,
Vladimir I.
Bregadze
d and
Alexey V.
Feofanov
*ab
aShemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya, 16/10, 117997 Moscow, Russia. E-mail: avfeofanov@yandex.ru
bBiological Faculty, Lomonosov Moscow State University, Vorobyevi Gori 1, Moscow, 119992, Russia
cM.V. Lomonosov Moscow State Academy of Fine Chemical Technology, Vernadskii Prosp. 86, 119571, Moscow, Russia
dA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov Str. 28, 119991, Moscow, Russia
First published on 11th October 2013
Abstract
Further development of boron neutron capture therapy (BNCT) requires new neutronsensitizers with improved ability to deliver 10B isotopes in cancer cells. Conjugation of boron nanoparticles with porphyrin derivatives is an attractive and recognized strategy to solve this task. We report on breakthroughs in the structural optimization of conjugates of chlorin e6 derivative with cobalt-bis(dicarbollide) nanoparticles resulting in the creation of dimethyl ester 13-carbomoylchlorin e6 [N-hexylamine-N′-ethoxyethoxy]-cobalt-bis(dicarbollide) (conjugate 1). Conjugate 1 is able to accumulate quickly and efficiently (distribution factor of 80) in cancer cells, thus delivering more than 109 boron atoms per cell when its extracellular concentration is more than 1 μmol L−1. Also 1 is an active photosensitizer and is phototoxic towards human lung adenocarcinoma A549 cells at 80 nmol L−1 (50% cell death). Photoinduced cytotoxicity of 1 is associated with lipid peroxidation, lysosome rupture and protease activity enhancement. Conjugate 1 fluoresces in the red region (670 nm), which is useful to monitor its accumulation and distribution in vivo. It is not toxic to cells without activation by neutrons or photons. Structural features that improve the functional properties of 1 are discussed. The properties of 1 warrant its preclinical evaluation as a multifunctional agent for BNCT, photodynamic therapy and fluorescent tumor diagnosis.
1. Introduction
Boron neutron capture therapy (BNCT) has become a recognized approach for the treatment of high-grade gliomas, melanoma, and recurrent head and neck cancer, although the number of medical centers practising BNCT is currently very limited.1–5Therapeutic effects of BNCT are realized due to selective accumulation of nonradioactive 10B isotope in a tumor followed by bombarding with boron atoms with thermal or epi-thermal neutrons.1,6 A neutron fuses with the 10B nucleus and forms excited 11B*, which undergoes fission quickly into α-particles and 7Li atoms. Fission products have a high linear energy transfer and do irreversible damage to biological cells. Compared to 10B isotopes, H-, C-, N- and O-atoms have very low cross sections of thermal neutron capture that minimize nonspecific interactions of neutrons with biological molecules.7 The emitted α-particles and 7Li atoms have a short free path in biological tissues (less than 9 and 5 μm, respectively7) and thereby destroy primarily those cells where their precursors, 10B atoms, accumulate.
BNCT efficacy critically depends on the concentration of 10B isotopes in cancer cells. According to estimations, boron concentration needs be as high as 20–35 μg of boron per gram of tumor tissue or 109 atoms per cell to achieve a therapeutic effect with BNCT.1 It means that BNCT agents, neutronsensitizers, should be efficient in the delivery of large amounts of boron atoms in cancer cells. Here, boron nanoparticles and nanoclusters like metal-bis(dicarbollide) complexes (18 boron atoms per nanoparticle) and polyhedral boranes (up to 12 boron atoms per nanocluster) are a good alternative to compounds with a few boron atoms per molecule like boronophenylalanine, sodium borate, boric acid and its derivatives.1,6 At the same time, functionalization of boron nanoparticles is required to increase their affinity to cancer cells and improve their ability for intracellular penetration and accumulation. Different strategies were proposed to solve this task including conjugation of boron nanoparticles with different organic compounds, lipids, amino acids, peptides, carbohydrates, nucleosides, nucleotides and porphyrins.1,6,8,9
Conjugation of boron nanoparticles with porphyrins is considered because many porphyrins are known to accumulate in cancer cells, and some of them are successfully used as photosensitizers for anticancer PDT.10 There are many similarities in the requirements of photosensitizers and neutronsensitizers for anticancer therapy, which should: accumulate highly in cancer cells; be nontoxic themselves for cells; produce sufficient amounts of damaging factors (reactive oxygen species and free radicals in the case of photosensitizers) at the local activation with specific stimulus (red light for photosensitizers). Thus, it is expected that conjugates of boron nanoparticles with porphyrins will gain improved accumulation in cancer cells and meet the key requirements of BNCT agents.
Additionally, conjugates with porphyrins fluoresce, which simplifies the study of their intracellular accumulation and accelerates optimization of their design. Currently, neutron sources for BNCT are very complex and expensive, and their number is limited,1 which hampers wide screening of non-fluorescent neutronsensitizers and selection of prospective candidates. Fluorescence is also very useful for preclinical and clinical studies of nanoconjugates, due to tumor-to-normal tissue accumulation contrast, and the pharmacodynamics and pharmacokinetics of such conjugates can be easy monitored. Also attractive is that good conjugates of boron nanoparticles with porphyrins can be applied to both anticancer BNCT and PDT.
In this paper, we report on breakthroughs in the development and structural optimization of conjugates of cobalt-bis(dicarbollide) with chlorin e6 derivatives (Fig. 1) and describe the properties of recently synthesized11 conjugates 1–3 including intracellular boron delivery and photoinduced cytotoxicity. The design of conjugate 1 is reported to be optimal among 1–10 (Fig. 1) and provides both a considerable photodynamic effect and the highest boron accumulation in A549 human lung adenocarcinoma cells. Structural features that improve the functional properties of 1 are highlighted. The mechanisms of cellular traffic and the photoinduced cytotoxicity of 1 are also studied.
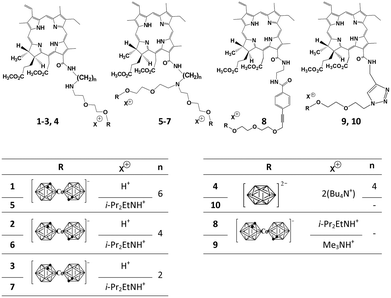 | ||
| Fig. 1 Structures of conjugates of chlorin e6 with boron nanoparticles cobalt-bis(dicarbollide) ([3,3′-Co(1,2-C2B9H11)2]−) and closo-dodecaborate ((B12H12)2−). | ||
2. Experimental
2.1. Reagents
Dimethyl ester 13-carbomoylchlorin e6 [N-hexylamine-N′-ethoxyethoxy]-cobalt-bis(dicarbollide) (1), dimethyl ester 13-carbomoylchlorin e6 [N-butylamine-N′-ethoxyethoxy]-cobalt-bis(dicarbollide) (2) and dimethyl ester 13-carbomoylchlorin e6 [N-ethylamine-N′-ethoxyethoxy]-cobalt-bis(dicarbollide) (3) were synthesized as described earlier,11 and characterized with analytical thin layer chromatography on Merck Kieselgel 60 F245 plates (chloroform–methanol 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 1H NMR spectroscopy.
