A core–shell–satellite structured Fe3O4@MS–NH2@Pd nanocomposite: a magnetically recyclable multifunctional catalyst for one-pot multistep cascade reaction sequences†
Ping
Li
,
Yu
Yu
,
Hua
Liu
,
Chang-Yan
Cao
and
Wei-Guo
Song
*
Beijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Nanostructures and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, Beijing,100190, P. R. China. E-mail: wsong@iccas.ac.cn; Fax: +86 10 62557908
First published on 22nd October 2013
Abstract
A hierarchical core–shell–satellite structured composite system Fe3O4@MS–NH2@Pd, which was composed of Pd nanoparticles well-dispersed on an amino group functionalized mesoporous silica (MS–NH2) nanosphere, and superparamagnetic Fe3O4 nanoparticles scattered inside the silica sphere, was prepared by using a facile procedure. The composite combined the catalytic properties of amino groups and Pd nanoparticles with superparamagnetic properties of magnetite into a single platform. This integrated nanosystem acted as an efficient magnetically recyclable noble metal-base multifunctional nanocatalyst and showed excellent catalytic activity, selectivity and stability for the direct synthesis of α-alkylated nitriles under mild conditions through facile one-pot multistep cascade reaction sequences.
1. Introduction
One-pot cascade reaction sequences, which combine multistep reactions in a single reaction vessel, are useful in efficient organic synthesis as they simplify the synthesis route, decrease the waste output and lower the operation cost as well.1–7 To make one-pot cascade reaction sequences proceed smoothly, the key lies in the multifunctional catalyst.8–12 Heterogeneous catalysts are capable of being multifunctional catalysts.13–16 Yet they often suffer from lower efficiency when compared with their homogeneous counterparts. To overcome this issue, heterogeneous nanometer-sized catalysts become highly attractive due to their high reactivity originating from high surface area-to-volume ratio.17–22 When bare nanometer-sized catalysts are employed, however, separation using conventional physical methods such as centrifugation or filtration becomes a time-consuming and tedious procedure. In addition, nanoparticles tend to aggregate and sinter during application, resulting in rapid decay in catalytic ability.23,24 Thus, a highly efficient, stable and conveniently recyclable nanocatalyst is particularly critical for multifunctional catalysis.25–28In recent years, incorporation of a magnetic element (e.g. Fe3O4) to provide an additional functionality to the original material has been widely used in the field of drug delivery,29–31 therapy diagnosis32–34 and catalysis.35–37 Specifically in catalysis, superparamagnetic nanoparticles serve as an excellent platform for supporting various nanocatalysts as they allow easy separation of the catalyst from the reaction media by using an external magnetic field.38–41 Along this line, magnetic composite nanocatalysts with hierarchical architecture would be fascinating as they can act as multifunctional integrated nanosystems which combine the catalytic properties with magnetic response on a single platform. Many excellent studies about magnetic composite nanocatalysts have been reported.42–47 However, fabrication of magnetic composite nanocatalysts with delicate structure, which combine two different types of catalytically active sites with magnetic functionality as multifunctional nanosystems for one-pot cascade reaction sequences, is still a great challenge.
