Correlating elastic properties and molecular organization of an ionic organic nanostructure†
Jeremy R.
Eskelsen
a,
Yun
Qi
b,
Samantha
Schneider-Pollack
c,
Samantha
Schmitt
d,
K. W.
Hipps
*a and
Ursula
Mazur
*a
aDepartment of Chemistry and Materials Science and Engineering Program, Washington State University, Pullman, WA 99164-4630, USA. E-mail: umazur@wsu.edu; hipps@wsu.edu; Fax: +1-509-335-8867; Tel: +1-509-335-5822 Tel: +1-509-335-3033
bCharles Evans & Associates, Sunnyvale, CA 9408, USA
cAlfred University, Alfred, NY 1480, USA
dDepartment of Material Science and Engineering, University of Wisconsin, Madison, WI 5370, USA
First published on 24th October 2013
Abstract
Mechanical and structural properties of ionically self-assembled nanostructures of meso-tetra(4-sulfonatophenyl)porphyrin (TSPP) and meso-tetra(4-pyridyl)porphyrin (TPyP) are presented. This is the first time that elastic modulus of an ionic porphyrin nanostructure has been reported. X-ray photoelectron spectroscopy (XPS), UV-visible spectra, and elemental analysis all support a stoichiometric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TSPP to TPyP composition. Atomic force microscopy (AFM) revealed that the porphyrin nanostructure is composed of stacked ribbons about 20 nm tall, 70 nm wide, and several microns in length. High resolution transmission electron microscopy (HRTEM) images showed clear lattice fringes 1.5 ± 0.2 nm in width aligned along the length of the nanorod. Selected area electron diffraction (SAED) and powder X-ray diffraction patterns of TSPP:TPyP are consistent with an orthorhombic system and space group Imm2 with lattice parameters a = 26.71 Å, b = 20.16 Å, and c = 8.61 Å. Crystallographic data is consistent with an arrangement of alternating face-to-face TSPP and TPyP molecules forming ordered columns along the length of the nanorods. The structural integrity of the solid is attributed to combined noncovalent interactions that include ionic, hydrogen bonding, and π–π interactions. The values of Young's modulus obtained for the crystalline TSPP:TPyP nanorods averaged 6.5 ± 1.3 GPa. This modulus is comparable to those reported for covalently bonded flexible polymeric systems. The robust bonding character of the TSPP:TPyP nanostructures combined with their mechanical properties makes them excellent candidates for flexible optoelectronic devices.
1 TSPP to TPyP composition. Atomic force microscopy (AFM) revealed that the porphyrin nanostructure is composed of stacked ribbons about 20 nm tall, 70 nm wide, and several microns in length. High resolution transmission electron microscopy (HRTEM) images showed clear lattice fringes 1.5 ± 0.2 nm in width aligned along the length of the nanorod. Selected area electron diffraction (SAED) and powder X-ray diffraction patterns of TSPP:TPyP are consistent with an orthorhombic system and space group Imm2 with lattice parameters a = 26.71 Å, b = 20.16 Å, and c = 8.61 Å. Crystallographic data is consistent with an arrangement of alternating face-to-face TSPP and TPyP molecules forming ordered columns along the length of the nanorods. The structural integrity of the solid is attributed to combined noncovalent interactions that include ionic, hydrogen bonding, and π–π interactions. The values of Young's modulus obtained for the crystalline TSPP:TPyP nanorods averaged 6.5 ± 1.3 GPa. This modulus is comparable to those reported for covalently bonded flexible polymeric systems. The robust bonding character of the TSPP:TPyP nanostructures combined with their mechanical properties makes them excellent candidates for flexible optoelectronic devices.
Introduction
One of the most important advantages of organic molecular semiconductors over their inorganic equivalents is that their electronic, mechanical, and optical properties can be chemically tuned by molecular design. This advantage can be further enhanced by arranging the individual photo- and electro-active molecules into organized assemblies that possess charge carrier properties superior to those of their corresponding bulk forms.1,2 The molecular interactions that mediate the optoelectronic properties of organic nanomaterials are also known to have pronounced effects on their mechanical properties such as elastic modulus, hardness, and bending strength.3–5 For high performance practical applications of organic semiconducting nanostructures (light-emitting diodes,6 field-effect transistors,7 photoswitches,1 sensors,8,9 solar cells,10,11 and memory devices12) fast and efficient carrier mobility needs to be coupled with low internal stress and superior tensile characteristics.13 For example, both increased elastic modulus and conductivity were observed for polypyrrole nanotubes with improved longitudinal alignment of the polymer chains via π–π interactions.5 Metal-like stiffness and transparent optical properties were reported for nanospheres formed from aromatic dipeptides.14 The rigid geometry of the nanospheres was constrained by efficient aromatic interactions and a network of hydrogen bonded carboxylate groups. Accordingly, good fundamental understanding of structure–property relationships is crucial in designing and building high performance, stable, and durable molecular devices and remains one of the key scientific challenges for advancing organic optoelectronic technology.In this report, we present a detailed structural study and elastic properties of a porphyrin based nanostructure with focus on the structure–property relationship of this system. Synthetic porphyrins are an important class of organic semiconductors that structurally and functionally resemble natural light harvesting chromophores and they are promising building blocks for organic electronics,15 photovoltaics,16,17 sensors,8,18,19 and catalysts.20 Porphyrin nanostructures may be prepared by a variety of methods including ionic self-assembly,21,22 phase-transfer ionic self-assembly,23 surfactant-assisted self-assembly,24 and vapour condensation recrystallization.25 Of particular interest to us are the porphyrin nanostructures created by ionic self-assembly, a rather simple solution-based synthetic method that utilizes a combination of structurally different ionic species, or a single zwitterionic species.26–31 Nanostructures composed from oppositely charged porphyrin ions (also called cooperative binary ionic, CBI, solids) are particularly appealing because they present a novel class of robust nanomaterials that have been shown to potentially serve as efficient light-harvesting components of dye-sensitized solar cells and organic photovoltaics.26,32 Favourable optoelectronic properties of some of the reported CBI solids may be attributed, in part, to their reported crystalline character.26,32 It is well known that organic molecular crystals exhibit higher charge mobility than amorphous and polycrystalline films and are therefore better candidates for the fabrication of high-performance electronic and optical devices.2,4,33 For example, copper phthalocyanine (CuPc) crystalline (β-phase) nanowires have higher charge mobility than films fabricated from the same compound.4 Nanometer size crystals, like the CBI systems, are especially attractive because they may possess fewer defects and grain boundaries that can act as energetic barriers for charge transport. In addition, the reduced dimensions of nanocrystals may impart better flexibility, a highly desirable mechanical property for high-performance flexible molecular optoelectronic devices.34,35 To date there has been little reported on the mechanical properties of porphyrin materials and, to our knowledge, no mechanical measurements available for ionic porphyrin nanostructures. Similarly, there is a scarcity of detailed molecular and submolecular structural data on the CBI systems.36,37 The main reason for the lack of structural data is because the size of the CBI crystals that can be isolated are at best suitable for powder and not single crystal X-ray diffraction. To date only one crystal structure of a single CBI solid prepared from zinc(II)-tetra(4-sulfonatophenyl)porphyrin (ZnTPPS) and tin(IV)-tetra(N-methyl-4-pyridiniumyl)porphyrin (SnTNMePyP) has been reported.32
The subject of this report is a binary system formed from metal free porphyrin tectons namely, meso-tetra(4-pyridyl)porphyrin, TPyP, and meso-tetra(4-sulfonatophenyl)porphyrin, TSPP, Fig. 1. It is important to note that solution pH plays a significant role in determining the numbers of protons (and therefore the charge) of these tectons. We will use the TSPP and TPyP notation for brevity, but will also identify the actual protonation state when needed. The TSPP:TPyP CBI material was prepared earlier but its stoichiometric and structural details are unknown.26,38 A combination of spectroscopic and microscopic analysis here furnishes a detailed molecular level model of how the TSPP and TPyP ionic tectons combine in the CBI nanostructure. X-ray photoelectron spectroscopy (XPS), electronic spectroscopy, atomic force microscopy (AFM), high resolution transmission electron microscopy (HRTEM), selected area electron diffraction (SAED), powder X-ray diffraction (XRD), and DFT calculations helped us to model the structure and organization of molecules within the nanostructured aggregates. XPS was particularly useful in identifying the protonation (via shifts in the N 1s peak) states of the TSPP and TPyP tectons, their stoichiometry in the ionically coupled solid, and the elemental composition of the porphyrin nanocomposite. Morphology and dimensions of the rod-like structures were acquired from their AFM images. HRTEM, SAED, and X-ray powder patterns were used to establish the crystallinity of the TSPP:TPyP nanorods and molecular orientation within nanostructured aggregates. The collective application of spectroscopy and microscopy furnished a detailed molecular level picture of how the TSPP and TPyP ionic tectons combine in the CBI nanostructure.
