Thermal stability of Mn2+ ion luminescence in Mn-doped core–shell quantum dots†
Xi
Yuan
ab,
Jinju
Zheng
c,
Ruosheng
Zeng
d,
Pengtao
Jing
a,
Wenyu
Ji
a,
Jialong
Zhao
*ae,
Weiyou
Yang
*c and
Haibo
Li
*e
aState Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, 3888 Eastern South Lake Road, Changchun 130033, China. E-mail: zhaojl@ciomp.ac.cn; Tel: +86-431-86176313
bUniversity of Chinese Academy of Sciences, Beijing 100039, China
cInstitute of Materials, Ningbo University of Technology, Ningbo 315016, China. E-mail: weiyouyang@tsinghua.org.cn; Tel: +86-574-87080966
dSchool of Chemistry and Material Science, Guizhou Normal University, Guiyang 550001, China
eKey Laboratory of Functional Materials Physics and Chemistry of the Ministry of Education, Jilin Normal University, Siping 136000, China. E-mail: lihaibo@jlnu.edu.cn; Tel: +86-434-3290232
First published on 7th October 2013
Abstract
The thermal stability of luminescence is important for the application of quantum dots (QDs) in light-emitting devices. The temperature-dependent photoluminescence (PL) intensities and decay times of Mn-doped ZnS, ZnSe, and ZnSeS alloyed core–shell QD films were studied in the temperature range from 80 to 500 K by steady-state and time-resolved PL spectroscopy. It was found that the thermal stability of Mn-doped QD emissions was significantly dependent on the shell thickness and the host bandgap, which was higher than that of workhorse CdSe QDs. Nearly no PL quenching took place in Mn:ZnS QDs with a thick ZnS shell, which kept a high PL quantum yield (QY) of ∼50% even at 500 K; and the thermally stable PL was also observed in highly luminescent Mn:ZnSe and Mn:ZnSeS QDs with a quenching temperature over 200 °C. Further, the stability of Mn-doped QDs with different shell thickness at high temperature was also examined through heating–cooling cycling experiments. The PL quenching in the thick shell-coated Mn-doped QDs was almost totally recovered. The PL quenching mechanisms of the Mn2+ ion emissions were discussed.
1. Introduction
Colloidal semiconductor quantum dots (QDs) have drawn considerable attention for their size- and composition-tunable photoluminescence (PL), rich electronic structures, and flexible solution processability.1–4 Doping transition metal ions, e.g. Mn2+, into semiconductor QDs introduces new optical, electronic, and magnetic properties to the host nanocrystals, exhibiting extensive applications in solar energy conversion,5 electroluminescent devices,6,7 and biological labels.8,9 In comparison with the current workhorse CdSe QDs, the Mn2+ ion doped QDs usually contain no heavy metal ions, and have advantages of no self-quenching due to the large Stokes shift,8–10 and suitability for applications where high density emitters are required, such as solid state lighting and lasers. In recent years, nearly pure and highly efficient (above 50%) Mn dopant emission has been achieved in Mn:ZnS,11 Mn:ZnSe,12,13 and Mn:ZnSeS QDs,14 moreover, narrow and widely tunable Mn emission from deep green to deep red has been observed.15 These properties make the heavy metal free Mn-doped QDs relevant to be used in light-emitting devices (LEDs). As we know, the luminescence efficiency of QDs is a key factor to determine the performance of LEDs. However, the luminescence efficiencies of Mn-doped QDs were reported to be strongly temperature-dependent, and generally decrease at elevated temperature.16–19 And yet, for most optical devices of QDs, the thermal effects are inevitable and the working temperature is higher than room temperature due to heat dissipation of the QDs themselves.20 The working temperature is even up to 450 K, when the QDs are applied as color converters in warm-white LEDs.21 Therefore, the QDs with a high luminescence efficiency and an improved thermal stability at high temperature are urgently needed for their ultimate commercialization of QD luminescent devices.The studies of the thermal stability of Mn-doped QDs were almost focused on the cryogenic temperature range of 5–300 K in early works. In this temperature region, serious thermal quenching of Mn2+ ion emissions observed by many groups16–19 was generally attributed to the thermal activation to the defect states; besides, temperature antiquenching due to thermal release of delocalized carriers to Mn2+ ions was also observed.22,23 The two competing factors determined the ultimate temperature dependent PL behaviors, which could be controlled by the particle size and the shell structure.22,24 Above room temperature, only a few reports were relative to luminescence properties of Mn-doped QDs. Chen reported that the emission in Mn:ZnS QDs was quenched completely at 140 °C,17 while the Mn:ZnSe QDs with little temperature quenching (PL spectra recorded in situ) have been achieved by Peng's group.13 However, previous reports have not provided the systemic research on the thermal stability of luminescence for Mn-doped QDs against the thermal quenching, the thermally induced structural changes, and the intrinsic PL quenching mechanisms under high temperature conditions. Hence, the method to improve the high-temperature thermal stability of Mn2+ ion emission is still unclear. Moreover, the stability of Mn-doped QDs at elevated temperature is significant for the QD applications in optical devices. Therefore, better understanding of the PL quenching mechanisms and improving the thermal stability under high temperature conditions for Mn-doped QDs are highly desired.