α-Alkylated nitriles, as important building blocks for the synthesis of carboxylic acids, ketones, amides and a variety of biologically active compounds, are particularly useful in organic synthesis.48 The traditional method for synthesizing α-alkylated nitriles is through the reaction between nitriles and alkyl halides in the presence of stoichiometric amounts of homogeneous inorganic bases such as NaH and NaNH2. This procedure suffers from many disadvantages from the standpoint of green chemistry. Thus, the design of an efficient, mild and green synthesis route and development of an environmentally friendly, highly active, conveniently recyclable heterogeneous catalyst system for the synthesis of α-alkylated nitriles are of great significance.
Herein, we present a magnetic multifunctional nanocatalyst, hierarchical core–shell–satellite structured Fe3O4@MS–NH2@Pd, which consists of Pd nanoparticles well-dispersed on an amino group functionalized mesoporous silica (MS–NH2) nanosphere, and superparamagnetic Fe3O4 nanoparticles being scattered inside the silica sphere. The MS–NH2 served as a protecting shell for Fe3O4 nanoparticles. Meanwhile, the amino groups on the MS–NH2 served as basic catalytically active sites for base-catalyzed reactions and were capable of supporting Pd nanoparticles with high dispersion and excellent stability via the coordination effect between amino groups and Pd nanoparticles. With such a hierarchical structure design, the composite system was an efficient magnetically recyclable multifunctional nanocatalyst and showed excellent catalytic activity for rapid direct synthesis of α-alkylated nitriles under mild conditions through facile one-pot domino reaction sequences.
2. Experimental section
2.1. Materials and reagents
Fe(NO3)3·9H2O, ethylene glycol (EG), ammonia solution (concentration of 25 wt%), ethanol, NH4NO3, PdCl2,NaBH4, sodium citrate and tetraethylorthosilicate (TEOS), methanol and para-xylene were purchased from Beijing Chemical Reagent Corporation (Beijing, China). 3-Aminopropyltrimethoxysilane (APTMS), cetyltrimethylammonium bromide (CTAB), cyclohexanone derivatives and malononitrile were obtained from Alfa Aesar. All chemicals were used as received without further purification. Ultrapure water was generated using a Millipore Milli-Q system with a Milli-pak filter of 0.22 μm pore size and used for all the preparation of aqueous solutions.2.2. Synthesis of Fe3O4 nanocrystals
Fe3O4 nanocrystals were prepared using a gas–liquid interfacial synthesis method according to our previous work.49 Typically, Fe(NO3)3·9H2O (0.404 g) was dissolved in EG (5 mL) in a 15 mL beaker, then the beaker was carefully placed in a 30 mL Teflon-lined autoclave which contained 6 mL of 25 wt% ammonia solution. The autoclave was sealed and placed in a programmable microwave oven (MDS-6, Shanghai Sino Microwave Chemistry Technology Co. Ltd). The oven was heated to 170 °C under microwave irradiation for 2 min and then kept at 170 °C for 0.5 h. The black precipitate was collected using a magnet and redispersed in ethanol with an end concentration of 9.0 mg mL−1 for further use.2.3. Synthesis of Fe3O4@MS–NH2
The Fe3O4@MS–NH2 composite was synthesized by our previously reported method with slight modifications.50,51 Briefly, 3 mL of Fe3O4 ethanolic dispersion was first added in a mixed solution containing H2O (150 mL), CTAB (0.3 g) and 25 wt% NH3·H2O (1200 μL). The mixture was ultrasonicated for 2 h to form a uniform suspension. The suspension was heated to 60 °C and a mixture of silica precursors composed of TEOS (400 μL) and APTMS (70 μL) was added. The as-obtained solution was then vigorously stirred at 60 °C for another 12 h. After being collected using a magnet, the synthesized material was transferred to ethanol solution containing NH4NO3 and kept at 80 °C for 10 h to remove the surfactant CTAB. After template extraction, the powder was washed with ethanol and dried under vacuum.2.4. Synthesis of Pd colloidal nanoparticles