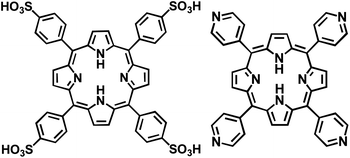 | ||
| Fig. 1 Free-base forms of meso-tetra(4-sulfonatophenyl)porphyrin, TSPP, left, and of meso-tetra(4-pyridyl)porphyrin, TPyP, right. | ||
Young's modulus (E) measurements of the TSPP:TPyP nanostructures were made using an AFM by applying a force to the nanorods while measuring the corresponding indentation. Both highly ordered pyrolytic graphite (HOPG) and mica substrates were used to test if the elastic modulus values of the nanostructures were affected by the underlying substrate. E showed no substrate dependence at the depth of indentation studied and no degradation of the porphyrin nanorods was observed after repeated deformation. The values of the Young's modulus obtained for the crystalline ionic TSPP:TPyP nanorods were comparable to those of covalently bonded polymeric systems but are an order of magnitude smaller than that of inorganic nanowires39 making them excellent candidates for flexible optoelectronic devices.
Experimental section
TSPP:TPyP nanostructure synthesis
The procedure for the preparation and isolation of the solid nanomaterial is described in the ESI.† Therein also are the solution electronic spectra of the TSPP:TPyP aggregates.Atomic force microscopy
AFM images were taken, using a Digital Instruments Multimode AFM in tapping mode. Silicon cantilevers with a driving frequency around 300 MHz and a force constant of 42 N m−1 were used in the measurement of the AFM images. Substrates for imaging were highly oriented pyrolytic graphite (HOPG) and mica purchased from SPI Supplies Inc. AFM samples were prepared by depositing 1–2 drops by Pasteur pipette of the TSPP/TPyP aggregate solution onto freshly peeled mica or HOPG for 1 minute followed by a 30 seconds spin at 3900 rpm. This process was repeated 10 times.Helium ion microscopy
Samples for HIM were prepared in the same fashion as for the AFM and were checked by AFM prior to imaging by HIM. The microscope used was an ORION® PLUS manufactured by Carl Zeiss located at Pacific Northwest National Lab in Richland, WA. The ORION® He ion microscope is capable of imaging with two different detectors, an Everhart–Thornley and a Rutherford backscattering detector. The Everhart–Thornley detector measures secondary electrons ejected from the sample by the incident He ions, while the Rutherford backscattering detector measures He ions scattered by the sample nuclei. Both modes were used to analyze the nanorods.TEM, HRTEM, and SAED
For transmission electron microscopy and high resolution imaging, nanorods were deposited onto Ni Formvar TEM grids for 1 min followed by wicking using the edge of a paper filter. All images were acquired using a Philips CM200 TEM at an acceleration voltage of 200 keV and outfitted with a controlled eucentric sample holder capable of a tilt from −45° to +45° along the A axis and −30° to +30° along the B axis. The SAED of the nanorods was also determined using the CM200 with line resolution of 0.19 nm with the same holder.XRD
X-ray powder diffraction data was gathered using a Rigaku MiniFlex 600 by Lori Fields Hatherley at Rigaku Americas Corporation. The sample was placed in a 0.2 mm deep, zero background holder. Copper Kα X-rays at 40 kV and 15 mA emission, with a scintillation detector and graphite monochromator were used for the analysis. A θ/2θ scan was ran from 2° to 60° with a step size of 0.02° and a 15 s per pt dwell time. The total time for analysis was 12.1 hours.Le Bail refinement and modelling
The CMPR program was used to obtain a starting space group and lattice dimensions.40 The lattice constants were then refined via a Le Bail fit41 in the program GSAS interfaced by EXPGUI.42,43 Peak profiles were modelled with a pseudo-Voigt peak shape44 with the Finger, Cox, Jephcoat asymmetry function45 to deal with the low-angle data. An 8-term shifted Chebyschev polynomial was used to model the amorphous background. S/L and H/L values were set at 0.005. The parameters refined were the lattice parameters and the peak shape parameters u, v, w, and x. The u, v, and w parameters were refined separately to prevent refining out of bounds. The constraints are u and w > 0 and v < 0. The total number of parameters refined was 12, not including the background. We modelled the crystal structure with the refined lattice constants and intermolecular distances for the isolated ions from DFT energy minimized structures. CrystalMaker®: a crystal and molecular structures program for Mac and Windows was used.46 This model is based on our XPS findings of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TSPP
1 TSPP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPyP ratio. Our model correctly reproduces all the data, but it is not a unique solution.
TPyP ratio. Our model correctly reproduces all the data, but it is not a unique solution.
Calculations and modelling
Structural calculations and geometry optimizations of the ionized species were performed using the commercial program Gaussian 03. All reported results are based on DFT calculations using the B3LYP functional and the 6-311G(d,p) basis (see ESI for results†).Mechanical measurements
Young's modulus measurements were made using a Bruker Multimode 8 AFM with Nanoscope V controller and a J “vertical” scanner and a lateral force Digital Instruments AFM head. Bruker's Nanoscope 8.15 software was used for the acquisition of data. Two different antimony doped silicon cantilevers with length, resonance frequency, and spring constant values of 116.3 μm, 131.8, kHz, 2.91 N m−1 and 116.7 μm, 137.9 kHz, 3.65 N m−1, respectively, were used for acquiring force–distance curves. Experimental details of the modulus data acquisition and analysis in given in the ESI.†TSPP:TPyP nanorod solutions were prepared at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 μM porphyrin concentration at pH 2 as described in the ESI.† The AFM samples and substrates were prepared by taping a piece of mica or HOPG to a round AFM puck using double stick tape. A fresh surface of mica or HOPG was exposed by pealing the top layer using adhesive tape. Using a Pasteur pipette, 1–2 drops of the nanorod solution (stirred) was applied to the freshly peeled surface and allowed to remain on the surface for 1 minute followed by spinning at 3800 rpm for 30 seconds. The deposition and spinning was repeated for 5–10 times depending on the substrate and the desired surface coverage. The nanorod surface coverage was inspected under an optical microscope prior to scanning.
15 μM porphyrin concentration at pH 2 as described in the ESI.† The AFM samples and substrates were prepared by taping a piece of mica or HOPG to a round AFM puck using double stick tape. A fresh surface of mica or HOPG was exposed by pealing the top layer using adhesive tape. Using a Pasteur pipette, 1–2 drops of the nanorod solution (stirred) was applied to the freshly peeled surface and allowed to remain on the surface for 1 minute followed by spinning at 3800 rpm for 30 seconds. The deposition and spinning was repeated for 5–10 times depending on the substrate and the desired surface coverage. The nanorod surface coverage was inspected under an optical microscope prior to scanning.
Results and discussion
Structural studies
Optical absorption studies show that the TSPP:TPyP solid nanostructures were prepared reproducibly in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio in a pH 2 aqueous HCl solution (described in the ESI†). At this hydrogen ion solution concentration, the TSPP exists in a diacid form where the inner nitrogen system is protonated and all four sulfonate groups are ionized, to give the porphyrin a net −2 charge, [H4TSPP]2−.47 The imino nitrogens of the TPyP core have a pKa value of 1.1.48 Thus, at pH 2 the TPyP remains a free base. The pyridyl nitrogens in TPyP are commonly believed to be protonated near pH 2.3 based on the measured pKa value of 5.25 for pyridine in aqueous solution,48 (to our knowledge, no one has ever directly measured the pKa of the pyridyl groups in the TPyP itself). Also, the pyridyl groups of the tin complex of TPyP were reported to protonate at pH 2.21 The preceding information suggests that the TPyP pyridyls must have a pKa greater than 2 but less than 5. One would expect, therefore, a significant concentration of TPyP ions with two or more protonated pyridyl groups namely H2[H2TPyP]2+ and H4[H2TPyP]4+, to be present at pH near 2.