1) and 1H NMR spectroscopy.
Rhodamine 123, chlorpromazine hydrochloride, Cremophor EL (polyoxyethylene derivative of hydrogenated castor oil), methyl-β-cyclodextrin and 2-deoxy-D-glucose were supplied by Sigma Chemical Co. (USA). Transferrin conjugated with Oregon Green (TOG), BODIPY® 581/591 C11 (BC11) and BZiPAR were purchased from Molecular Probes (USA) and used according to vendor protocols.
Other reagents were of the same origin as described elsewhere.12
2.2. Experiments in solution
The ability of 1–3 for photoinduced generation of singlet oxygen was estimated using a RNO–histidine assay.13 Rose Bengal was used as a reference compound with a known quantum yield of singlet oxygen generation (Φ(1O2) = 0.75).14 Conjugates 1–3 (6.6 μmol L−1 in 0.1% Cremophor) or Rose Bengal (1.3 μmol L−1) were mixed with histidine (11 mmol L−1) and 4-nitroso-N,N-dimethylaniline (30 μmol L−1) in a sodium phosphate buffer (50 mmol L−1, pH 7.0) and irradiated with a laser (532 nm, 0.8 mW). The bleaching of 4-nitroso-N,N-dimethylaniline was measured with absorption spectroscopy every 10 min. The value of Φ(1O2) for 1–3 was calculated as described elsewhere15 and averaged over three experiments. The same experimental protocol but without histidine was used to verify photoinduced generation of ˙OH radicals. Without histidine, 4-nitroso-N,N-dimethylaniline bleaching is induced mainly by ˙OH radicals.16The fluorescence spectra of conjugates in solutions of biological molecules were excited with a Nd3+-YAG laser (532 nm, 12 μW).
2.3. Experiments on cells
Human lung adenocarcinoma A549 cells were grown (37 °C, 5% CO2) in Eagle's minimum essential medium with phenol red, 8% fetal calf serum, 2 mmol L−1L-glutamine (so called complete medium). The cells were subcultured twice per week. On the day prior to an experiment, an exponentially growing monolayer of cells was plated on a round cover glasses placed in 24-well plates (for microscopic experiments) or seeded directly in 96-well plates (for cytotoxicity measurements). Sowing density was 5 × 104 cells per ml. Cells were incubated with 1–3 in a complete medium at 37 °C unless otherwise stated. The kinetics of conjugate uptake was studied at 0.5 μmol L−1 concentration of 1–3. Concentration dependence of conjugate uptake was measured after 2 h of cell incubation with 1–3. To study efflux, cells were incubated with 1–3 (0.5 μmol L−1) for 2 h, rinsed twice with a fresh medium and cultured further for 0.5–5 h.For staining cellular organelles, cells were preincubated with a conjugate (0.5 μmol L−1) for 1 h, and a specific probe was added to a culture medium: acridine orange (0.5 mg L−1) for 10 min, TOG (50 mg L−1) for 30 min or rhodamine 123 (5 μg L−1) for 15 min.
For the study of cellular transport, the cells were preincubated with chlorpromazine (10–50 μmol L−1 for 30 min), methyl-β-cyclodextrin (0.5–2 mmol L−1 for 30 min), sodium azide (10 mmol L−1 for 1 h) or 2-deoxy-D-glucose (100 mmol L−1 for 30 min) in a serum-deprived medium and further incubated in the presence of 1 (0.5 μmol L−1) for 1 h at 37 °C.
To estimate intracellular accumulation of conjugate 1 with an extraction technique, 4.5 × 106 cells were incubated with 1 (1 μmol L−1) in 5.5 ml of a complete medium for 2 h, washed, detached from a flask, pelleted and lysed with 0.25% Triton X-100 for 30 min. Concentration of 1 in cellular extract (Cex) was measured using fluorescence spectroscopy with reference solutions of 1 of known concentrations in 0.25% Triton X-100. The average cytoplasmic concentration (CACC) of 1 was calculated as
| CACC = CexVex/(NcVcyt), | (1) |
The repeated extraction of 1 from pelleted cell debris provided an additional 3% of 1 to the initial extraction.
For cell survival studies, conjugates were added to cells (0.5–16 μmol L−1 with a two-fold increment). Control cells were incubated with equivalent concentrations of Cremophor. The cytotoxicity was estimated after incubation of cells with 1–3 or Cremophor alone for 5 h in the dark. The photoinduced cytotoxicity was measured on cells incubated with 1–3, Photogem, Radachlorin, 13,15-N-(3′-hydroxypropylcycloimide) chlorin p6 or Cremophor alone for 2 h and irradiated with a halogen lamp (500 W) through a water filter (thickness of 5 cm) and a band-pass filter (transmission 630–1000 nm, 22 ± 2 mW cm−2, 20 ± 2 J cm−2). After irradiation the cells were further incubated for 3 h and examined for viability. All the experiments were performed in triplicate. To evaluate cell viability, cells were stained with Hoechst 33342 (stains all cells) and propidium iodide (stains dead cells) and analyzed with a fluorescence microscope as described elsewhere.12,17
To reveal protease activity in irradiated cells, the cells were incubated with a conjugate (0.2 μmol L−1 for 2 h), irradiated as described above and further incubated with BZiPAR (5 μmol L−1) for 15 min. Control cells were irradiated without a conjugate and incubated with BZiPAR or pre-incubated with a conjugate (0.2 μmol L−1 for 2 h) and further incubated with BZiPAR without irradiation. To examine cells for photoinduced lipid peroxidation, the cells were incubated with a conjugate (0.1 or 0.15 μmol L−1 for 2 h) and BC11 (1 μmol L−1 during the last 15 min of incubation) and irradiated as described above. Control cells were either incubated with a conjugate and BC11 without irradiation or incubated with BC11 and irradiated.