4 mL of 2 g L−1 PdCl2 and a certain amount of sodium citrate were added into 50 mL of deionized water. After ultrasonication, freshly prepared NaBH4 aqueous solution (20 mM) was added and the resultant suspension was stirred for 15 minutes at room temperature.2.5. Synthesis of Fe3O4@MS–NH2@Pd
Generally, 100 mg of Fe3O4@MS–NH2 was added into 50 mL of deionized water. The mixture was ultrasonicated for 2 h to obtain a homogeneous suspension. Then Pd colloidal solution was added dropwise and the mixture was stirred for 24 h at room temperature. The solid was magnetically collected, washed with deionized water and dried at 50 °C under vacuum.2.6. Catalytic activity test
Cyclohexanone (0.5 mmol), malononitrile (1 mmol), para-xylene (0.4 mmol, as internal standard), methanol (10 mL) and catalyst (25 mg) were added into a glass reactor. In the case of cyclohexanone derivatives, the resulting reaction mixture was vigorously stirred to perform the Knoevenagel condensation–hydrogenation cascade reaction sequence at 50 °C under H2 bubbling (80 mL min−1) for a given reaction time. For aromatic aldehydes and aliphatic aldehydes, the resulting reaction mixture was vigorously stirred for Knoevenagel condensation at 50 °C, followed by the hydrogenation reaction at 50 °C under H2 bubbling (80 mL min−1). The solid catalyst was separated using a Nd–Fe–B permanent magnet, and the filtrate was analyzed using a GC (Agilent 6890N) which was equipped with a capillary column (DB-5, 30.0 m × 320 μm × 0.25 μm) and a flame ionisation detector. The products were further confirmed using a GC-MS (SHIMADZU, GCMS-QP 2010S) equipped with a capillary column (DB-5 ms, 30.0 m × 320 μm × 0.25 μm).2.7. Characterization
Transmission electron microscopy (TEM) was carried out on a JEOL 1011F electron microscope running at 100 kV. EDX analysis was performed on a scanning electron microscope (SEM, JEOL-6701F) equipped with an energy-dispersive X-ray (EDS) analyzer (Oxford INCA). The wide-angle X-ray diffraction patterns were taken on a Rigaku model D/MAX-2500 V system (Cu Kα radiation). The small-angle XRD measurement was carried out on a Rigaku D/max-2400 diffractometer equipped with a secondary graphite monochromator with CuKα radiation (wavelength λ = 0.154 nm). Data were collected in a step-scan mode in the range of 1–8° with a step-width of 0.02 and speed of 1° min−1. Nitrogen adsorption–desorption isotherms were obtained on a Quantachrome Autosorb AS-1 at 77 K. The surface area of the product was measured by the Brunauer–Emmett–Teller (BET) method and the mesopore size distribution was obtained using the Non-Local Density Functional Theory (NLDFT) method. XPS data were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W Al Kα radiation. The Pd content in the material and Pd leaching in the reaction filtrate after each run were measured using an ICPE (Shimadzu ICPE-9000). The magnetic measurements were performed on a superconducting quantum interference device vibrating sample magnetometer (SQUID-VSM) at 300 K.3. Results and discussion
