Optically enhanced charge transfer between C60 and single-wall carbon nanotubes in hybrid electronic devices†
Christopher S.
Allen
*a,
Guoquan
Liu
a,
Yabin
Chen
b,
Alex W.
Robertson
a,
Kuang
He
a,
Kyriakos
Porfyrakis
a,
Jin
Zhang
b,
G. Andrew D.
Briggs
a and
Jamie H.
Warner
a
aDepartment of Materials, University of Oxford, Parks Road, Oxford, OX1 3PH, UK. E-mail: christopher.allen@materials.ox.ac.uk
bCenter for Nanochemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, P. R. China
First published on 11th November 2013
Abstract
In this article we probe the nature of electronic interactions between the components of hybrid C60–carbon nanotube structures. Utilizing an aromatic mediator we selectively attach C60 molecules to carbon nanotube field-effect transistor devices. Structural characterization via atomic force and transmission electron microscopy confirm the selectivity of this attachment. Charge transfer from the carbon nanotube to the C60 molecules is evidenced by a blue shift of the Raman G+ peak position and increased threshold voltage of the transistor transfer characteristics. We estimate this charge transfer to increase the device density of holes per unit length by up to 0.85 nm−1 and demonstrate further optically enhanced charge transfer which increases the hole density by an additional 0.16 nm−1.
Introduction
In order to fully exploit the potential of carbon nano-materials it is necessary to establish a comprehensive understanding of their interactions with other molecular species. Now that the fundamental physical properties of carbon nanotubes (CNTs) are relatively well understood,1–3 attention is increasingly turning towards hybrid CNT–molecular systems.4 Of primary concern for the development of CNT electronics is understanding how the presence of a particular molecule might affect conduction through a CNT and the mechanism behind this interaction.Motivated primarily by the potential for chemical sensing applications, the effect of the physisorption of various molecules on the properties of nanotube field-effect transistors (CNTFETs) has recently been studied.5–13 Changes in FET characteristics have been attributed to mechanisms such as charge-transfer doping14 or Schottky barrier modulation.5 However, for the development of complex hybrid CNT systems a more controllable functionalization process is required.
Both covalent and non-covalent routes are being explored for the robust and controllable functionalization of single-wall carbon nanotubes (SWCNTs).15 Non-covalent functionalization is of particular interest as this should preserve the sp2 lattice and consequently the excellent electronic properties of the host tube.16 Pyrene groups have been shown to be an effective anchor for attaching compounds to SWCNTs16–18 due to a strong π–π interaction between this aromatic molecule and the graphite basal plane.19 Charge transfer between compounds non-covalently bonded to CNTs has been studied both theoretically20 and experimentally by observing modification of the electronic13,21 and opto-electronic properties of CNTFETs.22,23 As the technical groundwork for producing hybrid CNT structures has been established the challenge of understanding these increasingly complex systems can be addressed.
Buckminsterfullerene (C60) and other closely related carbon allotropes have excellent electron accepting properties for use in organic photo-voltaic devices.24 Furthermore these molecules can be used as protective cages encapsulating atoms of a wide range of elements. Measurements of exceptionally long spin coherence times in these endohedral fullerene molecules,25 have led to proposals for their utilization as individual spin qubits, the basic building block of a quantum computer.26 However, electronically addressing anything but large ensembles of fullerene molecules remains challenging. The use of carbon nanotubes as conducting scaffolds for few or even individual molecules shows great potential for exciting advances in molecular electronics.17 Composite systems in which fullerenes interact non-covalently with the sidewalls of CNTs without any intermediary have been developed with potential applications in solar cell technology.27,28 There has also already been some success in attaching C60 to SWCNTs via non-covalent π–π interactions using various aromatic mediators including pyrene.29,30 Evidence of photo-induced charge transfer between the C60 molecule and SWCNT has been demonstrated via optical31–34 and electronic measurements.35
In this work we experimentally investigate the nature of charge transfer in a SWCNT–C60 hybrid system. Using a non-covalent π–π interaction we attach N-methyl-2-pyrenyl-C60 (Pyr-C60) molecules to CNTFET devices and observe signatures of optically activated charge transfer between the carbon nanotube and Pyr-C60.
Experimental
Ultra-long SWCNTs were grown by flow directed chemical vapor deposition onto a heavily doped SiO2/Si substrate using a Fe(OH)3 or FeCl3 catalyst.36 FET devices with Cr/Au electrodes were fabricated using standard electron beam lithography techniques and thermal evaporation. By using ultra-long carbon nanotubes we have been able to pattern multiple FET devices onto a single tube (Fig. 1a and b).Completed FET devices were wire bonded into a ceramic chip holder and electronic measurements performed in ambient conditions using a HP4142B modular DC source/monitor unit. Devices were screened via their electronic properties to locate semiconducting tubes. These were then functionalized by drop-casting a solution of 1.25 × 10−4 M N-methyl-2-pyrenyl-C60 (Fig. 1c)37 in toluene onto the chip and allowing to dry in air. A schematic of a completed, functionalized device is shown in Fig. 1d.
Atomic force microscopy images were recorded in tapping mode using a Park Scientific CP-II. Raman spectroscopy was performed using a Horiba Jobin Yvon LabRam Aramis system with 532 nm excitation wavelength.