1 stoichiometric ratio in a pH 2 aqueous HCl solution (described in the ESI†). At this hydrogen ion solution concentration, the TSPP exists in a diacid form where the inner nitrogen system is protonated and all four sulfonate groups are ionized, to give the porphyrin a net −2 charge, [H4TSPP]2−.47 The imino nitrogens of the TPyP core have a pKa value of 1.1.48 Thus, at pH 2 the TPyP remains a free base. The pyridyl nitrogens in TPyP are commonly believed to be protonated near pH 2.3 based on the measured pKa value of 5.25 for pyridine in aqueous solution,48 (to our knowledge, no one has ever directly measured the pKa of the pyridyl groups in the TPyP itself). Also, the pyridyl groups of the tin complex of TPyP were reported to protonate at pH 2.21 The preceding information suggests that the TPyP pyridyls must have a pKa greater than 2 but less than 5. One would expect, therefore, a significant concentration of TPyP ions with two or more protonated pyridyl groups namely H2[H2TPyP]2+ and H4[H2TPyP]4+, to be present at pH near 2.
Elemental analysis results summarized in Table 1 readily support the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition ratio of TSPP to TPyP found by UV-vis analysis. The expected and experimental proportions of nitrogen to sulphur are exactly 3
1 composition ratio of TSPP to TPyP found by UV-vis analysis. The expected and experimental proportions of nitrogen to sulphur are exactly 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The measured C
1. The measured C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio is slightly greater then the calculated value but could be due to trace carbon contamination. We did observe variable amounts of residual chlorine in our unheated samples but this element was easily removed by heating the solid composite to above 100 °C as shown in our XPS results that follow.
N ratio is slightly greater then the calculated value but could be due to trace carbon contamination. We did observe variable amounts of residual chlorine in our unheated samples but this element was easily removed by heating the solid composite to above 100 °C as shown in our XPS results that follow.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TSPP
1 TSPP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPyP nanorod stoichiometry obtained from XPS data compared with expected values and elemental analysis results
TPyP nanorod stoichiometry obtained from XPS data compared with expected values and elemental analysis results
In order to verify the stoichiometry of the TSPP:TPyP obtained from above experiments, we performed XPS studies on the nanostructures at room temperature and after heating to 150 °C. Firstly, the nominal amounts of chlorine XPS signal detected in the CBI samples at room temperature were reduced to a negligible level upon heating the sample (see Table 1 and ESI†) indicating that the anion is not an essential constituent of the nanostructures. There was also little evidence of chloride anions found in the EDX spectra of a related CBI nanostructure composed of [H4TPPS]2− and a tin substituted tetrapyridyl porphyrin, H4[SnTPyP]4+, synthons.21 The measured N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S and C
S and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N atomic ratios presented in Table 1 agree very well with the calculated atomic ratios consistent with 1
N atomic ratios presented in Table 1 agree very well with the calculated atomic ratios consistent with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 tecton stoichiometry. The excellent agreement between the experimental and calculated N
1 tecton stoichiometry. The excellent agreement between the experimental and calculated N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio gives us a great deal of confidence in making this stoichiometric assignment. The slight excess of carbon in the measured C
S ratio gives us a great deal of confidence in making this stoichiometric assignment. The slight excess of carbon in the measured C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratios in both heated and unheated samples (Table 1) is not surprising, as some background carbon is always present on the indium used to support the compound for XPS analysis. XPS spectra of other pertinent atomic species (Cl and O) present in the tectons are reported in the ESI.† Based on the O 1s XPS, we believe there are 4 waters present per TSPP:TPyP unit in the nanorod annealed to 150 °C.
N ratios in both heated and unheated samples (Table 1) is not surprising, as some background carbon is always present on the indium used to support the compound for XPS analysis. XPS spectra of other pertinent atomic species (Cl and O) present in the tectons are reported in the ESI.† Based on the O 1s XPS, we believe there are 4 waters present per TSPP:TPyP unit in the nanorod annealed to 150 °C.
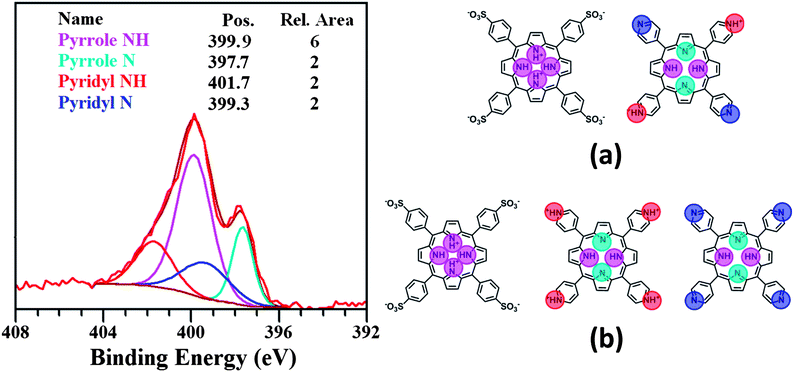
In addition to assaying elemental content, XPS was also used to evaluate and verify the state of protonation of the inner rings of the tectons in the nanostructures, as shown in Fig. 2. For the TSPP diacid only a single N 1s peak is expected at 400 eV, signalling that all four nitrogen atoms of the porphyrin are protonated.31 The neutral unprotonated H2[TPyP] should have three types of nitrogen atoms, two unprotonated and two protonated pyrrole N's and four nitrogen atoms in the pyridine rings bound to the porphyrin meso carbons. The reported nitrogen spectrum for this porphyrin vapour deposited on Au(111) showed three overlapping bands.49,50 This signal was deconvoluted into bands with area ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, one located at 399.4 eV, corresponding to the two protonated pyrrole nitrogens, the second one at 398.3 eV corresponding to the four pyridyl nitrogens, and the third one at 397.4 eV corresponding to the two unprotonated pyrrole ring nitrogens.49,50
2, one located at 399.4 eV, corresponding to the two protonated pyrrole nitrogens, the second one at 398.3 eV corresponding to the four pyridyl nitrogens, and the third one at 397.4 eV corresponding to the two unprotonated pyrrole ring nitrogens.49,50
The highest BE signal observed in Fig. 2 is an obvious shoulder at 401.7 eV. This N 1s peak position is typical for pyridinium cation nitrogen.51 We attribute the strong band near 400 eV to the protonated pyrrole nitrogens of the TSPP diacid and the TPyP free base. Also contributing to that signal is the N 1s binding energy of the unprotonated pyridyl nitrogens of the TPyP. The 397.7 eV peak is assigned to the unprotonated pyrrole nitrogens in TPyP since the TSPP diacid has all four ring N atoms associated with H atoms. Taking into account the atomic ratio data in Table 1 and guided by previous deconvolution results for the N 1s band for neutral TPyP, we have fitted the spectrum in Fig. 2 to four bands associated with four different nitrogens. These bands are linked with the signals from six protonated and two unprotonated pyrrole nitrogens and two protonated and two unprotonated pyridyl nitrogens with relative areas of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
Our spectroscopic and elemental analysis data clearly supports 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of TSPP to TPyP in the nanostructure. Since no other ionic species were detected besides a trace of chlorine, and because the relative amounts of the other elements were not significantly altered with heating, we must conclude that the formation of the charge neutral solid can only result from the combination of one [H4TSPP]2− and one H2[H2TPyP]2+ ion, or from two[H4TSPP]2− with one each H4[H2TPyP]4+ and H2TPyP. Both tecton combinations preserve the charge neutrality and stoichiometry in the solid state. In a pH 2 solution it is less clear which ionic species combinations predominate.
1 stoichiometry of TSPP to TPyP in the nanostructure. Since no other ionic species were detected besides a trace of chlorine, and because the relative amounts of the other elements were not significantly altered with heating, we must conclude that the formation of the charge neutral solid can only result from the combination of one [H4TSPP]2− and one H2[H2TPyP]2+ ion, or from two[H4TSPP]2− with one each H4[H2TPyP]4+ and H2TPyP. Both tecton combinations preserve the charge neutrality and stoichiometry in the solid state. In a pH 2 solution it is less clear which ionic species combinations predominate.