2.4. Microscopy
Fluorescence spectra and concentration of conjugates in cells were measured with the confocal spectral imaging technique15,18,19 using the experimental installation described elsewhere.20,21 The confocal spectral imaging technique allows one to study intracellular molecular interactions of fluorescent drugs and to take into account the influence of intracellular environment on the fluorescence quantum yield of drugs (important for quantitative analysis) through the analysis of their intracellular spectra. The technique is based on fluorescence spectrum acquisition at each point of the equatorial plane of a cell. Fluorescence was excited with a continuous Nd3+-YAG laser (532 nm, 12 μW) and measured at the lateral, axial and spectral resolutions of 0.6 μm, 3 μm and 1 nm, respectively. The 20 × 20 or 30 × 30 voxel spectral images were recorded and treated as described previously.12,15,18–21The concentration of the monomeric fluorescent form of conjugate within cells was deduced from the integrated intensity of intracellular fluorescence spectra. Calibration of fluorescence intensity as a function of a conjugate concentration was performed with the conjugate solutions in 1% Cremophor (50 mmol L−1 sodium phosphate buffer, pH 7.0) under the same conditions (excitation wavelength, laser power, lateral and axial resolution, integration time, objective, etc.) that were used in cell measurements. Solutions of the conjugates in 1% Cremophor were selected for calibration on the basis of the study of molecular interactions of conjugates and analysis of their intracellular spectra (see sections 3.1 and 3.2 below). Using the calibration results, conversion coefficients were calculated to convert the intensity of intracellular spectra of conjugates into concentrations and compute quantitative maps of intracellular distribution of conjugates. The quantitative map of conjugate distribution was computed for each measured cell, and an average cytoplasmic concentration of conjugate was calculated. Averaging over 30–40 cells was done for each analyzed regime of cell incubation.
Detailed intracellular distribution and localization of conjugates were studied with the LSM-710 confocal laser scanning microscope (Carl Zeiss AG, Germany) at 0.3 μm lateral and 1.5 μm axial resolution. Conjugate fluorescence was excited with a He–Ne laser (632.8 nm), and emission was registered in the 650–750 nm spectral range. Fluorescence of acridine orange, rhodamine 123, TOG or BZiPAR was excited with an Ar+-ion laser (488 nm), and emission was recorded in the 500–600 nm spectral range. Fluorescence of conjugates and acridine orange were recorded using a multi-track regime (consecutive scanning). Fluorescence of acridine orange in cells was within the 500–600 nm spectral range, because its concentration (0.5 μg ml−1) was well below the level leading to the formation of red fluorescent aggregates. Special experiments were performed to verify that the fluorescence of conjugates and organelle probes did not interfere with the used setup. Coefficients that characterize colocalization of conjugates and organelle probes were calculated as described elsewhere.22
Intracellular distributions of BC11, its peroxide and conjugate were measured with the confocal laser scanning microscope TSC SP2 (Leica, Germany) using a consecutive scanning regime. Conjugate fluorescence was excited and detected as described above. Fluorescent signals of BC11 and its photoinduced peroxide were excited with the 488 and 561 nm wavelengths and detected in the 500–530 and 585–630 nm ranges, respectively.
3. Results
3.1. Molecular properties of 1–3 in solutions
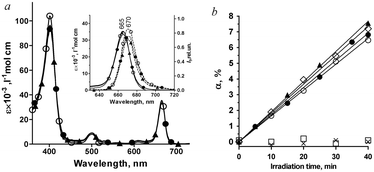
Conjugates 1–3 are insoluble in water, and thereby they were dissolved in 5% Cremophor aqueous emulsion (50 mmol L−1 sodium phosphate buffer, pH 7.0) at a concentration of 0.7 mmol L−1. Note, Cremophor is tolerable for cells in vitro at moderate concentrations22 and is used in clinical practice.23 As confirmed with absorption and fluorescence spectroscopy, Cremophor efficiently stabilizes a monomeric form of conjugates and prevents precipitation of 1–3 over at least six-months of storage. The absorption and fluorescence emission spectra of 1–3 in 1% Cremophor are very similar (Fig. 2a, Table 1), since the additional alkyl groups in 131-substituent do not affect π-electron density distribution in chlorin e6 chromophore. The molar extinction coefficient of 1–3 in 1% Cremophor solution is (35 ± 2)×103 L mol−1 cm−1 at 665 nm.| Solutiona | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| λ f/nm | R/% | FWHM/nm | λ f/nm | R/% | FWHM/nm | λ f/nm | R/% | FWHM/nm | |
| a Solutions were prepared in a sodium phosphate buffer (50 mmol L−1, pH 7.0), acetate buffer (50 mmol L−1, pH 4.0 and pH 5.0), sodium phosphate buffer with citric acid (50 mmol L−1, pH 3.0). λf is the maximum fluorescence emission. R is the ratio of the integrated fluorescence intensity of the studied conjugate in the examined solution to the intensity of this conjugate in 1% Cremophor solution (pH 7.0). FWHM is a full width at the half of maximum of the fluorescence spectrum. Abbreviation nb means no detectable binding. | |||||||||
| 1% Cremophor emulsion, pH 7.0 | 670 | 100 | 18 | 670 | 100 | 18 | 670 | 100 | 18 |
| 1% Cremophor emulsion, pH 5.0 | 670 | 97 | 18 | 670 | 100 | 18 | 670 | 99 | 18 |
| 1% Cremophor emulsion, pH 4.0 | 670 | 98 | 18 | 670 | 100 | 18 | 670 | 100 | 18 |
| 1% Cremophor emulsion, pH 3.0 | 670 | 93 | 18 | 670 | 95 | 18 | 670 | 96 | 18 |
| 0.5% Triton X-100 micelles, pH 7.0 | 670 | 98 | 18 | 670 | 96 | 18 | 670 | 99 | 18 |
| Bovine serum albumin, 10 g L−1 | 670 | 8 | 22 | 670 | 6,3 | 22 | 670 | 8 | 22 |
| Human serum albumin, 10 g L−1 | 668 | 3 | 22 | 668 | 1 | 22 | 668 | 7 | 22 |
| α-globulins, 10 g L−1 | 670 | 9 | 17 | 670 | 1 | 17 | 670 | 5 | 17 |
| Mixture of α- and β-globulins, 10 g L−1 | 670 | 8 | 17 | nb | — | — | 670 | 6 | 17 |
| Lysozyme, 10 g L−1 | 670 | 1 | 18 | nb | — | — | 670 | 2 | 18 |
| DNA, 10 g L−1 | 670 | 2 | 18 | nb | — | — | 670 | 1 | 18 |
| RNA, 10 g L−1 | nb | — | — | nb | — | — | nb | — | — |
Complexation with different biological molecules can govern pharmacokinetics and pharmacodynamics of drugs and, in particular, their intracellular penetration and localization.
Accordingly, we studied the binding of 1–3 to some proteins, nucleic acids and membrane-like systems. The results of the molecular interaction study are also important in order to interpret intracellular fluorescence spectra of conjugates and perform the quantitative analysis of intracellular accumulation of 1–3 using the confocal spectral imaging technique.