The carbon nanotubes used for the TEM studies were FH-P nanotubes obtained from Meijo Nanocarbon. They were dispersed by sonication in 1,2-dichloroethane for 20 minutes. Two drops of this solution were then applied to lacey carbon TEM grids.
TEM images were recorded at 80 keV using either a JEOL 2010 or Oxfords aberration corrected JEOL 2200 MCO.
Results and discussion
Atomic force microscopy
AFM scans of a completed CNTFET device prior to functionalization are shown in Fig. 2a and c. Line profiles across the pristine tube (Fig. 2b, black trace) give a mean height of 1.5 ± 0.1 nm, confirming the individual single wall nature of this tube. After the addition of the Pyr-C60 group (Fig. 2c) the apparent height of the SWCNT was measured as 2.8 ± 0.1 nm (Fig. 2b, red trace) an increase of ∼1.3 nm compared to the pristine device. This increase is almost twice the theoretical C60 diameter of 0.71 nm.38 AFM measurements of C60 molecules self-assembled in hexagonal close-packed mono- and bi-layers have been measured to have apparent heights of ∼1 nm and ∼2 nm respectively.39Insight into the degree of coverage achieved by functionalization can be gained by examining how apparent height varies along the tube. The height of the SWCNT was extracted from every line-scan in each of the AFM images (Fig. 2e, red line). The height of the pristine tube (Fig. 2f, blue trace) is fairly consistent across the entire AFM image with a standard deviation of σ = 0.16 nm, comparable with the substrate roughness of 0.15 nm (see ESI, Fig. S1†). The functionalized tube (Fig. 2f, red trace) is considerably rougher with standard deviation of σ = 0.78 nm and measured heights ranging from that of the pristine tube to over 4 nm. This inconsistency in measured height indicates incomplete coverage of the SWCNT by the Pyr-C60 moiety with some sections of the tube remaining pristine and others having a covering of thickness equivalent to over three times the diameter of a C60 molecule.
Analysis of the AFM data also allows us to comment on the selectivity of the deposition of the Pyr-C60 moiety to the CNT. After drop-casting Pyr-C60 in toluene on the device, the roughness of the Si substrate doubled from 0.15 nm to 0.30 nm (see ESI, Fig. S1†) indicating that some material has been deposited on the substrate. This is, however less than a quarter of the increase in roughness measured along the tube illustrating the preferential attachment of the Pyr-C60 groups to the SWCNT due to the relatively strong π–π bonding. Due to the finite size of the AFM tip used to image the SWCNT device it is not possible to get lateral resolution of the order necessary to resolve individual C60 molecules.
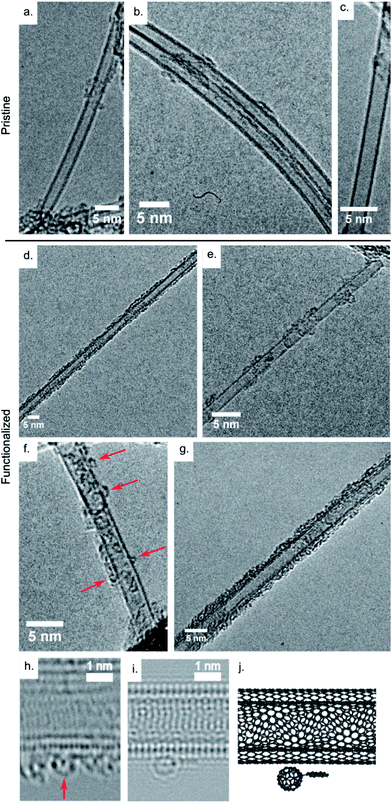
Transmission electron microscopy
Fig. 3a–c show high resolution transmission electron microscopy (HRTEM) images of individual or small bundles of pristine SWCNTs and double-wall carbon nanotubes (DWCNTs). All TEM images were recorded at an accelerating voltage of 80 keV, below the threshold for knock on damage in carbon nanotubes.40 Small amounts of amorphous material can be seen on the tube surfaces, a residue of the growth or dispersion procedures. Fig. 3d–g show HRTEM images of individual SW- and DWCNTs after functionalization with Pyr-C60 showing a high degree of coverage with amorphous material. Careful inspection of Fig. 3d–g reveals numerous circular projections with diameters of ∼1 nm (highlighted with red arrows in Fig. 3f) on the surface of the CNTs. We interpret these as projections of individual C60 molecules. The unambiguous determination of the presence of C60 molecules from HRTEM images is challenging as the presence of amorphous material complicates the interpretation. Fullerene molecules can react with neighbouring species under irradiation from an electron beam,41,42 further complicating the imaging of these structures. To confirm the presence of C60 molecules on the surface of the CNTs we have performed aberration corrected high resolution transmission electron microscopy (ac-HRTEM) of the functionalized tubes.Fig. 3h shows an ac-HRTEM image of a DWCNT after functionalization with the Pyr-C60 moiety. There is significant amorphous material on the surface of the DWCNT. Highlighted with a red arrow is a structure which we interpret as a C60 molecule. Fig. 3i shows an ac-HRTEM image simulation of the Pyr-C60 molecule adsorbed on the surface of a DWCNT, and Fig. 3j the atomic model used to create the simulation. In both the simulated and experimental image the projection of C60 molecule appears as a circular form with pronounced dark spots on its perimeter and a single dark spot at its centre. Line scans across the experimental and simulated images of the C60 molecules (ESI, Fig. S2†) give apparent diameters of 0.71 ± 0.05 nm and 0.68 ± 0.05 nm respectively. This good agreement provides strong evidence to support our interpretation.