The typical process for the preparation of the hierarchical core–shell–satellite structured material is schematically described in Scheme 1. Initially, highly dispersed Fe3O4 nanocrystals were prepared using a facile gas–liquid interfacial synthesis method. Second, amino group functionalized mesoporous silica was coated on Fe3O4 nanocrystals through a conventional co-condensation method with TEOS and APTMS as mixed silica precursors and CTAB as a template. Third, in the presence of an amino group functionalized mesoporous silica outer shell, the pre-synthesized Pd colloidal nanoparticles can be easily adsorbed onto the amino groups through strong interactions between metal and amine groups.The as-prepared material endowed facile catalyst recovery from the reaction mixture via an external magnetic field; meanwhile, Pd nanoparticles can firmly anchor on the functionalized mesoporous silica with high dispersion due to the existence of strong metal–amine coordination interaction. Thus, in addition to magnetic elements (offered by Fe3O4 nanocrystals), multifunctional catalytically active sites were obtained with Pd nanoparticles as noble metal active sites and unbound amino groups as basic active sites.
The TEM image in Fig. 1a shows that Fe3O4 magnetite nanoparticles were well-dispersed and ca. 9 nm in diameter with narrow size distribution. After the shell coating, the typical core–shell structure with Fe3O4 cores well-coated by a layer of silica (ca. 30 nm in thickness) with relatively disordered mesopores was observed (Fig. 1b and S1† in low magnification), and the size of the Fe3O4@MS–NH2 nanocomposite ranged from 50 to 80 nm. Then Pd nanoparticles were further assembled on the magnetic mesoporous silica. From the TEM images in Fig. 1c and S2,† Pd nanoparticles with less than 5 nm in diameter were finely distributed on the external surface of mesoporous silica. And the HRTEM image in Fig. 1d shows two kinds of crystal planes of Pd NPs, with the lattice fringes of 0.225 nm and 0.194 nm, corresponding to the (111) and (200) lattice planes of the face centered cubic (fcc) Pd crystal, respectively. In addition, the EDX analysis in Fig. S3† further confirmed that the composite nanoparticles contained the elements Fe, Pd, O and Si.
 | ||
| Fig. 1 TEM images of (a) Fe3O4 nanoparticles, (b) Fe3O4@MS–NH2, and (c) Fe3O4@MS–NH2@Pd nanocomposite, (d) HRTEM image of the Fe3O4@MS–NH2@Pd nanocomposite. | ||
The crystallinity and phase composition of the materials were characterized using wide-angle X-ray diffraction (WAXRD). Fig. 2 displays the WAXRD patterns of the samples. For Fe3O4, all the diffraction peaks were in good agreement with the face-centered cubic (fcc) structure of magnetite. After the silica coating, a new broad diffraction peak at around 23° appeared, which was assigned to the amorphous silica phase. In the case of Fe3O4@MS–NH2@Pd, apart from the characteristic diffractions of fcc Fe3O4, a weak and broad diffraction peak corresponding to the (111) reflection of the Pd crystal can be observed, further confirming the well-retained magnetite crystalline phase during the follow-up coating and immobilization process, and the success of loading of highly dispersed Pd nanoparticles on the support.
 | ||
| Fig. 2 Wide-angle XRD (WAXRD) patterns of (a) Fe3O4 nanoparticles, (b) Fe3O4@MS–NH2, and (c) Fe3O4@MS–NH2@Pd nanocomposite. | ||
The XPS technique was utilized to determine the oxidation state of surface elements in the material, the survey and the respective element XPS spectra are shown in Fig. 3. Pd 3d and N 1s spectra demonstrated that Pd species in the Fe3O4@MS–NH2@Pd composite was in the metallic form (Pd0), and N existed in the form of an amino group. Besides, ICP-AES and CHN elemental analyses were used to analyze the loading of Pd and N in the composite Fe3O4@MS–NH2@Pd, according to the analysis results, the loading amounts of elements Pd and N were 4.0 wt% and 0.9 wt%, respectively.