Interestingly, when [H4TPPS]2− and H4[SnTPyP]4+ were combined at pH 2 in HCl solution the ratio of the tectons was charge consistent with 2.0–2.5 TSPP to 1 SnTPyP as determined by EDX and optical spectroscopy.21 Clearly, incorporation of a metal into the ring of one of the tectons appears to affect charge stabilization on the porphyrins, which in turn affects the stoichiometry of the ionic coupling.
Morphology images from TEM, HIM, and AFM
Previous morphology studies of the TSPP:TPyP aggregates prepared in pH 2 solution reported inconsistent results. One study concluded that TSPP in combination with TPyP forms rods that have a rectangular cross-section and vary considerably in length.26 Another article claimed that the two tectons formed nanosheets and nanotubes and that the nanotubes resulted from wrapping of the nanosheets.38In Fig. 3 the TEM and HIM images of the TSPP:TPyP system deposited on HOPG reveal rectangular rod-like structures (not collapsed tubes). HIM is a new imaging methodology and we were pleased to discover that the porphyrin nanostructures behaved quite robustly in the ion beam and did not disintegrate even after long exposure times. The edges of the rectangular rods are prominent in the HIM image and corroborate the vertical edges deduced from the TEM results. Our TEM imaging data collected from multiple TSPP:TPyP preparations are consistent with Shelnutt's TEM measurements of the same CBI system.26 Snitka and coworkers, however, reported that the TSPP:TPyP ionic aggregate formed porphyrin nanosheets with high aspect ratios and multiwall nanotubes, based on their AFM, SEM, and TEM studies.38 We never observed any of these structures in our TSPP:TPyP samples.
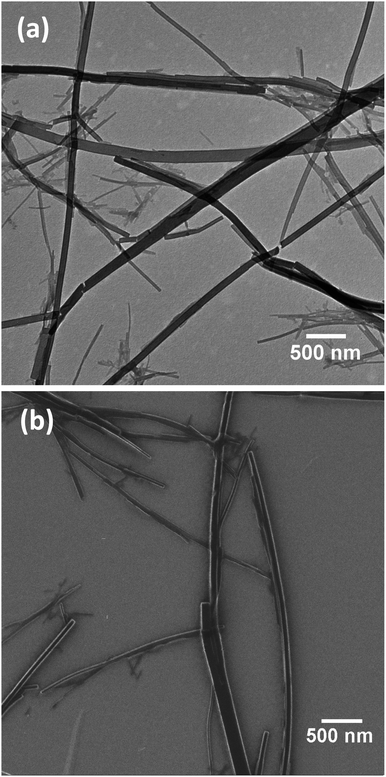 | ||
| Fig. 3 Low resolution 5 μm × 5 μm images of TSPP:TPyP nanorods deposited on HOPG acquired by TEM (a) and HIM (b). | ||
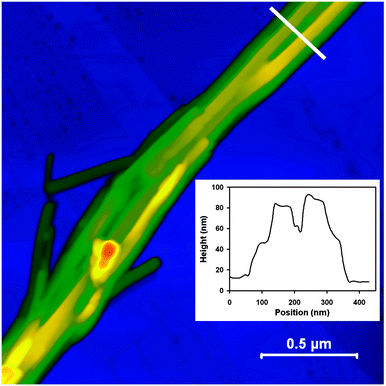
While the TEM and HIM images yield good information concerning widths and the lengths of the TSPP/TPyPP nanostructures they provide no height values. Our AFM images provide the first direct evidence that these rods are really composed of overlaid thin strips about 70 nm wide and 20 nm high (Fig. 4). Images acquired from different areas of the same sample, and from different samples, yielded the same nanorod dimensions. The rods are indeed rectangular in shape and have relatively smooth surfaces (see inset in Fig. 4). Rods tend to coalesce into tall stacks tens of nanometers high in the direction of their long axis. Their large size rendered the nanorod stacks unsuitable for imaging by scanning tunnelling microscopy, STM, the technique of choice for probing nano and subnanoscale dimensions. HRTEM, SAED, and powder diffraction patterns, however, allowed us to infer a molecular arrangement within the TSPP:TPyP nanostructures that is consistent with all the available data.
Nanorod internal structure based on HRTEM, SAED, and powder X-ray diffraction
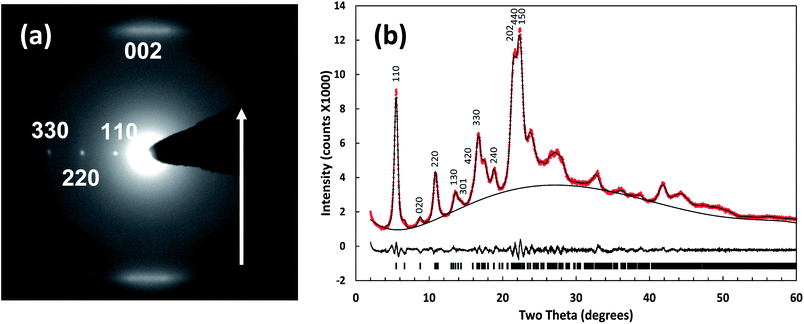
HRTEM of the porphyrin nanostructures showed clear fringes indicating that the rods are crystalline as shown in the ESI.† The spacing between the lines, 1.5 ± 0.2 nm, is consistent with the distance between two porphyrins positioned side by side with rings in perpendicular orientation relative to the substrate. It is important to note that the morphological and crystallographic properties of the TSPP:TPyP solid were not altered by heating (see ESI†).The SAED images of the nanorods show a clear crystalline pattern with lattice spacings: d = 1.5 ± 0.2 nm across the width of the rod and d = 0.44 ± 0.08 nm along the length of the rod (Fig. 5a). These spacings are consistent with columns of face-to-face stacked porphyrins aligned parallel to the long axis of the rod (c direction) with the macrocycles lying perpendicular to the substrate surface. The diffuse spots in the SAED scattering can be associated with a small degree of structural disorder in the crystalline nanorods. This disorder may be caused by amorphization of nanomaterial upon exposure to the electron beam, or, more likely by random displacement of the coherent columns of porphyrins relative to each other (in the z-direction) within the nanostructure. Borras et al. also observed an unperturbed zeroth order diffraction line and diffuse higher order lines in the SAED pattern of vapour deposited crystalline copper phthalocyanine nanowires.52 They attributed it to random offset in the adjacent phthalocyanine columns in the z-direction (long column axis). Pershan in his review of X-ray scattering from mesomorphic systems noted that X-ray scattering cross sections from disc-shaped molecules with either short or long range ordering usually exhibits a diffuse spot.53 Thus far, we have been unsuccessful in preparing crystals large enough to obtain a single-crystal X-ray diffraction pattern. We have, however, obtained reproducible powder patterns of several TSPP:TPyP samples, one of which is depicted in Fig. 5b. Table 2 compares the lattice spacings for TSPP:TPyP nanorods obtained from XRPD and SAED. The accuracy of the TEM in general is about 10% to 15%.54 The accuracy of the powder XRD is not as high as usually expected due to the broadness of the peaks due to the nanocrystalline size and the number of overlapping peaks due to the large lattice spacings. We estimate an error of about 1.5% from multiple attempts at refining the structure. The diffraction pattern is consistent with an orthorhombic crystal system. We have focused on the orthorhombic system because related porphyrin systems such as zinc-tetrakis(4-methoxycarbonylphenyl)porphyrin–pyridine complex55 and a meso-tetra-(N-methy-4-pyridyl)porphyrin tetratosylate and zinc-tetrakis(4-sufonatolphenyl)porphyrin composite37 were reported to crystallize into an orthorhombic structure. Using a trial space group of Imm2, we obtained lattice parameters a = 26.79 Å, b = 20.00 Å, and c = 8.42 Å using CMPR.40 The lattice parameters were refined using a Le Bail fit41 with a reduced X2 = 2.423 and Rp = 0.0200, see Fig. 5b. The refined lattice constants are a = 26.71 Å, b = 20.16 Å, and c = 8.61 Å.