Raman spectroscopy
The Raman G band spectra from the pristine SWCNT are shown in Fig. 4a. Raman spectra were recorded across the device (scan positions are shown in Fig. 4e) with the signal intensity corresponding to the area of exposed SWCNT. We could not resolve the radial breathing mode of the Raman spectra for this device. The Raman spectra shows a split G peak with a double-Lorentzian line shape as would be expected for a semiconducting SWCNT (Fig. 4b). The G peak of a semiconducting SWCNT comprises two dominant components, labeled G− and G+ corresponding to circumferential and axial vibrations respectively. We can estimate the tube diameter from the relative positions of the split G peak using the simple relationship proposed by Jorio et al.43ωG− = ωG+ − C/dt2, with ωG− and ωG+ correspond to the frequency of the G− and G+ peaks respectively. C is a constant which for semiconducting tubes takes the value of 47.7 cm−1 and dt is the tube diameter. Lorentzian fits to the two dominant G peaks in the Raman spectra of the pristine tube (Fig. 4b) give peak positions of ωG+ = 1591.7 cm−1 and ωG− = 1576.5 cm−1. From this we estimate a tube diameter of dt ∼1.8 nm, somewhat larger than previously measured by AFM.The position of Raman G+ band of a semiconducting SWCNT is related to its Fermi level due to strong electron-phonon coupling in resonant Raman spectroscopy.44 It is therefore possible to detect doping via Raman spectroscopy of graphitic structures. Indeed, shifts in the position of the G+ band with chemical doping have been reported in Raman studies of intercalated graphite,45 carbon nano-horns46 and nanotubes47,48 with hole doping causing a small blue shift in ωG+ and electron doping a small red shift.
The Raman spectra of the device after functionalization with Pyr-C60 are shown in Fig. 4c. Lorentzian fits give peak positions of ωG+ = 1594.4 cm−1 and ωG− = 1578.5 cm−1 (Fig. 4d), a blue shift of the G+ peak by ΔωG+ = +2.7 cm−1 (Fig. 4f). This shift in ωG+ with functionalization corresponds to electron transfer from the SWCNT to the attached Pyr-C60 moieties, resulting in hole doping of the tube. Raman studies of electrostatic doping of SWCNTs have found shifts in the G+ peak of ΔωG+ ∼ +3 cm−1 for a decrease in charge density of ∼0.4 e.nm−1.49 We therefore tentatively estimate a charge transfer from the SWCNT to Pyr-C60 of ∼0.36 e nm−1.
Within the spatial resolution of this measurement (laser spot size ∼1 μm), the position of the G peak is consistent across the device and there is no appreciable increase in the amplitude of the disorder related D peak with functionalization (ESI, Fig. S3a†). No shift in G peak position was seen on treatment of CNTFET devices with the toluene solvent (ESI, Fig. S3b†).
Electronic measurements
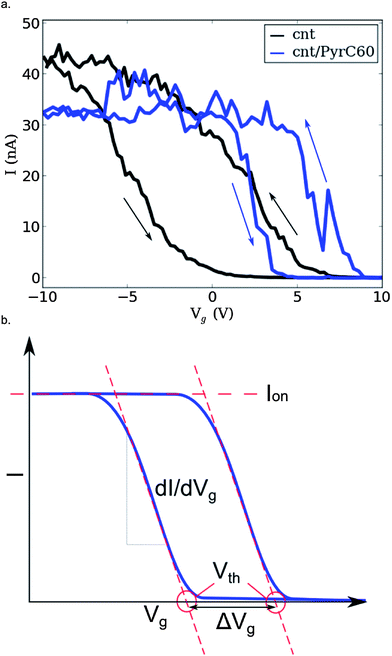
Fig. 5a shows the transfer characteristics of a CNTFET device with channel length of ∼250 nm before and after functionalization with the Pyr-C60 moiety The pristine device (black curve) shows p-type semiconducting behavior typical of CNTFETs measured in ambient conditions,50 and hysteresis due to accumulation of charge in response to the application of the gate voltage.51After functionalization with the Pyr-C60 moiety (Fig. 5a, blue curve) there is a change in the device transfer characteristics. In order to identify the mechanism underlying this change we concentrate on three key parameters shown schematically in Fig. 5b. Firstly the transconductance, dI/dVg, of a FET is related to the mobility of the device, the height of any Schottky barrier between the electrodes and carbon nanotube and the capacitive coupling to the gate electrode. Secondly, the gate voltage at which the device is turned on, the threshold voltage Vth, is a consequence of the band alignment between electrode and carbon nanotube. Finally, the on-state conductance of the device, Gon = Ion/Vsd, is determined by the sum of the contact resistances and the channel resistance.