In this work, we systematically investigated the thermal behaviours of Mn2+ ion emissions in MnS–ZnS, MnSe–ZnSe, and MnSe–ZnSeS alloyed core–shell QD films by the temperature-dependent steady-state and time-resolved PL spectroscopy from 80 to 500 K. The PL quenching mechanisms were explored by comparing the temperature-dependent PL intensities and PL lifetimes of the Mn-doped QDs with varying the shell thickness and host material. Then, the effects of the shell and host bandgap of the Mn-doped core–shell QDs on the thermal stability of Mn2+ ion emissions were discussed. Further, the stability of the QDs against the thermal degradation of the luminescence performance was examined by heating–cooling cycling experiments.
2. Experimental section
2.1 Sample preparation
The Mn-doped ZnS, ZnSe, and ZnSeS alloyed core–shell QDs used in this experiment were synthesized via a nucleation-doping strategy similar to our previous works.12,14,22 Briefly, a preformed MnS or MnSe nanocrystal core was initially coated with a ZnS, ZnSe, or ZnSeS shell, and then the doping was achieved by diffusion of Mn2+ ions from the core into the shell at elevated temperature (above 220 °C), so the shell also acted as the host for Mn2+ ions. The details of the QD synthesis are described in the ESI.† The Mn-doped QDs were collected by dispersing into chloroform solution for further optical measurements and film sample preparation. The film samples used for temperature dependent PL spectra and PL decay measurements were fabricated by dropping the Mn-doped QDs on silicon wafer substrates.2.2 Experimental setup
The absorption spectra were recorded by a U-3900 UV-VIS scanning spectrophotometer (Hitachi). Steady-state PL spectra, PL QY, and time-resolved PL spectra were measured by a Horiba Jobin Yvon Fluromax-4P with a QY accessory and a time-correlated single-photon counting (TCSPC) spectrometer. The continuous excitation source was a 150 W ozone-free xenon arc-lamp and a pulsed xenon lamp was utilized as the excitation source for the PL decay measurement. The size of the QDs was measured by a JEM-2100F transmission electron microscope (TEM). The energy dispersive X-ray spectroscopy (EDS) for the elemental analysis of the inorganic QDs was measured by using a Hitachi S-4800 scanning electron microscope. The film samples were mounted in a Janis VPF-800 vacuum liquid nitrogen cryostat with a controllable temperature region from 80 to 500 K, during the entire measurement.3. Results and discussion
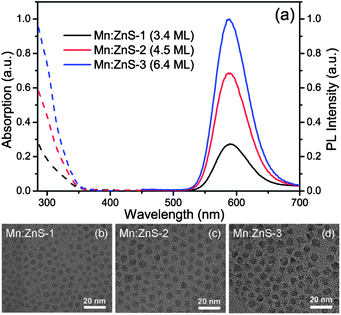
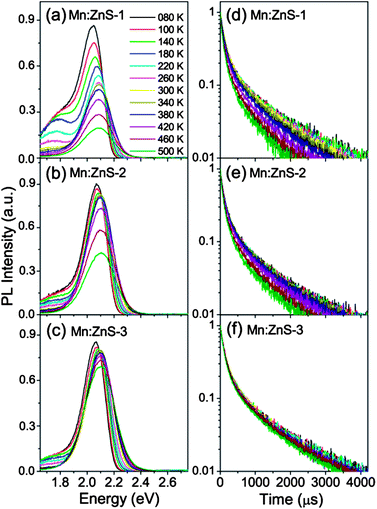
UV-visible absorption and PL spectra of MnS–ZnS core–shell QDs with different ZnS shell thicknesses in chloroform solution are shown in Fig. 1a. The core–shell QDs can also be called Mn:ZnS QDs because the shells also act as the host for Mn dopants.13 The thicknesses of the shells are estimated to be about 3.4, 4.5 and 6.4 monolayers (MLs), based on the TEM images of the MnS core (Fig. S1a, ESI†) and MnS–ZnS core–shell (Fig. 1b–d) QDs, and one monolayer thickness of 0.31 nm to ZnS materials.25 As seen in Fig. 1a, a large Stokes shift of about 270 nm (1.77 eV) is observed due to the significant energy differences between the bandgap of the host semiconductor and the energy of the Mn2+ ion 4T1 to 6A1 internal d–d transition. The PL QY of the MnS–ZnS core–shell QDs increases monotonously from 14% to 53%, through growing the ZnS shell around the MnS core, passivating the surface trap states.The left of Fig. 2 shows the temperature-dependent PL spectra of Mn:ZnS QDs with different shell thickness, recorded in the temperature range from 80 to 500 K (heating temperature over 500 K may induce the loss of capping ligands). As seen in Fig. 2a, the PL intensity of the thin shell (3.4 MLs) QDs quickly drops as the temperature increases. This means that there are a large amount of the nonradiative traps present on the surface of thin shell QDs, inducing strong thermal quenching. The PL intensity of medium shell (4.5 MLs) QDs almost keeps constant when temperature varies from 80 to 380 K, and decreases with increasing temperature from 420 to 500 K, as shown in Fig. 2b; resulting from that the surface nonradiative recombination centers are efficiently passivated as the shell thickness increases. Further, as seen in Fig. 2c, it is excitingly found that almost no PL quenching takes place from 80 to 420 K, and only a little PL quenching occurs as the temperature rises above 460 K in the thick shell (6.4 MLs) QDs, which means that the nonradiative recombination is significantly suppressed. This suggests that our Mn:ZnS QDs with a thick shell can keep high PL QY (∼50%) even at the high temperature range of 300–500 K, which means that the Mn:ZnS QDs can sufficiently satisfy the requirements (great thermal stability and high PL QY) for the applications in luminescence devices. In addition, besides the Mn2+ ion emission at about 2.1 eV, the surface trap PL at the low-energy side related to the defect in ZnS nanoparticles17 is observed, which can be eliminated with growing the ZnS shell around the MnS core, indicating that the surface defect states are efficiently passivated.