| Miller indices | XRPD reflection distances (Å) | SAED reflection distances (Å) |
|---|---|---|
| 110 | 16.11 ± 0.24 | 15.3 ± 1.5 |
| 220 | 8.06 ± 0.12 | 7.7 ± 0.8 |
| 330 | 5.37 ± 0.08 | 5.2 ± 0.5 |
| 440 | 4.03 ± 0.06 | 3.8 ± 0.4 |
| 002 | 4.30 ± 0.06 | 4.5 ± 0.5 |
| 004 | 2.15 ± 0.03 | 2.2 ± 0.2 |
| 130 | 6.53 ± 0.10 | 6.9 ± 0.7 |
| 020 | 10.10 ± 0.15 | 10.8 ± 1.1 |
| 040 | 5.05 ± 0.08 | 5.5 ± 0.6 |
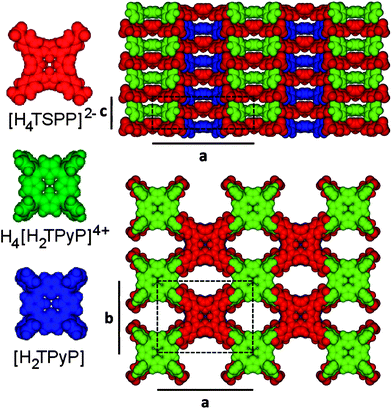
Using DFT calculated structures of the individual TSPP and TPyP molecules we built an initial model using the refined Imm2 space group. However, the symmetry elements of that group generated a molecular arrangement that was inconsistent with our XPS stoichiometric results. Because a 2[H4TSPP]2−:1H4[H2TPyP]4+:1[H2TPyP] combination is also plausible based on our spectroscopic data we selected a lower symmetry space group, Pmm2, a subgroup of Imm2 to better represent the crystal structure of the nanorods. A structural model of the TSPP:TPyP system based on the Pmm2 group is depicted in Fig. 6. The molecules are arranged with one [H4TSPP]2− centered at (0, 0, 0), one [H4TSPP]2− centred at (1/2, 1/2, 1/2), one H4[H2TPyP]4+ centred at (0, 0, 1/2), and one [H2TPyP] centered at (1/2, 1/2, 0). Water molecules may be present in the nanorod matrix but, for simplicity, they were not included in our crystal structure model. Our XPS data does indicate that there are four water molecules associated with each TSPP:TPyP unit in the nanorod sample annealed to 150 °C. Unheated rod samples prepared in UHV contained six waters per porphyrin dimer (see ESI†). Thus, it is possible that there are water molecules trapped in the channels within the structure that are slowly or incompletely removed by evacuation. It also must be noted that in the absence of a single crystal diffraction study, a structure consistent with the SAED and powder diffraction is not necessarily the correct structure.
Comparison of SAED data with the powder diffraction supports the arrangement of alternating face-to-face TSPP and TPyP molecules forming columns along the length of the nanorods. Ionic bonding, hydrogen bonding, and π–π interaction each contribute to aligning the ionic tectons (the macrocycles and their ring substituents) in cofacially packed columns, giving the CBI solid a well-defined rod-like structure. Moreover, the HRTEM image of a TSPP/TPyP in Fig. 6a is also compatible with this columnar formation. The well defined striped structure along the nanorod axis is formed by porphyrin columns of an approximate width of 1.5 nm. This value is consistent with a close packed arrangement of the porphyrin tectons (CPK models) depicted in Fig. 6. The dimensions of the individual [H4TSPP]2−, [H2TPyP], and H4[H2TPyP]4+ ions are 1.56 nm, 1.20 nm, and 1.23 nm, respectively, based on their calculated equilibrium geometries (see ESI†). The [H4TSPP]2− diacid has 2-fold symmetry because of the distortion (or saddling) in the porphyrin ring caused by inner proton repulsion,26,29 while the H4[H2TPyP]4+ and [H2TPyP] free base species are not significantly distorted.
One would expect that in the saddled conformation, the [H4TSPP]2− macrocycle approaches the H4[H2TPyP]4+ tecton closer than if both were planar. In fact the separation between the two synthons is small, only 0.43 nm (for reference the porphyrin spacing in bacteriochlorophyll is 0.35 nm (ref. 56)). This is a much shorter distance than the value of 0.689 nm reported for the intermolecular separation of neutral free base tetrapyridyl porphyrins cofacially packed in a monoclinic single crystal.25 In a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composite of meso-tetra(N-methyl-4-pyridyl)porphyrin tetratosylate and ZnTSPP the spacing between the oppositely charged tectons in a face to face arrangement was 0.489 nm based on powder diffraction data.37 Because of the shorter distance and more ideal bonding angle, the out of plane acidic protons of the H2[H4TSPP]2− ring interact strongly with the pyrrolic nitrogens of the H4[H2TPyP]4+ tecton. The closer macrocycle proximity also facilitates stronger π–π interactions. The sulfonate groups as well as the pyridyl groups on the porphyrin rings can easily adjust themselves to give the maximum ionic and H-bonding.
1 composite of meso-tetra(N-methyl-4-pyridyl)porphyrin tetratosylate and ZnTSPP the spacing between the oppositely charged tectons in a face to face arrangement was 0.489 nm based on powder diffraction data.37 Because of the shorter distance and more ideal bonding angle, the out of plane acidic protons of the H2[H4TSPP]2− ring interact strongly with the pyrrolic nitrogens of the H4[H2TPyP]4+ tecton. The closer macrocycle proximity also facilitates stronger π–π interactions. The sulfonate groups as well as the pyridyl groups on the porphyrin rings can easily adjust themselves to give the maximum ionic and H-bonding.
Elastic modulus measurement
Accurate assessment of the mechanical properties of organic nanostructures such as TSPP:TPyP is complicated by their small physical dimensions and the atomic force microscope is the ideal tool for quantifying the deformation behaviour of small volume materials. We employed a Bruker Multimode 8 AFM capable of collecting PeakForce quantitative nanomechanical mapping (PF-QNM) images of surfaces. The elastic property fitting models such as the Derjaguin, Muller, Toporov (DMT)57 and the Sneddon58 models are compatible with data acquired with the Bruker Multimode AFM. The DMT model works well for indentation experiments where the tip radius is much greater than the indentation depth.59 For tip radii much smaller than the indentation depth, the Sneddon model is more appropriate.60 Typical AFM tip radii used in our experiments were approximately 20 nm. Sample indent depth was limited to 2 nm. Since the tip radius was much larger than the indent depth, the DMT equation was chosen for determining the Young's modulus. See ESI† for detailed experimental procedure of data acquisition and analysis.We used two different antimony doped silicon cantilevers with length, resonance frequency, and spring constant values of 116.3 μm, 131.8, kHz, 2.91 N m−1 and 116.7 μm, 137.9 kHz, 3.65 N m−1, respectively, for acquiring force–displacement curves. The deflection sensitivity of each cantilever was determined by taking single ramps on both sapphire and mica substrates. These substrates are hard materials with Young's modulus values of 441 GPa (ref. 61) and 137 GPa,62 respectively. The spring constants of the AFM cantilevers were determined using the Sader method.63 In agreement with the literature, the best force–displacement curve measurements were obtained with dulled tips.64,65
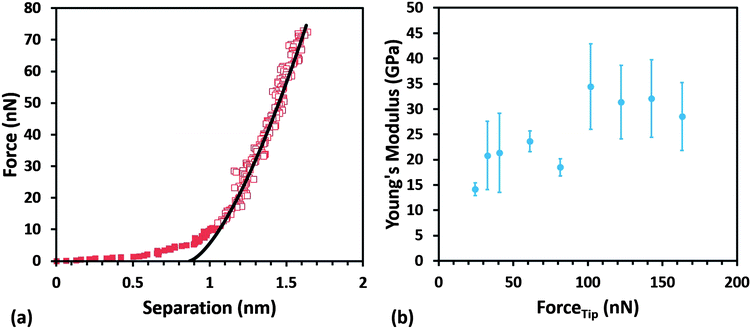
In order to test the accuracy of our experimental method and calculations employed, we first evaluated the Young's modulus of HOPG using different cantilevers and collecting several measurements with each. Fig. 7 depicts a representative force–displacement curve for HOPG; Young's modulus values of 23 ± 6 GPa and 25 ± 9 GPa were obtained using two different cantilevers. These values fit well into the range of 15–30 GPa reported in the literature for HOPG.66–69
TSPP:TPyP nanorod samples were deposited on both HOPG and freshly peeled mica. With the AFM in PF-QNM mode, the tip approach to the sample was monitored using the camera on the AFM. The sample was then scanned at a 2 μm scan size with minimal force using Bruker's ScanAsyst. The image was offset until the center of the scan was directly over top of the nanorod and the creep and drift were allowed to settle. Once the creep and drift stabilized, the AFM was switched into ramp mode. Ramp settings were identical to those used for the HOPG indent studies. These settings consisted of five single ramps per deflection error voltage at voltages ranging from 300 to 2000 mV. The TSPP:TPyP sample was imaged again and the tip centered in a different location on either the same or a different nanorod. The same data analysis procedures were used for the nanorod samples as were used on the clean HOPG standard to determine the reduced Young's modulus.