On functionalization there is a small drop in the on-state conductance from Gpon ∼ 8.6 μS in the pristine CNTFET device to Gfon ∼ 6.5 μS. There is also an increase in the transconductance of the device from which the device field-effect mobility can be calculated using52
 | (1) |
 | (2) |
ε = 2.45 is the average of the dielectric constant of silicon (ε = 3.9) and air (ε = 1), tox = 300 nm the oxide thickness and dt = 1.7 nm the tube diameter. After functionalization the peak field-effect mobility almost doubles from μpFE = 20.7 cm2 V−1 s−1 in the pristine device to μfFE = 37.7 cm2 V−1 s−1. There is a clear shift in the transfer curve to more positive Vg with the up sweep threshold voltage shifting from Vpth,u = −2.2 V for the pristine SWCNT device to Vfth,u = +4.7 V after functionalization, a shift of δVth,u = +6.9 V. Similarly the down sweep threshold voltage changes from Vpth,d = +5.7 V for the pristine device to Vfth,d = +8.4 V post functionalization, a shift of δVth,d = +2.7 V. There is an appreciable reduction in the transfer hysteresis with functionalization from ΔVpg = 7.9 V to ΔVfg = 3.7 V.
The effect of functionalization on the transfer characteristics of a CNTFET has been addressed in detail by Heller et al.5 Here we will consider the possible physical mechanism behind each of the observed changes individually. Firstly the drop in on-state conductance with functionalization is attributed to an increase in scattering along the carbon nanotube after attachment of the Pyr-C60 molecule. Secondly, the increase in transconductance dI/dVg can be explained in terms of a decrease in Schottky barrier height for hole conduction between the electrodes and SWCNT caused by the local modification of work-function at the electrode-SWCNT interface.53 Finally, the observed shift in threshold voltage δVth can be understood in terms of the transfer of charge from the SWCNT to the Pyr-C60 molecule modifying the Fermi level in the conduction channel thus altering the gate voltage required for full carrier depletion, effectively hole-doping the device.
We can make a simple estimation of the change in carrier density (in this case holes) on our device from the measurement of δVth.52 The change in charge on the CNTFET can be written δQ = CδVth and the corresponding change in carrier density δp = δQ/eL. For our device with estimated capacitance of C = 4.9 × 10−18 F, channel length of L = 250 nm and δVth,u = +6.9 V (δVth,d = +2.7 V) we calculate an increase in carrier density of δpu = 0.85 nm−1 (δpd = 0.33 nm−1) or the addition of 212 (83) holes to the 250 nm long CNTFET device. This maximum change in charge density of 0.85 nm−1 is over twice that estimated from the G+ peak shift in the Raman data.
A similar conclusion has been reached by Shen et al. to explain the combined p doping and drop in on-state conductance in composite CNT–C60 FET devices.35 Other studies on the effect of molecules adsorbed onto the surface of CNTFET devices have estimated charge transfer of 0.04 electrons per NH3 molecule,14 0.37 electrons per porphyrin molecule22 and 2.2 electrons per pyrene-CdSe moiety.23
The decrease in hysteresis of ΔVpg − ΔVfg = 2.5 V in the transfer curve after functionalization suggests that the Pyr-C60 molecule is to some extent displacing other surface mobile species which screen the applied gate voltage.
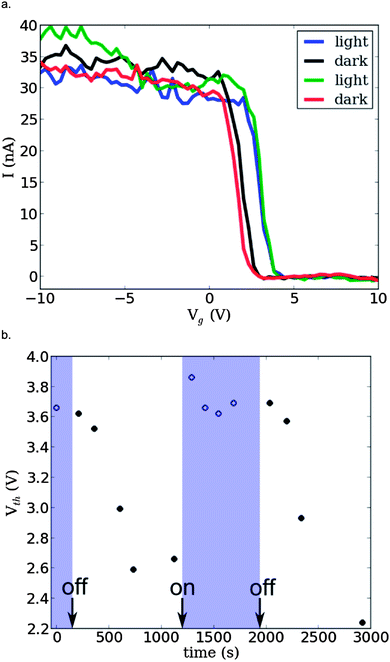
Fig. 6 shows the change in transfer characteristics of the device when measured in the dark and under illumination with white light (150 W halogen bulb). The peak mobility of the device is unchanged upon illumination (44.1 cm2 V−1 s−1 in the dark and 44.3 cm2 V−1 s−1 under illumination). The threshold voltage shifts from Vup,dth = 3.9 V to Vup,lth = 5.2 V on the up sweep, a shift of δVupth = 1.3 V, corresponding to the addition of ∼40 holes to the device or an increase in hole density of ∼0.16 nm−1. The change in threshold voltage for the down sweep is considerably less (Vdown,dth = 6.9 V, Vdown,lth = 70 V, δVdownth = 0.1 V). We also observe a significant reduction in the transfer hysteresis of the device from ΔVdg = 3.0 V when measured in the dark to ΔVlg = 1.8 V under illumination, a reduction of ∼1.2 V. There was no appreciable change in the transfer characteristics of the pristine device with illumination (see ESI, Fig. S4†).
 | ||
| Fig. 6 (a) Transfer characteristics of a functionalized carbon nanotube FET measured in the dark (black curve) and under illumination from a 150 W halogen lamp (blue curve). (b) Schematic energy level diagram for the optically activated charge separation process with redox potentials for a (6,5) SWCNT marked.58 | ||
Previous reports on similar Pyr-C60–SWCNT systems have explained photo-excited charge separation in terms of interactions between the host SWCNT and attached C60 molecules.31 It is, however, important to consider the role of the pyrene molecule in any observed effect,54 particularly any optical response.55,56 In order to determine the degree to which the response of our Pyr-C60–SWCNT system is due to just the pyrene molecule control experiments were performed in which SWCNT–FET devices were functionalized with ∼1.5 M pyrene in toluene. We observed a small negative shift of Vth on functionalization and no discernable optical response (see ESI, Fig. S5†). We therefore ascribe the optical enhancement of the doping effect to interactions between the host SWCNT and C60 molecule.