It is well known that the Mn2+ ion emission is due to energy transfer from the exciton in host to the Mn2+ ions.26,27 Therefore, the PL QY of Mn2+ ions (QYMn) is determined by both the energy transfer efficiency (ΦET) and Mn2+ ion emission efficiency (ΦMn), which can be expressed as:25 QYMn = ΦET × ΦMn. The energy transfer process (a few to tens of picoseconds) is significantlyly faster than the 4T1 to 6A1 transition process (milliseconds) of Mn2+ ions,26–29 thus, the PL decay curves can only reflect the Mn2+ ion recombination process. Besides, the PL decay lifetime of Mn2+ ion emissions (τMn = 1/(kMnr + kMnnr), kMnr and kMnnr are the radiative and nonradiative decay rate constant of Mn2+ ions) is related to the Mn2+ ion emission efficiency ΦMn = kMnr/(kMnr + kMnnr),25 therefore, we can use τMn to reflect ΦMn. To better understand the different thermal quenching issues in the Mn:ZnS QDs with various shell thicknesses, we measure their luminescence decay curves at different temperatures (80–500 K) shown in the right of Fig. 2. The PL recombination dynamics becomes faster at higher temperature, and the largest, medium, and smallest changes in PL decay with increasing temperature were observed in 3.4, 4.5, and 6.4 ML ZnS shell coated Mn:ZnS QDs, respectively. We analyzed the PL decays by a bi-exponential function with two time components (τi) and weights (Ai). The amplitude-weighted lifetimes were obtained by a relation τav = (A1τ1 + A2τ2)/(A1 + A2).
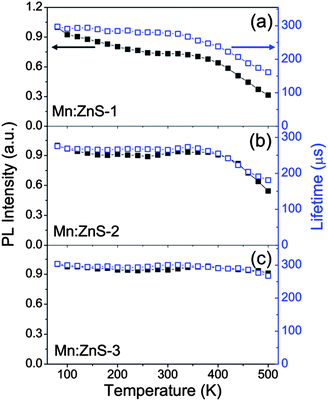
Both the temperature-dependent integrated PL intensities (normalized at 80 K) and lifetimes are plotted in Fig. 3. The PL intensity drops about 70% for thin shell QDs, 40% for medium shell QDs, and only about 10% for thick shell QDs, after heating up to 500 K, compared to that at 80 K. This suggests that the thin shell QDs with a low PL QY are more sensitive to the temperature than the thick shell QDs with a high PL QY. We know that both the energy transfer efficiency (ΦET= kET/(kQDr + kQDnr + kET), kET is the energy transfer rate constant, kQDr and kQDnr are the radiative and nonradiative decay rates (without energy transfer) of the host)25 and Mn2+ ion emission efficiency (ΦMn) are dependent on the nonradiative decay rates (kQDnr, kMnnr), which will become fast with increasing the temperature. Hence, using a thick shell to passivate the surface trap states and decrease the density of nonradiative recombination centers can effectively improve the PL QY and thermal stability of Mn-doped QDs. On the other hand, increasing the shell thickness can also make the Mn dopant centers sufficiently far away from the surface, thus the thermally activated carries trapping by surface states cannot effectively affect the Mn2+ ion emission. Moreover, we observed a similar trend in the PL intensities and lifetimes with the temperature, present in medium and thick shell QDs, indicating that the thermal quenching is mainly due to the decrease of ΦMn. Meanwhile, the PL intensity drops faster than the PL lifetime with increasing temperature in thin shell QDs, which reveals both the decrease of ΦMn and drop of ΦET are responsible for the PL thermal quenching. The ΦMn significantly impacts all three kinds of Mn:ZnS QDs, and ΦET only partially refers to the thin shell QDs. This issue may be attributed to the larger energy transfer rate (kET ∼ 1011 s−1) than Mn2+ ion radiative rate (kMnr ∼ 103 s−1).26–29 Therefore, we could amend the thermal stability of Mn2+ ion emission in QDs by increasing the shell thickness, decreasing the density of nonradiative recombination centers, making the Mn2+ ions sufficiently far away from the surface traps, and perhaps enhancing the energy transfer rate or radiative rate of Mn2+ ions.