When Cremophor is present in solution with biological molecules (proteins, nucleic acids) interactions of conjugates with Cremophor masks other interactions. Therefore, Cremophor was eliminated, and the following approach was employed in those experiments. Conjugates 1–3 are monomeric and fluoresce in 100% ethanol, but form non-fluorescent aggregates upon fifty-fold dilution of the ethanol solution in water. Appearance of fluorescence that occurs upon mixing of these aggregates with biological molecules indicates dissociation of the aggregates due to formation of complexes. The ratio of fluorescence intensity of a conjugate in the concentrated solution of examined molecules to its fluorescence intensity in 1% Cremophor emulsion (R, in percents) is introduced for the qualitative comparison of binding ability (Table 1). Analysis of absorption spectra and dependences of fluorescence intensity on the concentration of added molecules is used to verify formation of complexes with a low fluorescence quantum yield. Concentration of 1–3 was kept constant (6 μmol L−1) in these experiments. The efficiency of an such approach was proved earlier in the studies of different hydrophobic photosensitizers15,17,20,22,24 and boronated conjugates.12,21
Conjugate 2 does not bind to nucleic acids. Similarly, 1 and 3 demonstrate no binding with RNA and very weak binding with DNA (Table 1). A decrease in DNA concentration from 10 to 5 g L−1 leads to total disappearance of fluorescence of 1 and 3. The absorption spectra of the conjugates coincide in the presence and absence of nucleic acids.
Weak binding with bovine serum albumin and the absence of complexation with other tested proteins (Table 1) are the properties of 2. Binding with lysozyme is traceable for 1 and 3 at a high protein concentration only (Table 1). Conjugates 1 and 3 form complexes with globulins, human and bovine serum albumins (Table 1), but just a small portion of 1 and 3 participates in the binding in spite of a high concentration of proteins and their considerable molar excess over the conjugates. This conclusion comes from the following data. A decrease in protein concentration from 10 to 2 g L−1 (from ca. 0.16 to 0.03 mM) is accompanied with a directly proportional decrease in fluorescence intensity. It means that the binding is far from saturation. In the presence of proteins, the absorption spectra of 1 and 3 are not changed as compared to the spectra in water.
A distinct property of 1–3 is their intensive binding with Triton X-100 micelles and Cremophor emulsions (Table 1) that are used as simple membrane-like systems in our studies. Total dissociation of aggregates and achievement of maximal fluorescence intensity is observed at the concentration of Cremophor or Triton X-100 being equal to 0.1% or higher. Thus, the affinity of 1–3 to lipids is much higher than their affinity to water soluble proteins and nucleic acids. The spectral characteristics of 1–3 bound in lipid-like environment formed by Cremophor droplets and Triton X-100 micelles are quite similar, and they are not changed in the pH range of 3.0–7.0 (Table 1).
Derivatives of chlorin e6 are photosensitizers that produce reactive oxygen species under illumination with light.25 Accordingly, we have studied the photosensitizing properties of 1–3 in a solution. Using methods of chemical traps,13,15,16 the conjugates were found to preserve the ability of chlorin e6 in the photoinduced generation of singlet oxygen (Fig. 2b). To confirm this conclusion, addition of sodium azide, a well-known specific singlet oxygen quencher, totally suppressed the photoinduced conjugate-mediated bleaching of a chemical trap (Fig. 2b). In a lipid-bound form, 1–3 possess high quantum yields of singlet oxygen generation Φ(1O2) (Table 2). A length of amino–alkyl–amino linker in 1–3 does not affect the value of Φ(1O2) (Table 2). Also, it was found that 1–3 do not produce OH˙ radicals under illumination (Fig. 2b).
| Φ(1O2) | T up/h | T ef/h | K | LD50/nmol L | LD90/nmol L−1 | |
|---|---|---|---|---|---|---|
| a Φ(1O2) is the quantum yield of singlet oxygen generation. Tup (Tef) is the time for 50% uptake (50% efflux) of a conjugate in A549 cells. LD90 (LD50) is a conjugate concentration that provides 90% (50%) photoinduced cell death. K is a distribution factor, i.e. the ratio of the average cytoplasmic concentration of a conjugate to its extracellular concentration. b Adapted from published paper.12 c Adapted from published paper.21 | ||||||
| 1 | 0.76 ± 0.05 | 0.91 ± 0.08 | 3.4 ± 0.1 | 80 ± 4 | 80 ± 10 | 120 ± 10 |
| 2 | 0.72 ± 0.05 | 0.64 ± 0.05 | 3.2 ± 0.2 | 52 ± 2 | 240 ± 40 | 370 ± 20 |
| 3 | 0.70 ± 0.04 | 0.67 ± 0.07 | 1.8 ± 0.1 | 24 ± 1 | 520 ± 30 | 800 ± 50 |
| 4 | 0.85 ± 0.06 | 0.9 ± 0.1 | 4.3 ± 0.2 | 1.5 ± 0.2 | 2100 ± 200 | 3100 ± 200 |
| 5 | 9 ± 1 | ≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| 6 | 0.55 ± 0.05 | 0.7 ± 0.1 | 4.0 ± 0.3 | 5.0 ± 0.2 | ≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 7 | 0.20 ± 0.05 | ≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
| 8, 9, 10c | <0.2 | ≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≫16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|||
3.2. Intracellular accumulation and distribution of 1–3
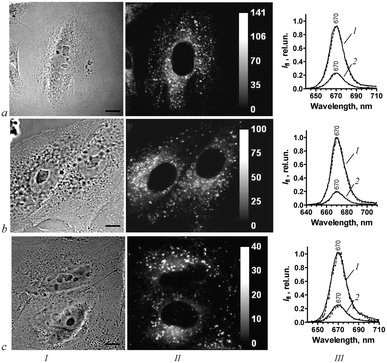
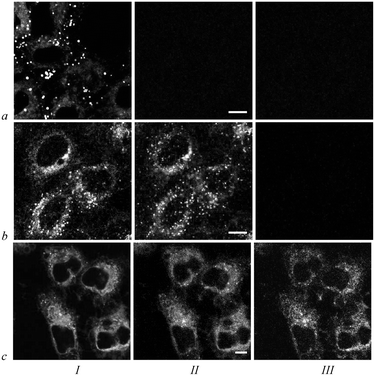
Incubation of A549 cells with 1–3 is accompanied by the appearance of a bright red fluorescence in the cells that indicates cellular accumulation of the conjugates. As proven with confocal laser scanning microscopy, conjugates accumulate in the cytoplasm (Fig. 3). They do not penetrate into the nucleus. In the cytoplasm, 1–3 have a similar mixed granular-diffuse distribution with predominant granular staining at the periphery.Vital fluorescent probes for cellular organelles,26 namely, acridine orange for lysosomes, rhodamine 123 for mitochondria and TOG for endosomes, were used to identify the origin of intracellular granules with conjugates. As shown using confocal laser scanning microscopy, most of the intracellular granules with 1–3 are lysosomes (Fig. 4, row a). Colocalization coefficients for conjugates and lysosomes (calculated as a ratio of the conjugate–acridine orange colocalization area to the total area of lysosomes stained with acridine orange) are 0.75 ± 0.4, 0.79 ± 0.03 and 0.77 ± 0.05 for 1, 2 and 3, respectively. Colocalization between conjugates and endosomes stained with TOG is minor (Fig. 4, row b). Corresponding colocalization coefficients are 0.10 ± 0.03, 0.07 ± 0.03 and 0.09 ± 0.02 for conjugates 1, 2 and 3, respectively. Conjugates do not accumulate in the mitochondria (Fig. 4, row c) and lipid droplets, cellular organelles for storage of neutral lipids (Fig. 4, row d).