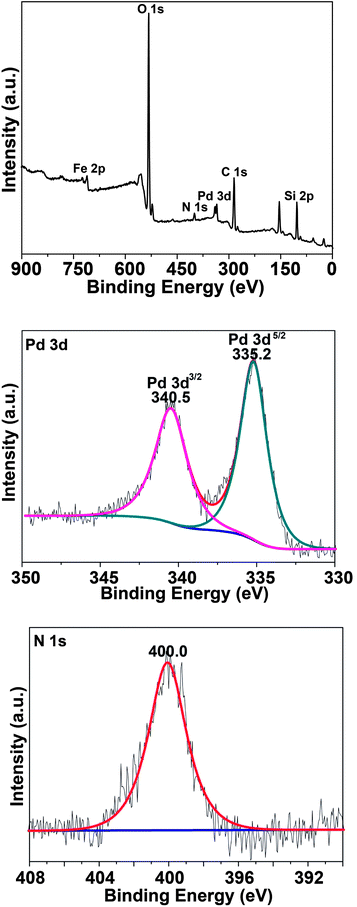 | ||
| Fig. 3 XPS spectra of the Fe3O4@MS–NH2@Pd composite: (a) wide scan spectrum, (b) Pd 3d, and (c) N 1s. | ||
The N2 adsorption–desorption isotherm of the Fe3O4@MS–NH2@Pd composite in Fig. 4a exhibited a type IV isotherm with a capillary condensation, demonstrating the mesoporous structure. In addition, the obvious steep increase in nitrogen uptake and a hysteresis loop (P/P0 above 0.85) in the isotherm was attributed to the interparticle spaces formed between silica nanoparticles. The BET surface area and pore volume of the composite were 372.0 m2 g−1 and 0.45 cm3 g−1, respectively. From the pore size analysis (inset of Fig. 4a), the Fe3O4@MS–NH2@Pd composite had a rather narrow pore size distribution centered at 3.66 nm, calculated from a non-local density functional theory (NLDFT) equilibrium model. Moreover, the existence of a broad diffraction peak indexed to (100) reflection in the SAXRD pattern (Fig. 4b) further confirmed the relatively ordered mesoporous structure of the Fe3O4@MS–NH2@Pd nanocomposite.
 | ||
| Fig. 4 N2 adsorption–desorption isotherm of the Fe3O4@MS–NH2@Pd composite. The inset shows the NLDFT pore size distribution curve. (b) Small-angle XRD pattern of the Fe3O4@MS–NH2@Pd composite. | ||
The magnetic behavior of the samples was measured using a superconducting quantum interference device magnetometer (SQUID-VSM) at 300 K in the applied magnetic field ranging from −30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 30
000 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. As illustrated in Fig. 5, no obvious remanence or coercivity was observed in the magnetization curves, indicating superparamagnetic behavior of all the samples at 300 K. The saturation magnetization values of Fe3O4 nanoparticles, Fe3O4@MS–NH2 and Fe3O4@MS–NH2@Pd were 64.2, 30.2 and 28.5 emu g−1, respectively. The systematic decrease of the saturated magnetization was due to the decreased content of Fe3O4 in the samples. Nevertheless, it should be noted that the multifunctional hybrid still showed relatively strong magnetization. As shown in the inset of Fig. 5, the composite Fe3O4@MS–NH2@Pd readily aggregated in a few seconds upon an external magnetic field, leaving the solution transparent and redispersed quickly again via shaking or ultrasonication when the magnetic field was removed, demonstrating desired magnetic control of Fe3O4@MS–NH2@Pd, which was particularly desirable for its practical applications in catalysis.
000 Oe. As illustrated in Fig. 5, no obvious remanence or coercivity was observed in the magnetization curves, indicating superparamagnetic behavior of all the samples at 300 K. The saturation magnetization values of Fe3O4 nanoparticles, Fe3O4@MS–NH2 and Fe3O4@MS–NH2@Pd were 64.2, 30.2 and 28.5 emu g−1, respectively. The systematic decrease of the saturated magnetization was due to the decreased content of Fe3O4 in the samples. Nevertheless, it should be noted that the multifunctional hybrid still showed relatively strong magnetization. As shown in the inset of Fig. 5, the composite Fe3O4@MS–NH2@Pd readily aggregated in a few seconds upon an external magnetic field, leaving the solution transparent and redispersed quickly again via shaking or ultrasonication when the magnetic field was removed, demonstrating desired magnetic control of Fe3O4@MS–NH2@Pd, which was particularly desirable for its practical applications in catalysis.