It was noted that serious PL thermal quenching occurred for Mn-doped QDs reported before.16–19 These results are vastly different from our Mn:ZnS QDs. Here, we noticed that, in the early works, the Mn-doped QDs generally showed low PL QY, suffering from the low doping yield and/or a significant portion of the doped ions near the surface of the QDs.30,31 The low doping yield means the slow energy transfer process28 and the doped ions near the surface make the Mn2+ ion emission efficiency susceptible to the temperature, therefore the strong thermal quenching presented in these Mn-doped QDs.
Besides the wide bandgap semiconductor ZnS, the ZnSe semiconductor is also a commonly used, heavy metal free host material for Mn dopants, and the Mn:ZnSe QDs have been used in solid state lighting.32,33 However, there are no reports about the thermal stability of Mn:ZnSe QD films at high temperature. The PL spectra and decay curves at different temperatures (80–500 K) of Mn:ZnSe (MnSe–ZnSe core–shell) QD films are shown in Fig. 4. The shell thickness of the Mn:ZnSe QDs with a PL QY of ∼56% is estimated to be 6.2 MLs from the TEM (Fig. S1b, ESI†). As seen in Fig. 4, the PL intensity of the Mn:ZnSe QDs shows little change with increasing the temperature from 80 to 380 K and quickly decreases at a temperature above 380 K; simultaneously, the PL decay exhibits the similar trend. It is interesting to see that, in Fig. 2c and 4, both Mn:ZnS and Mn:ZnSe QDs synthesized by the nucleation-doping strategy12,22 possess high PL QY (above 50%) and thick shell (above 6 MLs), however, Mn:ZnS QDs can keep the high PL QY at high temperature, while the PL QY of Mn:ZnSe QDs rapidly drops with increasing temperature. To better understand this issue, the temperature-dependent integrated PL intensities and lifetimes of Mn:ZnSe QDs are plotted in the ESI (Fig. S2).† The similar trend for the PL intensity and lifetime indicates that the decrease of ΦMn should be responsible for the thermal quenching of Mn:ZnSe QDs.
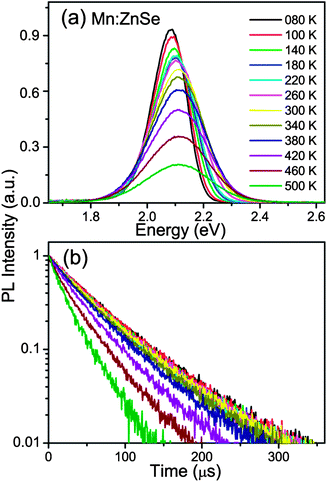 | ||
| Fig. 4 PL spectra (a) and decay curves (b) of Mn:ZnSe QDs at different temperatures from 80 to 500 K, under excitation at 280 nm. | ||
In general, thermal quenching behavior in QDs is attributed to multiphonon relaxation and thermally activated nonradiative recombination processes.19,20,34 The large 4T1–6A1 energy gap combines with the low phonon energies provided by ZnSe semiconductor lattices, at least 60 vibrational quanta are needed, hence the multiphonon relaxation is not expected to be the reason for the thermal quenching of Mn:ZnSe QDs. The thermally activated nonradiative recombination process induces the PL quenching by thermal escape of carriers from the luminescent center. For QDs, this process is generally viewed as the thermally activated escape of carriers to the trap state.20,35 Moreover, in phosphors, it also involves thermally activated photoionization from the localized dopant excited state to the host conduction band.36,37 In our work, the Mn:ZnS and Mn:ZnSe QDs exhibit similar PL QY and shell thickness, and are both synthesized by the nucleation-doping strategy,12,22 which indicates that the nature and location of the defects or traps should be similar. Therefore, their difference in thermal stability is not supposed to just arise from the thermally activated escape of carriers to trap states. The thermal quenching of Mn:ZnSe QDs should probably be caused by the photoionization from the localized dopant excited state to the host conduction band, because the bandgap of ZnSe (2.7 eV) is smaller than that of ZnS (3.7 eV). Based on this viewpoint, the host with a larger bandgap is expected to exhibit better PL thermal stability. So we examined the temperature-dependent PL spectra of Mn-doped ZnSe0.73S0.27 and ZnSe0.22S0.78 QDs (Fig. S3, ESI†), with the bandgap of 3.1 and 3.3 eV estimated from the absorption spectra, respectively, which were larger than that of ZnSe. The PL QY is 51% and 46%, and the shell thickness is 6.3 and 5.2 MLs (Fig. S1c and d, ESI†) for Mn:ZnSe0.73S0.27 and Mn:ZnSe0.22S0.78 QDs, respectively. Their integrated PL intensities (normalized at 300 K) as a function of temperature are plotted in Fig. 5; for comparison, the PL intensities of Mn:ZnSe and Mn:ZnS QDs at different temperature are also shown in this figure.