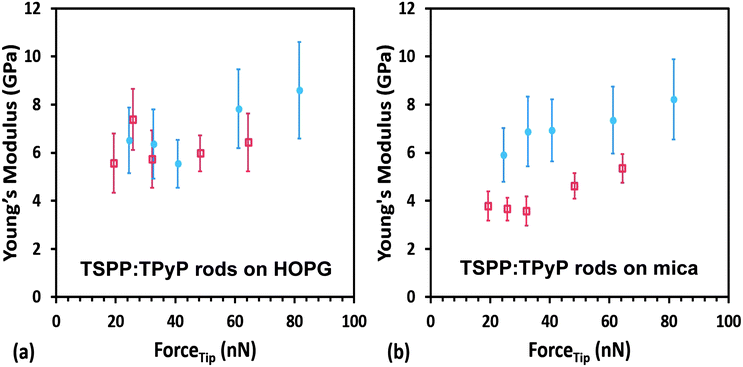
The average values of the Young's modulus obtained for TSPP:TPyP nanorods of variable thickness deposited on HOPG and mica are 6.6 ± 1.3 GPa and 6.4 ± 1.2 GPa respectively (see Fig. 8 and Table 3). Note that in Fig. 8b, the red data points represent the average modulus obtained from only two rod samples. The other E data reported in Fig. 8 is based on measurement collected from four or more nanorods. We observed that the modulus for the TSPP:TPyP samples sometimes increased erratically at ramp set points higher than 1000 mV. This may have been a result of a tip shape change. Based on this observation, only the values obtained from ramps measured at 300, 400, 500, 750, and 1000 mV were used in determining the average Young's modulus of each nanorod. The maximum tip force applied to each nanorod ranged from 19–68 nN using the cantilever with a spring constant of 2.91 N m−1 and 24–82 nN for the cantilever with a spring constant of 3.65 N m−1.
| HOPG substrate | Mica substrate | ||||||
|---|---|---|---|---|---|---|---|
| Nanorod height (nm) | Average E (GPa) | Indentation depth (nm) | Tip radius (nm) | Nanorod height (nm) | Average E (GPa) | Indentation depth (nm) | Tip radius (nm) |
| a Approximate nanorod height. b Obtained using a 2.91 N m−1 cantilever with a range of maximum applied force from 19–68 nN. c Obtained using 3.65 N m−1 cantilever with a range of maximum applied force from 24–82 nN. d Measurements made on the same nanorod. | |||||||
| 89c,d | 3.4 ± 0.6 | 1.0–1.9 | 21–35 | 38c,d | 6.0 ± 1.7 | 0.9–1.7 | 18–33 |
| 89c,d | 4.8 ± 1.7 | 1.0–1.6 | 20–32 | 38c,d | 11.8 ± 2.9 | 0.5–1.0 | 9–19 |
| a100b | 4.0 ± 1.0 | 0.8–1.2 | 27–39 | 62b | 5.5 ± 1.0 | 0.7–1.1 | 25–37 |
| a100b | 6.8 ± 1.4 | 0.6–1.1 | 20–38 | 62b | 2.9 ± 0.8 | 0.8–1.5 | 29–41 |
| 115b | 9.1 ± 2.2 | 0.4–0.9 | 14–33 | 68c | 5.3 ± 1.0 | 0.9–1.5 | 18–31 |
| 135b | 4.9 ± 2.2 | 0.6–1.9 | 22–44 | 107c | 3.3 ± 0.5 | 1.0–1.9 | 19–34 |
| 190c | 8.2 ± 2.5 | 0.6–1.1 | 11–23 | 220c,d | 6.4 ± 1.6 | 0.8–1.4 | 14–28 |
| 190c | 5.7 ± 1.9 | 0.7–1.4 | 13–28 | 220c,d | 7.9 ± 2.4 | 0.7–1.4 | 13–29 |
| 220c,d | 8.6 ± 2.4 | 0.6–1.2 | 11–24 | ||||
| Average | 6.6 ± 1.3 | Average | 6.4 ± 1.2 | ||||
It is gratifying that the average values of the elastic modulus of the TSPP:TPyP nanorods deposited on HOPG and on mica are almost identical. In addition, the summary of results in Table 3 shows that the modulus does not seem to correlate with the thickness of the rods. Both of these results indicate that we are truly measuring the mechanical property of the TSPP:TPyP samples and not that of the substrates used (HOPG and mica), both of which are much harder materials.
The TSPP:TPyP crystalline nanorods exhibited elastic deformation with a Young's modulus of 6.5 GPa. This value is comparable to that of polyfluoroethylene (7.5 GPa) and is a factor of 2 higher than the modulus for polyethylene (3 GPa). E is an intrinsic material property and is fundamentally related to internal bonding properties of the solid. The stronger the intermolecular bonds, the larger the Young's modulus. Table 4 compares the values of Young's modulus for TSPP:TPyP and other organic and inorganic materials. Although the noncovalent interaction in the ionic porphyrin solid are weaker than the covalent bonds in organic polymers their collective contribution gives the self assembled ionic TSPP:TPyP solid excellent structural integrity and E values comparable to those of covalently bonded materials.
| Material | Young's modulus (GPa) |
|---|---|
| Polypyrrole films70 | 1.2 |
| β-Phase CuPc nanowires (AFM)4 | 1.9 |
| Polyformaldehyde62 | 2.9 |
| Polyethylene71 | 3 |
| Polypyrrole nanotubes5 | 1.2–3.2 |
| Benzene single crystal (250 K) | 5.1 |
| CuPc film (nanoindenter)72 | 5.2 |
| TSPP:TPyP nanorods/mica (AFM) | 6.4 ± 1.2 |
| TSPP:TPyP nanorods/HOPG (AFM) | 6.6 ± 1.2 |
| Benzophenone single crystal73 | 6.7 |
| Polyfluoroethylene74 | 7.5 |
| Ammonium sulfate single crystal73 | 23.8 |
| HOPG (AFM) | 25 ± 9 |
| ZnTe nanowire39 | 63 ± 6 |
| pCuPc(OOc)8/W (nanoindenter)62 | 83 ± 5.7 |
| Calcium sulfate single crystal73 | 88.3 |
| pCuPc(OBu)8/mica (AFM)62 | 110 ± 15 |
In fact, poor alignment of polymer chains decreases the order in the nanostructures resulting in low values of modulus of elasticity, as in the case of polypyrrole (PPy) nanotubes.5 Improved ordering of the PPy chains along the nanotube axis resulted in more efficient π–π interactions and fewer defects. The more ordered PPy nanostructures displayed higher elastic modulus and improved charge mobilty.5 Multilayered Langmuir–Blodgett films made from peripherally substituted alkoxy copper phthalocyanines pCuPc(OOR)8 exhibited unusually high nearly metallic Young's modulus.62 The strength of these films was determined by the extent of π–π bonds interaction in their cofacial arrangement and interdigitation of the long paraffinic chains. Single crystalline nanowires of β phase copper phthalocyanine (CuPc), held together by π–π bonds, were reported to have an elastic modulus of 1.5 GPa.5 These nanowires were easily bent and relaxed without loosing their crystalline integrity or decrease in charge mobility.5 The elasticity and flexibility of CuPc nanowires was attributed to π–π bonding mediated organization of the phthalocyanine cores. The crystalline TSPP:TPyP nanorods have a higher elastic modulus than the CuPc nanowires, almost certainly because, in addition to the aromatic interactions, they also maintain electrostatic and hydrogen bonding. Like the CuPc nanowires, however, the TSPP:TPyP nanorods stiffness values are one order of magnitude smaller than that of inorganic nanowires, indicating that organic nanowire crystals are softer and hence more appropriate for flexible devices. Interestingly, we observed that the TSPP:TPyP is photoconducting i.e., these rods are insulating in the dark but become conducting under illumination. The results of our photoconductivity studies of the TSPP:TPyP system will be published elsewhere.