The mechanism of photo-excited charge separation in Pyr-C60–SWCNT hybrid systems (shown schematically in Fig. 6b) has been determined from optical measurements.31 The photo-excitation of an electron from the highest-occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) in the C60 molecule allows an electron from the valence band of the SWCNT to hop into the vacant C60 HOMO state. This leaves the SWCNT hole doped and causes the shift to more positive Vg observed in our CNTFET measurements. As this mechanism is dependent on the precise band alignment between the SWCNT and C60 molecule it is sensitive to the size of the band-gap in the semiconducting SWCNT and thus the tube chirality.57
Fig. 7a shows the reproducibility of the photo-switching process. In order to minimize hysteresis these measurements were performed using a pulsed gate technique (see ESI, Fig. S6a†)59 and we only show the up sweep for clarity (representative full hysteresis curves are shown in ESI, Fig. S6b†). There is a reproducible threshold voltage shift from Vth ∼3.7 V in the illuminated case to Vth ∼2.5 V in the dark, a change of δVth = 1.2 V, equivalent to that measured with the standard gate voltage sweep (Fig. 6a). Between each ‘light’ and ‘dark’ measurement shown in Fig. 7a the device was allowed to settle for approximately 15 minutes.
Fig. 7b shows the measured threshold voltages from pulsed gate measurements of the SWCNT–Pyr-C60 device as a function of time. The shaded regions correspond to periods of illumination and the times at which the halogen lamp was turned on and off are marked. During periods of illumination the measured Vth remains stable to within ± 0.2 V. Transfer curves measured immediately after the lamp is turned off show little discernible change in Vth. When measured after increasingly long periods in the dark Vg shifts towards lower values, plateauing at around 2.5 V. This observed behavior suggests that the charge transfer from the SWCNT to the C60 molecule which occurs on illumination is significantly faster than the transfer of charge back to the nanotube. This increase in time to recover the un-illuminated state is in qualitative agreement with other studies22,23 with persistent charge separation decay times as long as 1.8 × 103 s having been reported in functionalized SWCNT systems.60
Similar results were obtained from devices with 450 nm and 500 nm channel length fabricated on the same SWCNT as those presented in Fig. 5–7 (see ESI, Fig. S7 and S8†). However due to the inferior quality of the data we were unable to reliably extract doping densities.
Conclusions
In this work we have successfully incorporated fullerene molecules into CNTFET devices. Structural characterization has confirmed the presence of C60 molecules and the preferential nature of their attachment to the graphitic lattice of the carbon nanotubes.Through the change in Raman spectra and room temperature transport properties we have demonstrated charge transfer from individual SWCNTs to attached Pyr-C60 moieties. The absorbance of Pyr-C60 onto the surface a semiconducting SWCNT caused four distinct changes to the CNTFET transfer characteristics which can each be physical rationalized:
1. A small decrease in on state current after functionalization indicated an increase in scattering in the CNTFET conduction channel.
2. An increase in trans-conductance was attributed to a decrease in the SWCNT–electrode Schottky barrier height.
3. A shift in the threshold voltage can be explained in terms of hole doping of the carbon nanotube by the adsorbed Pyr-C60 molecule.
4. The decrease in device hysteresis was due to the displacement of other surface species by the adsorption of Pyr-C60 moieties onto the surface of the SWCNT.
The increase in density of holes after functionalization was found to be up to 0.85 nm−1. Due to the band alignment between C60 and the semiconducting SWCNT we were able to demonstrate optically excited charge transfer between the Pyr-C60 and SWCNT causing further hole doping of the device by an additional 0.16 nm−1.
We have demonstrated the feasibility of using electronic measurements to probe interactions between a SWCNT and exohedrally attached C60 molecules. This approach should be directly transferable to the wide range of endohedral metallo-fullerenes allowing for the study of the electronic states of exotic spin-active hybrid systems. Furthermore, by tailoring the size of the aromatic anchor group, it should be possible to control the density of C60 molecules decorating the SWCNT.
Acknowledgements
This work was supported by EPSRC (EP/H001972/1) and MOST (2011CB932601).Notes and references
- M. S. Dresselhaus, G. Dresselhaus, J. C. Charlier and E. Hernández, Electronic, Thermal and Mechanical Properties of Carbon Nanotubes, Philos. Trans. R. Soc., A, 2004, 362, 2065–2098 CrossRef CAS PubMed.
- P. Avouris, Z. Chen and V. Perebeinos, Carbon-Based Electronics, Nat. Nanotechnol., 2007, 2, 605 CrossRef CAS PubMed.
- M. P. Anantram and F. Leonard, Physics of Carbon Nanotube Electronic Devices, Rep. Prog. Phys., 2006, 69, 507–561 CrossRef CAS.
- T. Umeyama and H. Imahori, Photofunctional Hybrid Nanocarbon Materials, J. Phys. Chem. C, 2013, 117, 3195–3209 CAS.
- I. Heller, A. M. Janssens, J. Männik, E. D. Minot, S. G. Lemay and C. Dekker, Identifying the Mechanism of Biosensing With Carbon Nanotube Transistors, Nano Lett., 2008, 8, 591–595 CrossRef CAS PubMed.