Confocal spectral analysis revealed that intracellular fluorescence spectra of 1–3 are identical in a maximum wavelength and shape in every point of the cytoplasm including lysosomes and regions of diffuse distribution. The intracellular spectra coincides with the fluorescence spectra of 1–3 in 1% Cremophor (Fig. 3, column III) as well as with the spectra of 1–3 in Triton X-100 micelles (Table 1). At the same time 1–3 possess low affinity for proteins and nucleic acids. Therefore, it may be concluded that the conjugates are situated in lipids and lipid-like environments within cells, most probably in the lysosome membranes and cytoplasmic membranous structures. In accordance with the data obtained in vitro (Table 1), a low pH in lysosomes does not affect the fluorescent spectra of lipid-bound conjugates.
It is worth mentioning that we have no data indicating that conjugates 1–3 penetrate and accumulate within the cell in complexes with Cremophor. It seems that 1–3 are translocated from Cremophor drops to plasma membrane and transported further in the complexes with lipids. This is confirmed by experiments when conjugate 1 was added to cells from dimethylsulfoxide solution (data not shown): the same intracellular distribution and fluorescent spectra were detected. Indeed, in an extracellular medium the conjugates can leave Cremophor drops and bind to lipids and lipoproteins of fetal calf serum. In fact, Cremophor is a solubilizer that prevents irreversible aggregation of 1–3 in biological fluids and helps to form complexes of conjugates with lipids and lipoproteins upon manifold dilution of Cremophor emulsion.
The results of molecular (Table 1) and cellular spectral studies enable the use of the confocal spectral imaging technique15,18,19 for quantitative analysis of intracellular accumulation of 1–3. A linear growth of a cytoplasmic conjugate concentration is observed during incubation of cells with 2–3 and 1 for 1 and 1.5 h, respectively (Fig. 5a). Saturation of intracellular accumulation occurs after 2 h exposure of cells to 1–3 (Fig. 5a). A pattern of intracellular distribution described above (Fig. 3) is formed during the first 30–40 min of cell exposure to 1–3 and changes quantitatively only with a further increase in incubation time. The retention kinetics of 1 and 2 in cells is very similar, whereas efflux of 3 occurs ca. two-times faster (Table 2, Fig. 5a). The most important difference between conjugates is their average cytoplasmic concentration (ACC), which is highest for 1 and lowest for 3 during both uptake and efflux (Fig. 5). To characterize and compare the ability of 1–3 to accumulate in cells, the distribution factor K was calculated (Table 2). K is a maximal ratio of ACC to extracellular conjugate concentration. It is maximal and constant at the linear part of the ACC dependence on extracellular conjugate concentration (Fig. 5b), namely, at extracellular concentrations of 1–3 less than 1 μmol L−1. K values for the studied conjugates are very high (Table 2). Conjugate 1 surpasses conjugates 2 and 3 in this parameter by factors of 1.5 and 3.3, respectively. Local concentrations of conjugates are at least threefold higher in lysosomes than in the surrounding diffusely stained cellular structures (Fig. 3). Extremely high accumulation of 1 in cancer cells is independently confirmed with an extraction technique. According to the extraction data, ACC of 1 in A549 cells is 75 ± 9 μmol L−1 after incubation with 1 at 1 μmol L−1 for 2 h (i.e. K = 75 ± 9).
Each conjugate penetrating into a cell brings 18 boron atoms. Assuming that the cytoplasm and total volume of a cell are ca. 1.2 × 10−12 and 1.7 × 10−12 L, respectively, one can calculate that conjugate 1 meets the BNCT requirement to deliver 109 boron atoms per cell. This value is achieved at ca. 1 μmol L−1 extracellular concentration of 1. Conjugates 2 and 3 provide less but still high cell loading with boron.
3.3. Study of the mechanisms of intracellular penetration of conjugate 1
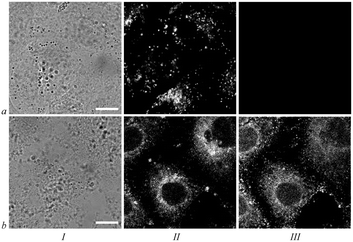
Efficient intracellular boron delivery with 1–3 brings up a question about mechanisms promoting cellular accumulation of the conjugates. We have performed a set of experiments with 1 to reveal the factors affecting its cellular transport.A decrease in the content of fetal calf serum in a culture medium from 8 to 0% does not disturb intracellular accumulation and distribution of 1 (Fig. 6a, b, i), and therefore association of 1 with serum components (proteins, lipids, lipoproteins) is not essential for the transfer of 1 from the extracellular medium to and across plasma membrane.
Extraction of cholesterol with methyl-β-cyclodextrin from a cell membrane and inhibition of clathrin recycling with chlorpromazine were used to block caveolin- and clathrin-dependent types of endocytosis, respectively, and thus to probe their involvement in cellular transport of 1. Neither uptake nor the intracellular pattern of 1 changed in these experiments (Fig. 6c, d, i), even at sub-toxic treatments with inhibitors leading to cell morphology distortion (data not shown). Hence, caveolar- and clathrin-dependent endocytosis is hardly involved in absorption of 1 by cells.
Temperature is a factor affecting both intracellular accumulation and distribution of 1 (Fig. 6e, f, i). A decrease in the temperature of a culture medium during incubation of A549 cells with 1 from 37 to 17 °C results in a threefold reduction of ACC and the disappearance of granular (lysosomal) accumulation of the conjugate (Fig. 6f, i). Reduced lysosomal accumulation of 1 is observed even at 27 °C, whereas ACC decreases just slightly (Fig. 6e, i).
To verify, if some energy-dependent mechanisms of transport are involved in conjugate uptake, ATP synthesis in the cytoplasm and mitochondria was inhibited with 2-deoxy-D-glucose and sodium azide, respectively. ATP depletion leads to a decrease in lysosomal accumulation of 1 (Fig. 6g, h) but exerts no effect on ACC (Fig. 6h). One can conclude that ATP-dependent mechanisms are involved in sequestration of 1 in lysosomes but play a negligible role in its cellular uptake.