The hierarchical composite, which combined the catalytic properties of amino groups and Pd nanoparticles with superparamagnetic properties of magnetite, was a fantastic multifunctional system. The multifunctional catalytic performance of the composite Fe3O4@MS–NH2@Pd was firstly investigated in a one-pot multistep synthesis of α-alkylated nitriles using cyclohexanone and malononitrile as the reactants. The tandem reaction sequence was composed of two steps: Knoevenagel condensation on basic active sites and then hydrogenation on Pd NPs. The reaction was conducted under mild and optimized conditions through a series of optimization experiments. At the same time, to clarify the multifunctional catalytic activity of Fe3O4@MS–NH2@Pd, the catalytic properties of a series of control samples were also evaluated and the results are summarized in Table 1.
| Entry | Catalyst | Conversion (%) | Yield of B1 (%) | Yield of C1 (%) |
|---|---|---|---|---|
| a Reaction conditions: cyclohexanone (0.5 mmol), malononitrile (1 mmol), MeOH (10 mL), catalyst (25 mg), reaction temperature = 50 °C, reaction time = 1 h, H2 bubbling (80 mL min−1). All the yields were determined by GC analysis using para-xylene as internal standard. b For the hydrogenation reaction alone starting from B1. | ||||
| 1 | Fe3O4@MS–NH2@Pd | 100 | 0 | ≈100 |
| 2 | Fe3O4 | 0 | 0 | 0 |
| 3 | Fe3O4@MS | 0 | 0 | 0 |
| 4 | Fe3O4@MS–NH2 | 100 | ≈100 | 0 |
| 5 | Fe3O4@MS@Pd | 0 | 0 | 0 |
| 6b | Fe3O4@MS@Pd | 100 | — | ≈100 |
The Fe3O4@MS–NH2@Pd nanocomposite was able to convert A1 to the desired product C1 with 100% conversion and nearly 100% yield (Table 1, entry 1), demonstrating the superb catalytic activity and selectivity of our multifunctional system. Fe3O4 NPs and Fe3O4@MS could not catalyze the cascade reaction (Table 1, entries 2 and 3), due to the lack of catalytically active sites. In addition, core–shell structured material Fe3O4@MS–NH2 without Pd sites could efficiently catalyze the first step base-catalyzed reaction (Knoevenagel condensation) to intermediate product B1, but could not convert B1 to C1 (Table 1, entry 4). In the case of the Fe3O4@MS@Pd nanocomposite without amine groups, no reactions could be carried out (Table 1, entry 5). However, the hydrogenation reaction starting from B1 showed that Fe3O4@MS@Pd is an excellent catalyst for catalytic hydrogenation reactions (Table 1, entry 6). The above results demonstrate the multifunctional properties of the Fe3O4@MS–NH2@Pd nanocomposite system in tandem catalysis.
With the above initial satisfactory catalytic results, the core–shell–satellite structured composite Fe3O4@MS–NH2@Pd was investigated for a broader range of reactants. A variety of carbonyl compounds were tested, as listed in Table 2. When reacting with malononitrile, for the cases of cyclohexanone and cyclohexanone derivatives, the desired products could be obtained with good to excellent yields (70–100%) in the presence of Fe3O4@MS–NH2@Pd (Table 2, entries 1–5). Aromatic aldehydes, with either electron-withdrawing or electron-rich substituents, such as –CF3, –CN, –Me and –OMe, showed 98–100% yields for the corresponding products (Table 2, entries 6–11). In addition, aliphatic aldehydes can also afford satisfactory yields under very mild reaction conditions (Table 2, entries 12), demonstrating the decent catalytic activity and versatility of our multifunctional system Fe3O4@MS–NH2@Pd.
| Entry | Carbonyl compound | R1 | t(i) (h) | t(ii) (h) | Product | Conv. of A (%) | Yield of C (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: carbonyl compound (0.5 mmol), nitrile (1 mmol), MeOH (12 mL), catalyst (25 mg). (i) 50 °C. (ii) 50 °C, H2 (80 mL min−1). Conversion and yield were determined by GC analysis using para-xylene as internal standard. | |||||||
| 1 |