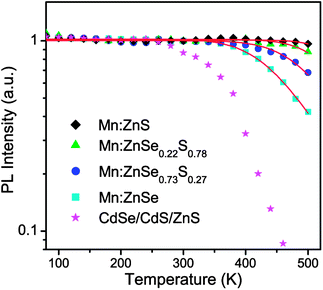 | ||
| Fig. 5 The integrated PL intensities of Mn:ZnS (black rhombuses), Mn:ZnSe0.22S0.78 (green triangles), Mn:ZnSe0.73S0.27 (blue circles), Mn:ZnSe (cyan squares), and CdSe–CdS–ZnS (magenta stars) core–shell QDs at different temperatures from 80 to 500 K, under excitation at 280 nm. The red solid lines represent fitting curves using eqn (1). | ||
As seen in Fig. 5, with raising the host bandgap, the Mn:ZnSe, Mn:ZnSe0.73S0.27, Mn:ZnSe0.22S0.78, and Mn:ZnS QDs show the onset of temperature quenching of PL around 380, 400, 460, and 480 K; and after heating to 500 K, the PL intensity drops off 58%, 32%, 13%, and 5%, respectively, compared to that at 300 K. The temperature dependence of the luminescence intensity can be described by an Arrhenius equation:19,38
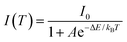 | (1) |
It is exciting to see that four different highly efficient Mn-doped QD systems all possess high luminescence quenching temperatures (defined as the temperature at which the emission reaches half of its maximum intensity) over 200 °C, higher than Mn-doped nanocrystals and workhorse CdSe QDs reported so far. The factors resulting in the low quenching temperatures of Mn-doped QDs reported before have been discussed in the above. For the pristine CdSe QDs, we plot the integrated PL intensity of CdSe–CdS–ZnS core–shell QDs with a high luminescence QY (67%) as a function of temperature in Fig. 5, corresponding PL spectra are shown in the ESI (Fig. S4).† The undoped CdSe QDs have a quenching temperature of about 100 °C, much lower than our Mn-doped QDs. The great difference between the quenching temperatures of the undoped and Mn-doped QDs can be understood by the different emission mechanisms for the two cases. For the undoped QDs, the emission arises from the direct recombination of the exciton; while for the Mn-doped QDs, the emission is attributed to the Mn2+ ion d–d transition, which is excited by the host exciton energy transfer.39,40 The fast energy transfer process (a few to tens of picoseconds)28,29 will effectively compete with nonradiative decay by trap states, which makes Mn2+ ion emission more robust than exciton luminescence in undoped QDs.41 Furthermore, the d-electrons are almost localized in the Mn2+ ion and do not spread over the entire nanocrystal,40 thus the Mn2+ ion emission is less sensitive to nonradiative decay channels on the QD surface. Therefore, the Mn-doped QDs exhibit better PL thermal stability than the undoped QDs, and Mn2+ ion doping can become a way to enhance the PL thermal stability of QDs.
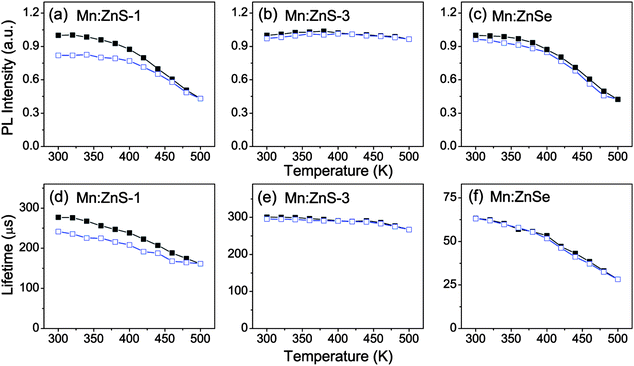
For practical applications, the thermal stability of the structure is also an important factor for the QDs to be used at elevated temperature, because the thermally induced structural changes can give rise to the formation of surface states or defects.20 The PL quenching arising from the structural changes is irreversible, which distinguishes from the reversible quenching caused by thermal activation. We recorded the temperature-dependent PL spectra and PL decay curves of Mn:ZnS QDs with different shell thickness and Mn:ZnSe QDs in heating–cooling cycle processes (Fig. S5, ESI†), and plotted the corresponding integrated PL intensities and PL lifetimes in Fig. 6. The irreversible quenching process was present in Mn-doped QDs with a thin shell, as seen in Fig. 6a. There is about 18% permanent loss of intensity after the heating and cooling cycle, accompanied by 13% permanent shortening of lifetime. The permanent drop in both PL intensity and lifetime gives evidence for the irreversible structural change in the thin shell coated Mn:ZnS QDs. Interestingly, in Mn-doped QDs with a thick shell, such as Mn:ZnS with 6.4 ML ZnS and Mn:ZnSe with 6.2 ML ZnSe shells, both the PL intensity and lifetime almost fully recover upon cooling, which suggests that the irreversible quenching process is absent, as seen in Fig. 6b and c. Similar behavior is also present in Mn:ZnSeS QDs with a thick shell (Fig. S6, ESI†). The results mean that irreversible quenching at high temperature is not inevitable, which depends on the shell structure. The density of thermally induced surface states or traps is larger for thin shell QDs, which may be attributed by the smaller size, and the thick shell can efficiently suppress the effect of thermally created surface states on the Mn2+ ion emission. Besides, the PL intensity drop of CdSe QDs is only partly reversible, 45% permanent loss of intensity is observed (Fig. S6, ESI†). This indicates that Mn2+ ion emission is more robust than exciton luminescence in CdSe QDs, consistently with that the Mn2+ ion emission is less susceptible to the trap states.