Conclusions
We have prepared a crystalline porphyrin nanostructure composed of TSPP and TPyP tectons in a one to one stoichiometric ratio. Our AFM images provide the first direct evidence that the nanostructures are rods composed of overlaid thin strips about 70 nm wide and 20 nm high, and on the order of a micron in length. The HRTEM SAED images of the nanorods show a clear crystalline pattern with lattice spacings: d = 1.5 ± 0.2 nm across the width of the rod and d = 0.41 ± 0.08 nm along the length of the rod. The TSPP:TPyP are thermally stable and do not lose their crystallinity when heated to temperatures up to 150 °C. Comparison of SAED data with the powder X-ray diffraction supports an arrangement of alternating face-to-face TSPP and TPyP molecules forming columns along the length of the nanorods. Ionic bonding, hydrogen bonding, and π–π interactions each contribute to aligning the ionic tectons (the macrocycles and their ring substituents) in cofacially packed columns, giving the CBI solid a well-defined rod-like structure. By correlating the diffraction pattern with the orientation of the nanorods, we arrive at the conclusion that the porphyrins within the columns are stacked perpendicular to the substrate surface and the stacking axis is the long axis of the rod. We are attempting to grow single crystals of the TSPP:TPyP solid to further refine its crystal structure.The close proximity of the [H4TSPP]2− and H4[H2TPyP]4+ or [H2TPyP] tectons in the nanorod structure is thought to result from the distorted structure of the [H4TSPP]2− diacid. In its saddled conformation the [H4TSPP]2− can form strong hydrogen bonds and π–π interactions with the H4[H2TPyP]4+ and [H2TPyP] tectons. The low rotational barriers for charged groups on the porphyrin rings allow the sulfonate and the pyridyl substituents on the porphyrin rings to easily adjust their orientation in the TSPP:TPyP composite to maximize the ionic and H-bonding interactions.
We employed AFM based nanoindentation to obtain information on the elastic properties of the TSPP:TPyP nanostructure. By acquiring statically significant sampling of the elastic compliance measurements we have obtained reproducible values of the Young's modulus. The values of E obtained for the crystalline TSPP:TPyP nanorods averaged 6.6 ± 1.3 GPa and 6.4 ± 1.2 GPa when deposited on HOPG and mica, respectively, indicating that we are truly measuring the mechanical property of the TSPP:TPyP samples and not that of the substrates. These modulus values are comparable to those reported for covalently bonded flexible polymeric systems and crystalline molecular solids like CuPc and benzophenone. The robust bonding character of the TSPP:TPyP nanostructures combined with their fine elastic properties makes them excellent candidates for flexible optoelectronic devices.
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grants CHE-11152951 and CHE-1048600. SPS and SS thank the NSF-REU program grants DMR-1062898 and DMR-0755055 for funding. We thank the Pacific Northwest National Laboratory for access to their Helium ion microscope. We are grateful to the Francheschi Microscopy and Imaging Center at Washington State University for the use of their transmission electron microscopes.Notes and references
- H. Yu, Z. Bao and J. H. Oh, Adv. Funct. Mater., 2013, 23, 629–639 CrossRef CAS.
- R. Li, W. Hu, Y. Liu and D. Zhu, Acc. Chem. Res., 2010, 43, 529–540 CrossRef CAS PubMed.
- M. Kanan, T. Wakamalsu, R. G. G. Fall and I. Ihara, Appl. Phys. Express, 2011, 4, 111603 CrossRef.
- Q. Tang, Y. Tong, Y. Zheng, Y. He, Y. Zhang, H. Dong, W. Hu, T. Hassenkam and T. Bjornholm, Small, 2011, 7, 189–193 CrossRef PubMed.
- S. Cuenot, S. Demoustier-Champagne and B. Nyste, Phys. Rev. Lett., 2000, 85, 1690–1693 CrossRef CAS.
- O. Fenwick, J. K. Sprafke, J. Binas, D. V. Kondratuk, F. Di Stasio, H. L. Anderson and F. Cacialli, Nano Lett., 2011, 11, 2451–2456 CrossRef CAS PubMed.
- J. S. Jung, J. W. Lee, K. Kim, M. Y. Cho, S. G. Jo and J. Joo, Chem. Mater., 2010, 22, 2219–2225 CrossRef CAS.
- A. B. Braunschweig, A. L. Schmucker, W. D. Wei and C. A. Mirkin, Chem. Phys. Lett., 2010, 486, 89–98 CrossRef CAS PubMed.
- C. Di Natalea, D. Monti and R. Paolesse, Mater. Today, 2010, 13, 46–52 CrossRef.
- J.-S. Hu, H.-X. Ji and L.-J. Wan, J. Phys. Chem. C, 2009, 113, 16259–16265 CAS.
- M. Jurow, A. E. Schuckman, J. D. Batteas and C. M. Drain, Coord. Chem. Rev., 2010, 254, 2297–2310 CrossRef CAS PubMed.
- C. Li, J. Ly, B. Lei, W. Fan, D. Zhang, J. Han, M. Meyyappan, M. Thompson and C. Zhou, J. Phys. Chem. B, 2004, 108, 9646–9649 CrossRef CAS.
- E. M. Lupton, L. Chen and F. Liu, J. Phys. Chem. Lett., 2010, 1, 1326–1331 CrossRef CAS.
- L. Adler-Abramovich, N. Kol, I. Yanai, D. Barlam, R. Z. Shneck, E. Gazit and I. Rousso, Angew. Chem., Int. Ed., 2010, 49, 9939–9942 CrossRef CAS PubMed.
- F. Banhart, Nanoscale, 2009, 1, 201–213 RSC.
- S. Guozhen and C. Di, Recent Pat. Nanotechnol., 2010, 4, 20–31 CrossRef.
- H. Xi, D. Z. Wei, W. Xu and D. Zhu, J. Phys. Chem. C, 2008, 112, 19934–19938 CAS.
- C. A. Berven, V. Dobrokhotov, D. N. McIlroy, S. Chava, R. Abdelrahaman, A. Heieren, J. Dick and W. Barredo, IEEE Sens. J., 2008, 9, 930–935 CrossRef.
- D. K. Avasthi, A. Kumar, R. Singhal, A. Tripathi and D. S. J. Misra, J. Nanosci. Nanotechnol., 2010, 10, 3767–3779 CrossRef CAS PubMed.
- A. Z. Moshfegh, J. Phys. D: Appl. Phys., 2009, 42, 233001-1–233001-30 CrossRef.
- Z. Wang, C. J. Medforth and J. A. Shelnutt, J. Am. Chem. Soc., 2004, 126, 15954–15955 CrossRef CAS PubMed.
- C. F. J. Faul and M. Antonietti, Adv. Mater., 2005, 15, 673–683 CrossRef.
- Z. Wang, K. J. Ho, C. J. Medforth and J. A. Shelnutt, Adv. Mater., 2006, 18, 2557–2560 CrossRef CAS.
- J.-S. Hu, Y.-G. Guo, H.-P. Liang, L.-J. Wan and L. Jiang, J. Am. Chem. Soc., 2005, 127, 17090–17095 CrossRef CAS PubMed.
- S. M. Yoon, I.-C. Hwang, K. S. Kim and H. C. Choi, Angew. Chem., Int. Ed., 2009, 48, 2506–2509 CrossRef CAS PubMed.
- C. J. Medforth, Z. Wang, K. E. Martin, Y. Song, J. L. Jacobsen and J. A. Shelnutt, Chem. Commun., 2009, 7261–7277 RSC.
- A. D. Schwab, D. E. Smith, C. S. Rich, E. R. Young, W. F. Smith and J. C. de Paula, J. Phys. Chem. B, 2003, 107, 11339–11345 CrossRef CAS.