|
CN | — | 1 |

|
≈100 | ≈100 |
| 2 |

|
CN | — | 1 |

|
≈100 | ≈100 |
| 3 |
|
CN | — | 1 |

|
≈100 | ≈100 |
| 4 |

|
CN | — | 1 |

|
96.2 | 78.1 |
| 5 |

|
CN | — | 2 |
|
91.5 | 70.7 |
| 6 |

|
CO2Et | 0.5 | 0.5 |

|
≈100 | ≈100 |
| 7 |

|
CO2Et | 0.75 | 0.5 |

|
≈100 | ≈100 |
| 8 |
|
CO2Et | 0.5 | 0.5 |

|
≈100 | ≈100 |
| 9 |

|
CO2Et | 0.5 | 0.5 |
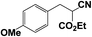
|
≈100 | ≈100 |
| 10 |

|
CO2Et | 0.5 | 0.5 |

|
98.0 | 98.0 |
| 11 |
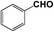
|
CN | 0.5 | 0.5 |
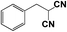
|
96.8 | 96.8 |
| 12 |

|
CO2Et | 0.5 | 0.5 |

|
97.1 | 97.1 |
The reusability and stability of the heterogeneous system is a crucial requirement for applications. The recyclability of the Fe3O4@MS–NH2@Pd catalyst was investigated in the one-pot domino reaction using cyclohexanone and malononitrile as substrates. After each cycle of the reaction, the catalyst was recovered by simple magnetic separation, washed and then reused in the next reaction run. As shown in Fig. 6, the results demonstrate that the catalyst can be reused for four runs without obvious loss of catalytic activity and selectivity. Magnetic separation made the recovery of the catalyst from the reaction media much more convenient compared with conventional separation methods such as filtration and centrifugation. Moreover, ICP-AES analysis showed Pd leaching in the mother liquid after each run was negligible. From the TEM image in Fig. S4,† the morphology of the composite catalyst was well retained after being reused for four times. Obviously, the above results demonstrate the good recyclability of the Fe3O4@MS–NH2@Pd multifunctional nanocomposite. This can be ascribed to the amino groups on the mesoporous silica, which can effectively stabilize and protect the Pd NPs from leaching and aggregation during catalysis.
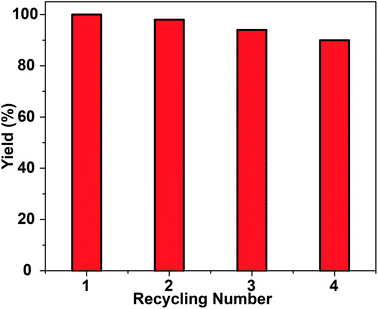 | ||
| Fig. 6 Recycling performance of the Fe3O4@MS–NH2@Pd multifunctional catalyst in the one-pot domino reaction between cyclohexanone and malononitrile. | ||
4. Conclusions
In summary, we have prepared a hierarchical core–shell–satellite structured composite system Fe3O4@MS–NH2@Pd by using a simple route. The magnetically recyclable multifunctional catalyst combined the catalytic properties of amino groups and Pd nanoparticles with superparamagnetic properties of magnetite into a single platform, showing excellent catalytic activity for the direct synthesis of α-alkylated nitriles under mild conditions via a tandem condensation–hydrogenation pathway in a single vessel. Further extensions of this multifunctional nanocatalyst system to a variety of organic transformations are currently under investigation.Acknowledgements
We thank the financial support from the National Basic Research Program of China (2009CB930400), National Natural Science Foundation of China (NSFC 21273244, 21121063), and the Chinese Academy of Sciences (KJCX2-YW-N41).Notes and references
- F.-X. Felpin and E. Fouquet, ChemSusChem, 2008, 1, 718 CrossRef CAS PubMed.
- K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993 RSC.
- M. J. Climent, A. Corma and S. Iborra, ChemSusChem, 2009, 2, 500 CrossRef CAS PubMed.
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2010, 111, 1072 CrossRef PubMed.
- C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed.
- S. Shylesh and W. R. Thiel, ChemCatChem, 2011, 3, 278 CrossRef CAS.
- C. Zhao and J. A. Lercher, Angew. Chem., Int. Ed., 2012, 51, 5935 CrossRef CAS PubMed.
- K. Motokura, M. Tada and Y. Iwasawa, J. Am. Chem. Soc., 2009, 131, 7944 CrossRef CAS PubMed.
- P. Li, C.-Y. Cao, Z. Chen, H. Liu, Y. Yu and W.-G. Song, Chem. Commun., 2012, 48, 10541 RSC.
- Y. Yang, X. Liu, X. Li, J. Zhao, S. Bai, J. Liu and Q. Yang, Angew. Chem., Int. Ed., 2012, 51, 9164 CrossRef CAS PubMed.