The temperature dependence of the PL peak energies for Mn:ZnS QDs with different shell thickness and Mn:ZnSe QDs in the heating–cooling cycle processes are shown in Fig. 7a. The PL peak of Mn2+ ions is observed to gradually shift to higher energy as the temperature increases from 80 to 500 K, which is generally attributed to the thermal expansion of the host lattice decreasing crystal field strength,19 similar to the temperature behavior of Mn2+ ion emission in bulk semiconductors.42 Upon cooling to 300 K, the shift is totally reversible for thick shell coated QDs, which indicates that no structure change occurs near the Mn2+ ions, because the structure change will induce the variation in the ligand field. The results also suggest that the Mn2+ ions do not diffuse during our experiments, because the ligand field splitting progressively increases from the core to the surface,15 while a permanent red shift is found in the thin shell QDs, which may be caused by the increase of surface defect state emission (as seen in Fig. S5, ESI†). On the other hand, the full widths at the half-maximum (FWHM) of these QDs broaden with increasing the temperature, as seen in Fig. 7b. The temperature dependence of the bandwidth can be written as:43
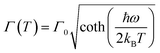 | (2) |
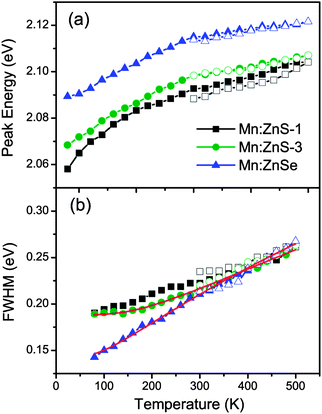 | ||
| Fig. 7 Temperature dependence of emission peak energy (a) and FWHM (b) of Mn:ZnS-1 (3.4 ML ZnS, black squares), Mn:ZnS-3 (6.4 ML ZnS, green circles), and Mn:ZnSe (blue triangles) QDs. The solid symbols refer to heating processes, the open symbols represent cooling processes. The red solid lines represent fitting curves using eqn (2). | ||
Here Γ0 is the bandwidth at 0 K and ħω is the energy of the lattice vibration (or phonon) that couples with the electronic transition. The bandwidth as a function of temperature can be fitted well by eqn (2), as seen by the red solid curves in Fig. 7b. The parameters ħω are obtained to be 46 and 28 meV for Mn:ZnS and Mn:ZnSe QDs, respectively, similar to the values reported for bulk Mn:ZnS (41 meV) and Mn:ZnSe (31 meV),43,44 the small difference may be caused by the strain at the core–shell interface.45 The variation in bandwidth at high temperature is reversible for thick shell QDs, while irreversible for the thin shell ones, as seen in Fig. 7b, which confirms that the thick shell can efficiently suppress the effect of thermal creation surface states on the Mn2+ ion emission.
4. Conclusions
In summary, we have studied the thermal stability of Mn2+ ion emissions in Mn-doped core–shell QDs with different shell structures at the temperature ranging from 80 to 500 K. By comparing the temperature-dependent PL intensity and lifetime, the thermal quenching of highly efficient Mn-doped QDs is mainly caused by the thermally activated escape of carriers from the excited Mn2+ ions, which presented at elevated temperature and can be controlled by the shell thickness and the host bandgap. The thermal stability of Mn2+ ion emission can be improved by increasing the shell thickness, reducing the density of nonradiative recombination centers, making the Mn2+ ions sufficiently far away from the surface traps, and enhancing the host bandgap. The highly efficient Mn:ZnSe, Mn:ZnSeS, and Mn:ZnS QDs with thick shells possessed high PL quenching temperature over 200 °C; excitingly, the Mn:ZnS QDs kept a high PL QY ∼50% even at 500 K. Further, the Mn-doped QDs exhibited excellent thermal robustness in the heating–cooling experiments, better than pristine CdSe core–shell QDs. The PL intensity, peak energy, and FWHM for thick shell coated Mn-doped QDs can almost completely recover after heating up to 500 K. Therefore, heavy metal-free Mn-doped QDs are promising to be used in high-efficiency and environmentally safe QD optical devices, in which the strong thermal effects are inevitable.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 11274304, and 11204298, 61106066, 61205025, and 21101038). We thank Mr Rong'an Shen for his help in the preparation of Mn-doped QDs.Notes and references
- S. Coe, W. K. Woo, M. G. Bawendi and V. Bulović, Nature, 2002, 420, 800–803 CrossRef CAS PubMed.