- J. R. Eskelsen, Y. Wang, Y. Qi, M. Ray, M. Handlin, K. W. Hipps and U. Mazur, J. Porphyrins Phthalocyanines, 2012, 16, 1233–1243 CrossRef CAS.
- B. A. Friesen, B. C. Wiggins, J. L. McHale, U. Mazur and K. W. Hipps, J. Phys. Chem. C, 2011, 115, 3990–3999 CAS.
- C. M. Drain, A. Varotto and I. Radivojevic, Chem. Rev., 2009, 109, 1630–1658 CrossRef CAS PubMed.
- B. A. Friesen, B. Wiggins, J. McHale, U. Mazur and K. W. Hipps, J. Am. Chem. Soc., 2010, 132, 8554–8556 CrossRef CAS PubMed.
- Y. Tian, M. C. Beavers, T. Busani, K. E. Martin, J. L. Jacobsen, B. Q. Mercado, B. S. Swartzentruber, F. van Swol, C. J. Medforth and J. A. Shelnutt, Nanoscale, 2012, 4, 1695–1700 RSC.
- S. Liu, W. M. Wang, A. L. Briseno, S. C. B. Mannsfeld and Z. Bao, Adv. Mater., 2009, 21, 1217–1232 CrossRef CAS.
- A. L. Briseno, S. C. B. Mannsfeld, X. Lu, Y. Xiong, S. A. Jenekhe, Z. Bao and Y. Xia, Nano Lett., 2007, 7, 668–675 CrossRef CAS PubMed.
- R. Li, W. Hu, Y. Liu and D. Zhu, Science, 2004, 305, 1269–1273 CrossRef PubMed.
- K. E. Martin, Z. Wang, T. Busani, R. M. Garcia, Z. Chen, Y. Jiang, Y. Song, J. L. Jacobsen, T. T. Vu, N. E. Schore, B. S. Swartzentruber, C. J. Medforth and J. A. Shelnutt, J. Am. Chem. Soc., 2010, 132, 8194–8201 CrossRef CAS PubMed.
- W. M. Hikal and H. J. Harmon, Polyhedron, 2009, 28, 113–120 CrossRef CAS PubMed.
- V. Snitka, V. Mizariene, I. Bruzaite, V. Lendraitis and M. Rackaitis, Int. J. Nanomanuf., 2010, 5, 194–204 CrossRef CAS.
- K. Davami, B. Mortazavi, H. M. Ghassemi, R. S. Yassar, J.-S. Lee, Y. Remondc and M. Meyyappan, Nanoscale, 2012, 4, 897–903 RSC.
- B. H. Toby, J. Appl. Crystallogr., 2005, 38, 1040–1041 CrossRef CAS.
- A. Le Bail, H. Duroy and J. L. Fourquet, Mater. Res. Bull., 1988, 23, 447–452 CrossRef CAS.
- A. C. Larson and R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory, Report LAUR 86±748, 2000 Search PubMed.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- P. Thompson, D. E. Cox and J. B. Hasting, J. Appl. Crystallogr., 1987, 20, 79–83 CrossRef CAS.
- L. W. Finger, D. E. Cox and A. P. Jephcoat, J. Appl. Crystallogr., 1994, 27, 892–900 CrossRef CAS.
- CrystalMaker Software Ltd, Oxford, England, http://www.crystalmaker.com.
- M. M. Kruk and S. E. Braslavsky, J. Phys. Chem. A, 2006, 110, 3414–3425 CrossRef CAS PubMed.
- E. B. Fleischer and L. E. Webb, Inorg. Chem., 1963, 67, 1131–1133 CAS.
- J. P. Macquet, M. M. Millard and T. Theophanide, J. Am. Chem. Soc., 1978, 100, 4741–4746 CrossRef CAS.
- L. Yang, J. Xiao, T. E. Shubina, M. Chen, Z. Shi, M. Schmid, H.-P. Steinruck, J. M. Gottfried and N. Lin, J. Am. Chem. Soc., 2012, 134, 6401–6408 CrossRef PubMed.
- Y. Zubavichus, M. Zharnikov, Y. Yang, O. Fuchs, E. Umbach, C. Heske, A. Ulman and M. Grunze, Langmuir, 2004, 20, 11022–11029 CrossRef CAS PubMed.
- A. Borras, O. Groning, M. Aguirre, F. Gramm and P. Groning, Langmuir, 2010, 26, 5763–5771 CrossRef CAS PubMed.
- P. S. Pershan, International Tables for Crystallography B, 2010, 547–566 Search PubMed.
- Transmission Electron Microscopy: A Textbook for Materials Science, ed. D. B. Williams and C. B. Carter, Springer Science + Business Media, 1996, pp. 165–167 Search PubMed.
- Z. M. Ou, H. Yao and K. Kimura, J. Photochem. Photobiol., A, 2007, 189, 7–14 CrossRef CAS PubMed.
- A. Amunts, O. Drory and N. Nelson, Nature, 2007, 447, 58–63 CrossRef CAS PubMed.
- B. V. Derjaguin, V. M. Muller and Y. U. P. Toporov, J. Colloid Interface Sci., 1975, 53, 314–326 CrossRef CAS.
- I. N. Sneddon, Int. J. Eng. Sci., 1965, 3, 47–57 CrossRef.
- K. L. Johnson, K. Kendall and A. D. Roberts, Proc. R. Soc. London, Ser. A, 1971, 324, 301–313 CrossRef CAS.
- M. Radmacher, IEEE Eng. Med. Biol. Mag., 1997, 16, 47–57 CrossRef CAS.
- W. C. Oliver and G. M. Pharr, J. Mater. Res., 1992, 7, 1546–1583 CrossRef.
- T. Oshiro, A. Backstrom, A.-M. Cumberlidge, K. W. Hipps, U. Mazur, S. P. Pevovar, D. F. Bahr and J. Smieja, J. Mater. Res., 2004, 19, 1461–1470 CrossRef CAS.
- J. E. Sader, J. W. M. Chon and P. Mulvaney, Rev. Sci. Instrum., 1999, 70, 3967–3969 CrossRef CAS.
- M. E. Dokukin and I. Sokolov, Macromolecules, 2012, 45, 4277–4288 CrossRef CAS.
- M. Dokukin and I. Sokolov, Langmuir, 2012, 28, 16060–16071 CrossRef CAS PubMed.
- PF-QNM User Guide Rev. F, Bruker Corporation, 2009, p. 51 Search PubMed.
- T. Tsuji and K. Yamanaka, Nanotechnology, 2001, 12, 301–307 CrossRef CAS.
- D. M. Schaefer, A. Patil, R. P. Andres, and R. Reifenberger, in Atomic Force Microscopy/Scanning Tunneling Microscopy, ed. S. H. Cohen, M. T. Bray, and M. L. Lightbody, Plenum Press, 1994, pp. 411–421 Search PubMed.
- K. Yamanaka, T. Tsuji, A. Noguchi, T. Koike and T. Mihara, Rev. Sci. Instrum., 2000, 71, 2403–2408 CrossRef CAS.
- M. Gandhi, G. M. Spinks, R. P. Burford and G. G. Wallace, Polymer, 1995, 36, 4761–4765 CAS.
- V. Jardret, H. Zahouani, J. L. Loubet and T. G. Mathia, Wear, 1998, 218, 8–14 CrossRef CAS.
- M. Kanari, H. Kawamata, T. Wakamatsu and I. Ihara, Appl. Phys. Lett., 2007, 90, 061921 CrossRef.
- Single Crystal Elastic Constants and Calculated Aggregate Properties: A Handbook, ed. G. Simmons and H. Wang, The MIT Press, 2nd edn, 1971, pp. 304–309 Search PubMed.
- A. Floresa, F. J. Balta Calleja, G. E. Attenburrow and D. C. Bassett, Polymer, 2000, 41, 5431–5435 CrossRef.
Footnote |
| † Electronic supporting information (ESI) available: Additional UV-visible data examining the relative stoichiometry involved in forming the TSPP:TPyP binary aggregates at pH 2 as well as additional XPS, diffraction, and nanomechanical data and analysis. See DOI: 10.1039/c3nr05047e |
| This journal is © The Royal Society of Chemistry 2014 |