3.4. Intrinsic and photoinduced cytotoxicity of conjugates
Conjugates 1–3 are not toxic to A549 cells at the tested concentrations (<16 μmol L−1, Fig. 7) without activation with photons or neutrons. Since chlorin e6 in the conjugates retains its capability for photoinduced generation of singlet oxygen (Table 2, Fig. 2b), the photoinduced cytotoxicity of 1–3 was studied. The results of this study (Fig. 7) show that photoinduced concentration-dependent killing of cells is a distinct property of 1–3. Photoinduced cytotoxicity increases in the order 3 < 2 < 1 (Fig. 7, Table 2).3.5. Mechanism of photoinduced cytotoxicity of 1
As revealed with BC11, a ratiometric lipid peroxidation sensor, photoactivation of 1 in cells is accompanied with peroxidation of unsaturated lipids (Fig. 8c). The quantity of lysosomes with 1 decreases, and the distribution of 1 becomes diffuse after irradiation of cells with light (Fig. 8 and 9). These observations indicate the damage to the lysosomes and leakage of 1 from the lysosomes to the cytoplasm. Protease activity develops in the cytoplasm after irradiation (Fig. 9) as shown with BZiPAR, a fluorogenic substrate for probing protease activity in living cells. Control cells irradiated without a conjugate and incubated with BZiPAR showed no protease-induced fluorescence of BZiPAR (data not shown). Similarly, no protease-induced BZiPAR fluorescence was revealed in the cells pre-incubated with a conjugate and further incubated with BZiPAR without irradiation (Fig. 9, row a).4. Discussion
Our efforts in the development of conjugates of chlorin e6 derivative with boron nanoparticles11,12,21,27,28 resulted in the creation of conjugate 1 having great a ability to accumulate in cancer cells. This property was achieved through optimization of the conjugate structure. It was revealed that intracellular accumulation of the conjugates depends on the type of boron nanoparticles, the number of conjugated nanoparticles and the structure of the linker between chlorin e6 and the nanoparticles.Concerning nanoparticle type, conjugates with cobalt-bis(dicarbollide) (2 and 6, Fig. 1) bear more boron atoms and demonstrate improved intracellular accumulation as compared to structurally similar conjugate 4 (Fig. 1) with a closo-dodecaborate nanoparticle (Table 2). Cellular uptake of conjugates 1–3 with one cobalt-bis(dicarbollide) nanoparticle is much better than uptake of conjugates 5–7 with two nanoparticles (Table 2), and the resulting intracellular boron delivery ameliorates in the case of 1–3 in spite of a twofold decrease in boron cargo per conjugate. Cobalt-bis(dicarbollide) is mono-anion with a delocalized charge, whereas closo-dodecaborate is di-anion. Accordingly, one of the reasons of improved penetration of 1–3 in cells is their reduced negative charge as compared to dianionic 4 and 5–7. Obviously, cobalt-bis(dicarbollide) is better suited than closo-dodecaborate for the creation of conjugates delivering boron in cancer cells, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between chlorin e6 and cobalt-bis(dicarbollide) is most optimal.
1 stoichiometry between chlorin e6 and cobalt-bis(dicarbollide) is most optimal.
Besides an evident fusion function, a linker between chlorin e6 and a nanoparticle is found to control intracellular delivery of conjugates. Flexible linkers in 1–7 promote internalization of the conjugates, whereas linkers with rigid aromatic cycles in 8–10 block it (Table 2). From comparison of 1–3 and 5–7 (Fig. 1, Table 2) it is clear that the increased length of a polyalkyl chain in the linker is another important structural parameter enhancing cellular accumulation of conjugates. A linker with an elongated polyalkyl chain seems to facilitate anchoring of conjugates in cellular membranes and/or improve their affinity to some membrane-bound carriers.
Mechanisms that govern efficient cellular uptake of 1–3 are currently unclear. Data obtained for 1 (Fig. 6) indicate that mechanisms of caveolar- and clathrin-dependent endocytosis are hardly involved in conjugate cellular traffic. It is revealed that total uptake of 1 is a temperature sensitive process, whereas its accumulation in lysosomes is energy-dependent.
Conjugates 1–3 are characterized by rather fast uptake and relatively long retention in A549 cells (Table 2, Fig. 5a). Both properties are convenient for medical applications of conjugates as photo- or neutron-sensitizers: tumor cells will absorb an injected conjugate quickly and efficiently; due to slowed efflux, a delay between conjugate administration and tumor treatment with light/neutrons can be optimized. Cellular retention of 1–3 is enhanced, probably due to hydrolysis of methylacetate groups to acetate anions with cellular esterases: the conjugates become more charged, and their efflux slows down.
According to the obtained results (Table 2, Fig. 7), conjugates 1–3 are not toxic themselves for cells but very potent in photoinduced cell killing. A decrease in photoinduced cytotoxicity in the row 1–3 is definitely related to a decrease in capacity of the conjugates for intracellular accumulation (Table 2, Fig. 5), because other some characteristics of 1–3 including spectral properties, interactions with molecules, Φ(1O2) and intracellular localization are similar.
Photoinduced cytotoxicity distinguishes 1–3 from previously studied conjugates 5, 6 with their two cobalt-bis(dicarbollide) nanoparticles, which have high Φ(1O2) and accumulate well in cells but produce no photocytotoxic effect (Table 2). It is supposed that in a cellular environment cobalt complexes of 5 and 6 induce an antioxidative effect associated with quenching of free radicals or (and) reactive oxygen species other than singlet oxygen.12,21 In line with this conclusion, conjugate 4 without a cobalt complex is photocytotoxic for A549 cells (Table 2), whereas the absence of photoinduced cytotoxicity for conjugate 10 without a cobalt complex is primarily related to its low intracellular accumulation (Table 2). To explain the appearance of photoinduced cytotoxicity for 1–3, one should suppose that a single cobalt complex in 1–3 is not able to quench efficiently all free radicals and reactive oxygen species formed in cells during irradiation with light. A partial antioxidative effect of the cobalt complex is still felt because, for example, a 34-fold increase in ACC of 2 leads to only an eight-fold enhancement of its activity as compared to conjugate 4. The negative influence of the cobalt complex on photocytotoxicity decreases evidently when the distance between chlorin e6 and cobalt-bis(dicarbollide) becomes large enough. Thus comparing 1 and 4, a 53-fold rise in ACC of 1 is accompanied with a 26-fold increase in its activity.