- Y. Shirasaki, G. J. Supran, M. G. Bawendi and V. Bulović, Nat. Photonics, 2013, 7, 13–23 CrossRef CAS.
- A. G. Pattantyus-Abraham, I. J. Kramer, A. R. Barkhouse, X. H. Wang, G. Konstantatos, R. Debnath, L. Levina, I. Raabe, M. K. Nazeeruddin, M. Grätzel and E. H. Sargent, ACS Nano, 2010, 4, 3374–3380 CrossRef CAS PubMed.
- K. Dohnalová, A. N. Poddubny, A. A. Prokofiev, W. D. Boer, C. P. Umesh, J. M. Paulusse, H. Zuilhof and T. Gregorkiewicz, Light: Sci. Appl., 2013, 2, e47 CrossRef.
- P. K. Santra and P. V. Kamat, J. Am. Chem. Soc., 2012, 134, 2508–2511 CrossRef CAS PubMed.
- H. Yang and P. H. Holloway, J. Phys. Chem. B, 2003, 107, 9705–9710 CrossRef CAS.
- H. Yang, P. H. Holloway and B. B. Ratna, J. Appl. Phys., 2003, 93, 586–592 CrossRef CAS.
- N. Pradhan, D. M. Battaglia, Y. C. Liu and X. G. Peng, Nano Lett., 2007, 7, 312–317 CrossRef CAS PubMed.
- A. R. Maity, S. Palmal, S. K. Basiruddin, N. S. Karan, S. Sarkar, N. Pradhana and N. R. Jana, Nanoscale, 2013, 5, 5506–5513 RSC.
- S. Cao, J. J. Zheng, J. L. Zhao, L. Wang, F. M. Gao, G. D. Wei, R. S. Zeng, L. H. Tian and W. Y. Yang, J. Mater. Chem. C, 2013, 1, 2540–2547 RSC.
- W. J. Zhang, Y. Li, H. Zhang, X. G. Zhou and X. H. Zhong, Inorg. Chem., 2011, 50, 10432–10438 CrossRef CAS PubMed.
- R. S. Zeng, M. Rutherford, R. G. Xie, B. S. Zou and X. G. Peng, Chem. Mater., 2010, 22, 2107–2113 CrossRef CAS.
- N. Pradhan and X. G. Peng, J. Am. Chem. Soc., 2007, 129, 3339–3347 CrossRef CAS PubMed.
- R. S. Zeng, T. T. Zhang, G. Z. Dai and B. S. Zou, J. Phys. Chem. C, 2011, 115, 3005–3010 CAS.
- A. Hazarika, A. Layek, S. De, A. Nag, S. Debnath, P. Mahadevan, A. Chowdhury and D. D. Sarma, Phys. Rev. Lett., 2013, 110, 267401 CrossRef.
- D. J. Norris, N. Yao, F. T. Charnock and T. A. Kennedy, Nano Lett., 2001, 1, 3–7 CrossRef CAS.
- W. Chen, A. G. Joly, J. Malm, J. Bovin and S. P. Wang, J. Phys. Chem. B, 2003, 107, 6544–6551 CrossRef CAS.
- M. Tanaka and Y. Masumotoa, Chem. Phys. Lett., 2000, 324, 249–254 CrossRef CAS.
- W. Chen, F. H. Su, G. H. Li, A. G. Joly, J. Malm and J. Bovin, J. Appl. Phys., 2002, 92, 1950–1955 CrossRef CAS.
- Y. M. Zhao, C. Riemersma, F. Pietra, R. Koole, C. M. Donegá and A. Meijerink, ACS Nano, 2012, 6, 9058–9067 CrossRef CAS PubMed.
- V. Bachmann, C. Ronda and A. Meijerink, Chem. Mater., 2009, 21, 2077–2084 CrossRef CAS.
- J. J. Zheng, X. Yuan, M. Ikezawa, P. T. Jing, X. Y. Liu, Z. H. Zheng, X. G. Kong, J. L. Zhao and Y. Masumoto, J. Phys. Chem. C, 2009, 113, 16969–16974 CAS.