- J. Kong, Nanotube Molecular Wires as Chemical Sensors, Science, 2000, 287, 622–625 CrossRef CAS.
- M. E. Roberts, M. C. LeMieux and Z. Bao, Sorted and Aligned Single-walled Carbon Nanotube Networks for Transistor-based Aqueous Chemical Sensors, ACS Nano, 2009, 3, 3287–3293 CrossRef CAS PubMed.
- K. Bradley, M. Briman, A. Star and G. Grüner, Charge Transfer from Adsorbed Proteins, Nano Lett., 2004, 4, 253–256 CrossRef CAS.
- M. Bockrath, J. Hone, A. Zettl, P. McEuen, A. Rinzler and R. Smalley, Chemical Doping of Individual Semiconducting Carbon-nanotube Ropes, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, R10606–R10608 CrossRef CAS.
- C. Klinke, J. Chen, A. Afzali and P. Avouris, Charge Transfer Induced Polarity Switching in Carbon Nanotube Transistors, Nano Lett., 2005, 5, 555–558 CrossRef CAS PubMed.
- B. R. Kang, W. J. Yu, K. K. Kim, H. K. Park, S. M. Kim, Y. Park, G. Kim, H.-J. Shin, U. J. Kim and E.-H. Lee, et al., Restorable Type Conversion of Carbon Nanotube Transistor Using Pyrolytically Controlled Antioxidizing Photosynthesis Coenzyme, Adv. Funct. Mater., 2009, 19, 2553–2559 CrossRef CAS.
- C. Klinke, A. Afzali and P. Avouris, Interaction of Solid Organic Acids with Carbon Nanotube Field Effect Transistors, Chem. Phys. Lett., 2006, 430, 75–79 CrossRef CAS PubMed.
- A. Star, T.-R. Han, J.-C. Gabriel, K. Bradley and G. Grüner, Interaction of Aromatic Compounds with Carbon Nanotubes: Correlation to the Hammett Parameter of the Substituent and Measured Carbon Nanotube FET Response, Nano Lett., 2003, 3, 1421–1423 CrossRef CAS.
- K. Bradley, J.-C. Gabriel, M. Briman, A. Star and G. Grüner, Charge Transfer from Ammonia Physisorbed on Nanotubes, Phys. Rev. Lett., 2003, 91, 218301 CrossRef.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chemistry of Carbon Nanotubes, Chem. Rev., 2006, 106, 1105–11036 CrossRef CAS PubMed.
- R. J. Chen, Y. Zhang, D. Wang and H. Dai, Noncovalent Sidewall Functionalization of Single-walled Carbon Nanotubes for Protein Immobilization, J. Am. Chem. Soc., 2001, 123, 3838–3839 CrossRef CAS.
- M. Urdampilleta, S. Klyatskaya, J.-P. Cleuziou, M. Ruben and W. Wernsdorfer, Supramolecular Spin Valves, Nat. Mater., 2011, 10, 502–506 CrossRef CAS PubMed.
- K. A. Shiral Fernando, Y. Lin, W. Wang, S. Kumar, B. Zhou, S.-Y. Xie, L. T. Cureton and Y.-P. Sun, Diminished Band-gap Transitions of Single-walled Carbon Nanotubes in Complexation with Aromatic Molecules, J. Am. Chem. Soc., 2004, 126, 10234–10235 CrossRef PubMed.
- C. A. Hunter and J. K. M. Sanders, The Nature of π–π Interactions, J. Am. Chem. Soc., 1990, 112, 5525–5534 CrossRef CAS.
- J. Zhao, J. P. Lu, J. Han and C.-K. Yang, Noncovalent Functionalization of Carbon Nanotubes by Aromatic Organic Molecules, Appl. Phys. Lett., 2003, 82, 3746 CrossRef CAS.
- A. Wurl, S. Goossen, D. Canevet, M. Sallé, E. M. Pérez, N. Martín and C. Klinke, Supramolecular Interaction of Single-Walled Carbon Nanotubes with a Functional TTF-Based Mediator Probed by Field-Effect Transistor Devices, J. Phys. Chem. C, 2012, 116, 20062–20066 CAS.
- D. S. Hecht, R. J. A. Ramirez, M. Briman, E. Artukovic, K. S. Chichak, J. F. Stoddart and G. Grüner, Bioinspired Detection of Light Using a Porphyrin-Sensitized Single-Wall Nanotube Field Effect Transistor, Nano Lett., 2006, 6, 2031–2036 CrossRef CAS PubMed.
- L. Hu, Y.-L. Zhao, K. Ryu, C. Zhou, J. F. Stoddart and G. Grüner, Light-Induced Charge Transfer in Pyrene/CdSe–SWNT Hybrids, Adv. Mater., 2008, 20, 939–946 CrossRef CAS.
- C.-Z. Li, H.-L. Yip and A. K.-Y. Jen, Functional Fullerenes for Organic Photovoltaics, J. Mater. Chem., 2012, 22, 4161 RSC.
- J. J. L. Morton, A. M. Tyryshkin, A. Ardavan, K. Porfyrakis, S. A. Lyon and G. A. D. Briggs, Electron Spin Relaxation of N@C60 in CS2, J. Chem. Phys., 2006, 124, 14508 CrossRef PubMed.