Lipid peroxidation, disruption of lysosomes and proteolysis are toxic processes that contribute to the photoinduced destruction of cells with 1. The effects are obviously related to high affinity of 1 to lipid structures (Table 1) and its enhanced accumulation in lysosomes (Fig. 4, row a). Due to the high reactivity of photoinduced reactive oxygen species, damage of molecules and cellular structures occurs initially in close proximity to their sites of formation (i.e. sites of 1 localization). Lysosome rupture can result from either photoperoxidation of lysosome membrane lipids or photoinduced osmotic imbalance across a lysosomal membrane associated with increased K+ uptake.29 Conjugate 1 is redistributed from damaged lysosomes to the cytoplasm during irradiation (Fig. 8 and 9) and, very probably, enhances photoinduced injury of other cellular structures. Development of protease activity in the cytoplasm (Fig. 9) arises at least partially because of protease leakage from permeabilized lysosomes and promotes cell death initiated by photodynamic treatment with 1.
To characterize better the potential of conjugate 1 as a photosensitizer we have measured photoinduced cytotoxicity of clinically used photosensitizers Photogem,30 Radachlorin (chlorin e6-based photosensitizer)30,31 and a highly active experimental photosensitizer 13,15-N-(3′-hydroxypropylcycloimide) chlorin p6.22,32 Due to excellent intracellular accumulation and good photodynamic properties, conjugate 1 is 160-, 300- and 4-fold more active than Photogem (LD50 = 13 μmol L−1), Radachlorin (LD50 = 24 μmol L−1), 13,15-N-(3′-hydroxypropylcycloimide) chlorin p6 (LD50 = 0.33 μmol L−1), respectively (Fig. 7). Conjugate 1 surpasses in photocytotoxicity many advanced experimental photosensitizers studied by us earlier.15,22,24
Conjugate 1 provides the highest boron delivery in cancer cells among conjugates 1–10 (Fig. 1). It brings more than 1 × 109 boron atoms per cell when its extracellular concentration is just 1 μM and creates conditions that are necessary and sufficient for cell death induced by the flux of thermal neutrons. Intracellular accumulation of boron is very important for efficient BNCT. According to theoretical estimations, if a neutronsensitizer is located extracellularly (e.g. adsorbed on the plasma membrane) instead of intracellularly its amount should be ten-fold more to kill a cancer cell.7In vivo studies confirm the significance of intracellular accumulation of boron.33
5. Conclusions
Conjugate 1 is a promising candidate for the development of neutronsensitizer for BNCT, because it is able to deliver more than 109 boron atoms per cell. Further, in vivo investigation of conjugate 1 as a photosensitizer for anticancer PDT is warranted, since it is very potent in the photoinduced killing of cancer cells. Currently, some clinical photosensitizers, which are in fact fluorophores, are successfully used for fluorescent diagnosis and fluorescence-guided tumor resection.34–36 Red fluorescence and intensive accumulation of 1 in cancer cells are a solid background to consider this conjugate for similar applications. Similarly, the properties of conjugate 1 allow one to classify it as a multifunctional agent for PDT, BNCT and fluorescent tumor diagnosis.Abbreviations
| ACC | Average cytoplasmic concentration; |
| ATP | Adenosine triphosphate; |
| BC11 | Lipid peroxidation sensor BODIPY® 581/591 C11; |
| BNCT | Boron-neutron capture therapy; |
| DNA | Deoxyribonucleic acid; |
| FWHM | A full width at the half of maximum of the fluorescence spectrum; |
| PDT | Photodynamic therapy; |
| RNA | Ribonucleic acid; |
| TOG | Transferrin conjugated with Oregon Green 488. |
Symbols
| C ex | Concentration of conjugate in cellular extract; |
| C ACC | Average cytoplasmic concentration of conjugate; |
| K | Maximal ratio of CACC to extracellular concentration of conjugate; |
| LD90 | Conjugate concentration that provides 90% photoinduced cell death; |
| LD50 | Conjugate concentration that provides 50% photoinduced cell death; |
| N c | Number of cells; |
| T up | Time for 50% uptake of a conjugate in cells; |
| T ef | Time for 50% efflux of a conjugate from cells; |
| R | Ratio of the integrated fluorescence intensity of the studied conjugate in the examined solution to the intensity of this conjugate in 1% Cremophor solution; |
| V ex | Extract volume; |
| V cyt | Cytoplasm volume of a cell; |
| Φ(1O2) | Quantum yield of singlet oxygen generation; |
| λ f | Maximum of fluorescence emission. |
Acknowledgements
This work was funded by the Russian Foundation for Basic Research (10-04-01436, 13-04-00670). Some experiments were performed at the User Facilities Center of M.V. Lomonosov Moscow State University (contract 16.552.11.7081) on equipment funded by M.V. Lomonosov Moscow State University Program of Development.Notes and references
- R. F. Barth, M. G. Vicente, O. K. Harling, W. S. Kiger 3rd, K. J. Riley, P. J. Binns, F. M. Wagner, M. Suzuki, T. Aihara, I. Kato and S. Kawabata, Radiat. Oncol., 2012, 7, 146 CrossRef PubMed.
- J. W. Hopewell, T. Gorlia, L. Pellettieri, V. Giusti, B. H-Stenstam and K. Sköld, Appl. Radiat. Isot., 2011, 69, 1737 CrossRef CAS PubMed.
- S. Kawabata, S. Miyatake, R. Hiramatsu, Y. Hirota, S. Miyata, Y. Takekita, T. Kuroiwa, M. Kirihata, Y. Sakurai, A. Maruhashi and K. Ono, Appl. Radiat. Isot., 2011, 69, 1796 CrossRef CAS PubMed.
- L. Kankaanranta, T. Seppälä, H. Koivunoro, K. Saarilahti, T. Atula, J. Collan, E. Salli, M. Kortesniemi, J. Uusi-Simola, P. Välimäki, A. Mäkitie, M. Seppänen, H. Minn, H. Revitzer, M. Kouri, P. Kotiluoto, T. Seren, I. Auterinen, S. Savolainen and H. Joensuu, Int. J. Radiat. Oncol., Biol., Phys., 2012, 82, e67 CrossRef PubMed.
- P. R. Menéndez, B. M. Roth, M. D. Pereira, M. R. Casal, S. J. González, D. B. Feld, G. A. Santa Cruz, J. Kessler, J. Longhino, H. Blaumann, R. Jiménez Rebagliati, O. A. Calzetta Larrieu, C. Fernández, S. I. Nievas and S. J. Liberman, Appl. Radiat. Isot., 2009, 67, S50 CrossRef PubMed.
- I. B. Sivaev and V. V. Bregadze, Eur. J. Inorg. Chem., 2009, 11, 1433 CrossRef.
- T. Kobayashi and K. Kanda, Radiat. Res., 1982, 91, 77 CrossRef CAS.
- H. Nakamura, N. Ueda, H. S. Ban, M. Ueno and S. Tachikawa, Org. Biomol. Chem., 2012, 10, 1374 CAS.