- J. S. Park, S. W. Mho, J. C. Choi, H. L. Park, G. C. Kim, S. H. Lee and J. S. Kim, J. Korean Phys. Soc., 2007, 50, 571–574 CrossRef CAS.
- F. H. Su, B. S. Ma, Z. L. Fang, K. Ding, G. H. Li and W. Chen, J. Phys.: Condens. Matter, 2002, 14, 12657–12664 CrossRef CAS.
- Y. G. Yang, O. Chen, A. Angerhofer and Y. C. Cao, Chem.–Eur. J., 2009, 15, 3186–3197 CrossRef CAS PubMed.
- N. Pradhan and D. D. Sarma, J. Phys. Chem. Lett., 2011, 2, 2818–2826 CrossRef CAS.
- R. Beaulac, P. I. Archer, S. T. Ochsenbein and D. R. Gamelin, Adv. Funct. Mater., 2008, 18, 3873–3891 CrossRef CAS.
- H.-Y. Chen, S. Maiti and D. H. Son, ACS Nano, 2012, 6, 583–591 CrossRef CAS PubMed.
- Y. Hefetz, W. C. Goltsos, A. V. Nurmikko, L. A. Kolodziejski and R. L. Gunshor, Appl. Phys. Lett., 1986, 48, 372–374 CrossRef CAS.
- F. V. Mikulec, M. Kuno, M. Bennati, D. A. Hall, R. G. Griffin and M. G. Bawendi, J. Am. Chem. Soc., 2000, 122, 2532–2540 CrossRef CAS.
- S. C. Erwin, L. J. Zu, M. I. Haftel, A. L. Efros, T. A. Kennedy and D. J. Norris, Nature, 2005, 436, 91–94 CrossRef CAS PubMed.
- H. Menkara, R. A. Gilstrap, Jr, T. Morris, M. Minkara, B. K. Wagner and C. J. Summers, Opt. Express, 2011, 19, 972–981 CrossRef PubMed.
- B. Yang, J. Zhang, Y. Cui and K. Wang, Appl. Opt., 2011, 50, 137–141 CrossRef PubMed.
- D. Valerini, A. Cretí, M. Lomascolo, L. Manna, R. Cingolani and M. Anni, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 235409 CrossRef.
- P. T. Jing, J. J. Zheng, M. Ikezawa, X. Y. Liu, S. Z. Lv, X. G. Kong, J. L. Zhao and Y. Masumoto, J. Phys. Chem. C, 2009, 113, 13545–13550 CAS.
- E. J. McLaurin, V. A. Vlaskin and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 14978–14980 CrossRef CAS PubMed.
- V. A. Vlaskin, N. Janssen, J. V. Rijssel, R. Beaulac and D. R. Gamelin, Nano Lett., 2010, 10, 3670–3674 CrossRef CAS PubMed.
- D. Valerini, A. Cretí, M. Lomascolo, L. Manna, R. Cingolani and M. Anni, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 235409 CrossRef.
- R. Beaulac, P. I. Archer and D. R. Gamelin, J. Solid State Chem., 2008, 181, 1582–1589 CrossRef CAS PubMed.
- K. P. Kadlag, M. J. Rao and A. Nag, J. Phys. Chem. Lett., 2013, 4, 1676–1681 CrossRef CAS.
- H.-Y. Chen and D. H. Son, Isr. J. Chem., 2012, 52, 1016–1026 CrossRef CAS.
- G. Morello, M. Anni, P. D. Cozzoli, L. Manna, R. Cingolani and M. D. Giorgi, J. Phys. Chem. C, 2007, 111, 10541–10545 CAS.
- J. F. Suyver, J. J. Kelly and A. Meijerink, J. Lumin., 2003, 104, 187–196 CrossRef CAS.
- W. C. Zhou, R. B. Liu, D. S. Tang, X. X. Wang, H. M. Fan, A. Pan, Q. L. Zhang, Q. Wan and B. S. Zou, Nanotechnology, 2013, 24, 055201 CrossRef CAS PubMed.
- H.-Y. Chen, S. Maiti, C. A. Nelson, X. Y. Zhu and D. H. Son, J. Phys. Chem. C, 2012, 116, 23838–23843 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis of Mn:ZnS, Mn:ZnSe, and Mn:ZnSeS QDs. TEM images of MnS core and Mn-doped ZnSe, ZnSe0.73S0.27, and ZnSe0.22S0.78 QDs. Temperature-dependent PL intensities and lifetimes of Mn:ZnSe QDs. Temperature-dependent PL spectra of Mn:ZnSe0.73S0.27, Mn:ZnSe0.22S0.7, and CdSe/CdZnS/ZnS core–shell QDs. Temperature-dependent integrated PL intensities of Mn:ZnS (4.5 ML ZnS), Mn:ZnSe0.73S0.27, Mn:ZnSe0.22S0.7, and CdSe/CdZnS/ZnS core–shell QDs in heating–cooling circle experiments. See DOI: 10.1039/c3nr04319c |
| This journal is © The Royal Society of Chemistry 2014 |