- S. C. Benjamin, A. Ardavan, G. A. D. Briggs, D. A. Britz, D. Gunlycke, J. Jefferson, M. A. G. Jones, D. F. Leigh, B. Lovett and A. N. Khlobystov, et al., Towards a Fullerene-based Quantum Computer, J. Phys.: Condens. Matter, 2006, 18, S867–S883 CrossRef CAS.
- T. Umeyama, N. Tezuka, S. Seki, Y. Matano, M. Nishi, K. Hirao, H. Lehtivuori, N. V. Tkachenko, H. Lemmetyinen and Y. Nakao, et al., Selective Formation and Efficient Photocurrent Generation of [70]Fullerene-Single-Walled Carbon Nanotube Composites, Adv. Mater., 2010, 22, 1767–1770 CrossRef CAS PubMed.
- V. C. Tung, J.-H. Huang, I. Tevis, F. Kim, J. Kim, C.-W. Chu, S. I. Stupp and J. Huang, Surfactant-Free Water-Processable Photoconductive All-Carbon Composite, J. Am. Chem. Soc., 2011, 133, 4940–4947 CrossRef CAS PubMed.
- G. Bottari, D. Olea, V. López, C. Gomez-Navarro, F. Zamora, J. Gómez-Herrero and T. Torres, Ordering phthalocyanine-C60 Fullerene Conjugates on Individual Carbon Nanotubes, Chem. Commun., 2010, 46, 4692–4694 RSC.
- D. M. Guldi, E. Menna, M. Maggini, M. Marcaccio, D. Paolucci, F. Paolucci, S. Campidelli, M. Prato, G. M. A. Rahman and S. Schergna, Supramolecular Hybrids of [60]fullerene and Single-wall Carbon Nanotubes, Chem.–Eur. J., 2006, 12, 3975–3983 CrossRef CAS PubMed.
- A. S. D. Sandanayaka, E. Maligaspe, T. Hasobe, O. Ito and F. D'Souza, Diameter Dependent Electron Transfer in Supramolecular Nanohybrids of (6,5)- or (7,6)-Enriched Semiconducting SWCNT as Donors and Fullerene as Acceptor, Chem. Commun., 2010, 46, 8749–8751 RSC.
- F. D'Souza, R. Chitta, A. S. D. Sandanayaka, N. K. Subbaiyan, L. D'Souza, Y. Araki and O. Ito, Supramolecular Carbon Nanotube-Fullerene Donor–Acceptor Hybrids for Photoinduced Electron Transfer, J. Am. Chem. Soc., 2007, 129, 15865–15871 CrossRef CAS PubMed.
- F. D'Souza and O. Ito, Photosensitized Electron Transfer Processes of Nanocarbons Applicable to Solar Cells, Chem. Soc. Rev., 2012, 41, 86–96 RSC.
- F. D'Souza, S. K. Das, A. S. D. Sandanayaka, N. K. Subbaiyan, D. R. Gollapalli, M. E. Zandler, T. Wakahara and O. Ito, Photoinduced Charge Separation in Three-Layer Supramolecular Nanohybrids: Fullerene–Porphyrin–SWCNT, Phys. Chem. Chem. Phys., 2012, 14, 2940–2950 RSC.
- Y. Shen, J. S. Reparaz, M. R. Wagner, A. Hoffmann, C. Thomsen, J.-O. Lee, S. Heeg, B. Hatting, S. Reich and A. Saeki, et al., Assembly of Carbon Nanotubes and Alkylated Fullerenes: Nanocarbon Hybrid Towards Photovoltaic Applications, Chem. Sci., 2011, 2, 2243 RSC.
- Y. Yao, X. Dai, C. Feng, J. Zhang, X. Liang, L. Ding, W. Choi, J.-Y. Choi, J. M. Kim and Z. Liu, Crinkling Ultralong Carbon Nanotubes into Serpentines by a Controlled Landing Process, Adv. Mater., 2009, 21, 4158–4162 CrossRef CAS.
- G. Liu, A. N. Khlobystov, A. Ardavan, G. A. D. Briggs and K. Porfyrakis, Photochemical Stability of N@C60 and Its Pyrrolidine Derivatives, Chem. Phys. Lett., 2011, 508, 187–190 CrossRef CAS PubMed.
- W. Krätschmer, L. D. Lamb, K. Fostiropoulos and D. R. Huffman, Solid C60: a New Form of Carbon, Nature, 1990, 347, 354–358 CrossRef.
- S. Burke, J. Mativetsky, R. Hoffmann and P. Grütter, Nucleation and Submonolayer Growth of C60 on KBr, Phys. Rev. Lett., 2005, 94, 096102 CrossRef CAS.
- F. Banhart, Irradiation Effects in Carbon Nanostructures, Rep. Prog. Phys., 1999, 62, 1181–1221 CrossRef CAS.
- M. Koshino, Y. Niimi, E. Nakamura, H. Kataura, T. Okazaki, K. Suenaga and S. Iijima, Analysis of the Reactivity and Selectivity of Fullerene Dimerization Reactions at the Atomic Level, Nat. Chem., 2010, 2, 117–124 CrossRef CAS PubMed.
- C. S. Allen, Y. Ito, A. W. Robertson, H. Shinohara and J. H. Warner, Two-Dimensional Coalescence Dynamics of Encapsulated Metallofullerenes in Carbon Nanotubes, ACS Nano, 2011, 5, 10084–10089 CrossRef CAS PubMed.