- V. Bregadze, A. Semioshkin and I. Sivaev, Appl. Radiat. Isot., 2011, 69, 1774 CrossRef CAS PubMed.
- A. E. O'Connor, W. M. Gallagher and A. T. Byrne, Photochem. Photobiol., 2009, 85, 1053 CrossRef CAS PubMed.
- M. A. Grin, R. A. Titeev, D. I. Brittal, O. V. Ulybina, A. G. Tsiprovskiy, M. Ya. Berzina, I. A. Lobanova, I. B. Sivaev, V. I. Bregadze and A. F. Mironov, Mendeleev Commun., 2011, 21, 84 CrossRef CAS PubMed.
- A. V. Efremenko, A. A. Ignatova, A. A. Borsheva, M. A. Grin, V. I. Bregadze, I. B. Sivaev, A. F. Mironov and A. V. Feofanov, Photochem. Photobiol. Sci., 2012, 11, 645 CAS.
- A. Blum and L. I. Grossweiner, Photochem. Photobiol., 1985, 41, 27 CrossRef CAS.
- E. Gandin, Y. Lion and A. Van de Vorst, Photochem. Photobiol., 1983, 37, 271 CrossRef CAS.
- A. Feofanov, A. Grichine, T. Karmakova, A. Pljutinskaya, V. Lebedeva, A. Filyasova, R. Yakubovskaya, A. Mironov, M. Egret-Charlier and P. Vigny, Photochem. Photobiol., 2002, 74, 633 CrossRef.
- W. Bors, M. Saran, E. Lengfelder, C. Michel, C. Fuchs and C. Frenzel, Photochem. Photobiol., 1978, 28, 629 CrossRef CAS.
- A. A. Ignatova, A. S. Maslova, M. P. Kirpichnikov and A. V. Feofanov, Russ. J. Bioorg. Chem., 2009, 35, 746–751 CrossRef CAS.
- A. Feofanov, S. Charonov, F. Fleury, I. Kudelina and I. Nabiev, Biophys. J., 1997, 73, 3328 CrossRef CAS.
- A. Grichine, A. Feofanov, T. Karmakova, N. Kazachkina, E. Pecherskih, R. Yakubovskaya, A. Mironov, M. Egret-Charlier and P. Vigny, Photochem. Photobiol., 2001, 73, 267 CrossRef CAS.
- A. V. Feofanov, A. I. Nazarova, T. A. Karmakova, A. D. Pliutinskaia, A. I. Grishin, R. I. Yakubovskaya, V. S. Lebedeva, R. D. Ruziev, A. F. Mironov, J. C. Maurizot and P. Vigny, Russ. J. Bioorg. Chem., 2004, 30, 374 CrossRef CAS.
- A. V. Efremenko, A. A. Ignatova, M. A. Grin, A. F. Mironov, V. I. Bregadze, I. B. Sivaev and A. V. Feofanov, Confocal microscopy and spectral imaging technique: contribution to the development of neutron sensitizers for anticancer BNCT, in Current microscopy contributions to advances in science and technology, ed. A. Méndez-Vilas, Formatex Research Center, Badajoz, 2012, pp. 84–90 Search PubMed.
- A. Feofanov, G. Sharonov, A. Grichine, T. Karmakova, A. Pljutinskaya, V. Lebedeva, R. Ruziyev, R. Yakubovskaya, A. Mironov, M. Refregier, J.-C. Maurizot and P. Vigny, Photochem. Photobiol., 2004, 79, 172 CrossRef CAS.
- M. Martin-Facklam, J. Burhenne, R. Ding, R. Fricker, G. Mikus, I. Walter-Sack and W. E. Haefeli, Br. J. Clin. Pharmacol., 2002, 53, 576 CrossRef CAS.
- A. Nazarova, A. Ignatova, A. Feofanov, T. Karmakova, A. Pljutinskaya, O. Mass, M. Grin, R. Yakubovskaya, A. Mironov and J.-C. Maurizot, Photochem. Photobiol. Sci., 2007, 6, 1184 CAS.
- E. S. Nyman and P. H. Hynninen, J. Photochem. Photobiol., 2004, 73, 1 CrossRef CAS PubMed.
- The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, ed. I. D. Johnson and M. T. Z. Spence, Life Technologies Corporation, 11th edn, 2010, ch. 12, pp. 495–524 Search PubMed.
- V. I. Bregadze, A. A. Semioshkin, J. N. Las'kova, M. Ya. Berzina, I. A. Lobanova, I. B. Sivaev, M. A. Grin, R. A. Titeev, D. I. Brittal, O. V. Ulybina, A. V. Chestnova, A. A. Ignatova, A. V. Feofanov and A. F. Mironov, Appl. Organomet. Chem., 2009, 23, 370 CrossRef CAS.
- M. A. Grin, R. A. Titeev, D. I. Brittal, A. V. Chestnova, A. V. Feofanov, I. A. Lobanova, I. B. Sivaev, V. I. Bregadze and A. F. Mironov, Russ. Chem. Bull., 2010, 59, 219 CrossRef CAS PubMed.
- G. J. Zhang and J. Yao, Biochim. Biophys. Acta, 1997, 1326, 75 CrossRef CAS.
- E. V. Filonenko, V. V. Sokolov, V. I. Chissov, E. A. Lukyanets and G. N. Vorozhtsov, Photodiagn. Photodyn. Ther., 2008, 5, 187 CrossRef PubMed.
- E. V. Kochneva, E. V. Filonenko, E. G. Vakulovskaya, E. G. Scherbakova, O. V. Seliverstov, N. A. Markichev and A. V. Reshetnickov, Photodiagn. Photodyn. Ther., 2010, 7, 258 CrossRef CAS PubMed.
- T. Karmakova, A. Feofanov, A. Pankratov, N. Kazachkina, A. Nazarova, R. Yakubovskaya, V. Lebedeva, R. Ruziyev, A. Mironov, J. C. Maurizot and P. Vigny, J. Photochem. Photobiol., 2006, 82, 28 CrossRef CAS PubMed.
- S. Kawabata, W. Yang, R. F. Barth, G. Wu, T. Huo, P. J. Binns, K. J. Riley, O. Ongayi, V. Gottumukkala and M. G. Vicente, J. Neuro-Oncol., 2011, 103, 175 CrossRef PubMed.
- M. I. Kurzhupov, V. A. Loshakov, E. V. Filonenko, A. M. Zaitsev and A. G. Khanmurzaeva, Voprosy Neirokhirurgii imeni N.N. Burdenko, 2012, 762, 50 Search PubMed.
- K. Roessler, A. Becherer, M. Donat, M. Cejna and I. Zachenhofer, Neurol. Res., 2012, 34, 314 CAS.
- H. Kostron, Methods Mol. Biol., 2010, 635, 261 CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2014 |