- E. Merino, E. Verde-Sesto, E. M. Maya, M. Iglesias, F. Sánchez and A. Corma, Chem. Mater., 2013, 25, 981 CrossRef CAS.
- P. Li, H. Liu, Y. Yu, C.-Y. Cao and W.-G. Song, Chem.–Asian J., 2013, 8, 2459 CrossRef CAS PubMed.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- U. Diaz, D. Brunel and A. Corma, Chem. Soc. Rev., 2013, 42, 4083 RSC.
- A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sánchez, ChemCatChem, 2013, 5, 3092 CrossRef CAS.
- P. Li, C.-Y. Cao, H. Liu, Y. Yu and W.-G. Song, J. Mater. Chem. A, 2013, 1, 12804 CAS.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- H. C. Zeng, Acc. Chem. Res., 2012, 46, 226 CrossRef PubMed.
- J. Liu, H. Q. Yang, F. Kleitz, Z. G. Chen, T. Yang, E. Strounina, G. Q. Lu and S. Z. Qiao, Adv. Funct. Mater., 2012, 22, 591 CrossRef CAS.
- H. Rong, S. Cai, Z. Niu and Y. Li, ACS Catal., 2013, 1560 CrossRef CAS.
- H. Wang, Y. Wang, Z. Zhu, A. Sapi, K. An, G. Kennedy, W. D. Michalak and G. A. Somorjai, Nano Lett., 2013, 13, 2976 CrossRef CAS PubMed.
- Y. Zhang, S. Wang, W. Zhu, J. Ke, J. Gu, A.-X. Yin and C.-H. Yan, Chem. Commun., 2013, 49, 7168 RSC.
- R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2003, 125, 8340 CrossRef CAS PubMed.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 12663 CrossRef CAS PubMed.
- J. Guerra and M. A. Herrero, Nanoscale, 2010, 2, 1390 RSC.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578 RSC.
- J. S. Chen, C. Chen, J. Liu, R. Xu, S. Z. Qiao and X. W. Lou, Chem. Commun., 2011, 47, 2631 RSC.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889 CrossRef CAS PubMed.
- J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47, 8438 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, S. Budi Hartono and G. Q. Lu, Angew. Chem., Int. Ed., 2010, 49, 4981 CrossRef CAS PubMed.
- L. Wang, J. Bao, L. Wang, F. Zhang and Y. Li, Chem.–Eur. J., 2006, 12, 6341 CrossRef CAS PubMed.
- Y.-S. Lin, S.-H. Wu, Y. Hung, Y.-H. Chou, C. Chang, M.-L. Lin, C.-P. Tsai and C.-Y. Mou, Chem. Mater., 2006, 18, 5170 CrossRef CAS.
- N. Lee, H. R. Cho, M. H. Oh, S. H. Lee, K. Kim, B. H. Kim, K. Shin, T.-Y. Ahn, J. W. Choi, Y.-W. Kim, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2012, 134, 10309 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425 CrossRef CAS PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- R. B. N. Baig and R. S. Varma, Chem. Commun., 2013, 49, 752 RSC.
- L. L. Chng, N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2013, 46, 1825 CrossRef CAS PubMed.
- T. Mitsudome and K. Kaneda, ChemCatChem, 2013, 5, 1681 CrossRef CAS.
- R. B. Nasir Baig and R. S. Varma, Green Chem., 2013, 15, 398 RSC.
- J. Ge, Q. Zhang, T. Zhang and Y. Yin, Angew. Chem., Int. Ed., 2008, 47, 8924 CrossRef CAS PubMed.
- V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC.
- L. Zhou, C. Gao and W. Xu, Langmuir, 2010, 26, 11217 CrossRef CAS PubMed.
- Y. Deng, Y. Cai, Z. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8466 CrossRef CAS PubMed.
- B. Liu, W. Zhang, F. Yang, H. Feng and X. Yang, J. Phys. Chem. C, 2011, 115, 15875 CAS.
- Y. Jang, J. Chung, S. Kim, S. W. Jun, B. H. Kim, D. W. Lee, B. M. Kim and T. Hyeon, Phys. Chem. Chem. Phys., 2011, 13, 2512 RSC.
- K. Motokura, N. Fujita, K. Mori, T. Mizugaki, K. Ebitani, K. Jitsukawa and K. Kaneda, Chem.–Eur. J., 2006, 12, 8228 CrossRef CAS PubMed.
- Z.-M. Cui, L.-Y. Jiang, W.-G. Song and Y.-G. Guo, Chem. Mater., 2009, 21, 1162 CrossRef CAS.
- Z. Chen, Z.-M. Cui, F. Niu, L. Jiang and W.-G. Song, Chem. Commun., 2010, 46, 6524 RSC.
- S.-W. Bian, Z. Ma, L.-S. Zhang, F. Niu and W.-G. Song, Chem. Commun., 2009, 45, 1261 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of the materials. See DOI: 10.1039/c3nr04427k |
| This journal is © The Royal Society of Chemistry 2014 |