- A. Jorio, A. Souza Filho, G. Dresselhaus, M. Dresselhaus, A. Swan, M. Ünlü, B. Goldberg, M. Pimenta, J. Hafner and C. Lieber, et al., G-Band Resonant Raman Study of 62 Isolated Single-wall Carbon Nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 23–27 Search PubMed.
- J. Park, J. Sasaki, R. Saito, W. Izumida, M. Kalbac, H. Farhat, G. Dresselhaus and M. Dresselhaus, Fermi Energy Dependence of the G-Band Resonance Raman Spectra of Single- Wall Carbon Nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 081402 CrossRef.
- M. Dresselhaus and G. Dresselhaus, Intercalation Compounds of Graphite, Adv. Phys., 1981, 51, 1–186 CrossRef.
- S. Bandow, A. M. Rao, G. U. Sumanasekera, P. C. Eklund, F. Kokai, J. Takahashi, M. Yudasaka and S. Iijima, Evidence for Anomalously Small Charge Transfer in Doped Single-wall Carbon Nanohorn Aggregates with Li, K and Br, Appl. Phys. A, 2000, 71, 561–564 CrossRef CAS.
- A. Rao, P. Eklund, S. Bandow, A. Thess and R. Smalley, Evidence for Charge Transfer in Doped Carbon Nanotube Bundles from Raman Scattering, Nature, 1997, 191, 257–259 Search PubMed.
- T. Pichler, A. Kukovecz, H. Kuzmany and H. Kataura, Charge Transfer in Doped Single Wall Carbon Nanotubes, Synth. Met., 2003, 135–136, 717–719 CrossRef CAS.
- J. C. Tsang, M. Freitag, V. Perebeinos, J. Liu, P. Avouris, Doping and P. Renormalization, Carbon Nanotubes, Nat. Nanotechnol., 2007, 2, 725–730 CrossRef CAS PubMed.
- P. G. Collins, K. Bradley, M. Ishihami and A. Zettl, Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes, Science, 2000, 287, 180 CrossRef.
- Y. Pascal-Levy, E. Shifman, M. Pal-Chowdhury, I. Kalifa, T. Rabkin, O. Shtempluck, A. Razin, V. Kochetkov and Y. Yaish, Water-Assisted Mobile Charge Induced Screening and Origin of Hysteresis in Carbon Nanotube Field-Effect Transistors, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 1–9 CrossRef.
- R. Martel, T. Schmidt, H. R. Shea, T. Hertel and P. Avouris, Single- and Multi-wall Carbon Nanotube Field-effect Transistors, Appl. Phys. Lett., 1998, 73, 2447–2449 CrossRef CAS.
- J. Chen, C. Klinke, A. Afzali and P. Avouris, Self-aligned Carbon Nanotube Transistors with Charge Transfer Doping, Appl. Phys. Lett., 2005, 86, 123108 CrossRef.
- M. B. Lerner, J. M. Resczenski, A. Amin, R. R. Johnson, J. I. Goldsmith and A. T. C. Johnson, Toward Quantifying the Electrostatic Transduction Mechanism in Carbon Nanotube Molecular Sensors, J. Am. Chem. Soc., 2012, 137, 14318–14321 CrossRef PubMed.
- Rajesh, T. Sarkar and A. Mulchandani, Photo-Induced Charge Transport in ZnS Nanocrystals Decorated Single Walled Carbon Nanotube Field-Effect Transistor, Appl. Phys. Lett., 2011, 99, 173110 CrossRef.
- Y.-L. Zhai and J. F. Stoddart, Noncovalent Functionalization of Single-Walled Carbon Nanotubes, Acc. Chem. Res., 2009, 42, 1161–1171 CrossRef PubMed.
- C. M. Isborn, C. Tang, A. Martini, E. R. Johnson, A. Ortero-de-la-Roza and V. C. Tung, Carbon Nanotube Chirality Determines Efficiency of Electron Transfer to Fullerene in All-Carbon Photovoltaics, J. Phys. Chem. Lett., 2013, 4, 2914–2918 CrossRef CAS.
- Y. Tanaka, Y. Hirana, Y. Niidome, K. Kato, S. Saito and N. Nakashima, Experimentally Determined Redox Potentials of Individual (n,m) Single-Walled Carbon Nanotubes, Angew. Chem., Int. Ed. Engl., 2009, 48, 7655–7659 CrossRef CAS PubMed.
- D. Estrada, S. Dutta, A. Liao and E. Pop, Reduction of Hysteresis for Carbon Nanotube Mobility Measurements Using Pulsed Characterization, Nanotechnology, 2010, 21, 085702 CrossRef PubMed.
- R. F. Khairoutdinov, L. V. Doubova, R. C. Haddon and L. Saraf, Persistent Photoconductivity in Chemically Modified Single-Wall Carbon Nanotubes, J. Phys. Chem. B, 2004, 108, 19976–19981 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: AFM line scans of the substrate before and after functionalization; scheme for measuring amorphous carbon coverage from TEM images; diameter comparisons of ac-TEM image and simulation of C60 molecule; Raman spectra D peak comparison; optical response of transfer properties of pristine devices; comparison between swept and pulsed Vg measurements; functionalization effect and optical response of the transfer characteristics of a 550 nm and a 450 nm CNTFET device. See DOI: 10.1039/c3nr04314b |
| This journal is © The Royal Society of Chemistry 2014 |