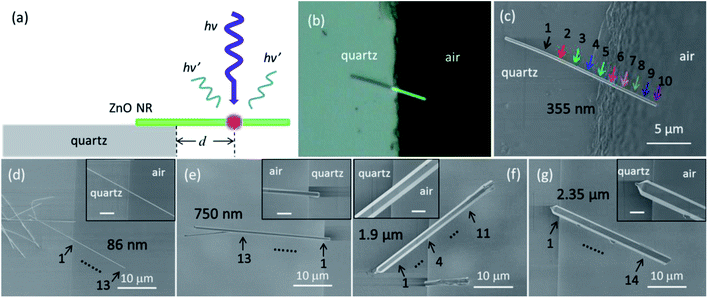
Substrate-induced effects on the optical properties of individual ZnO nanorods with different diameters†
Duan
Zhao
a,
Chao
Zhang
a,
Xiaoxian
Zhang
b,
Le
Cai
a,
Xiao
Zhang
a,
Pingshan
Luan
a,
Qiang
Zhang
a,
Min
Tu
a,
Yanchun
Wang
a,
Weiya
Zhou
a,
Zhiyuan
Li
a and
Sishen
Xie
*a
aBeijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: ssxie@iphy.ac.cn; Fax: +86-10-82640215; Tel: +86-010-8264-9081
bDepartment of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109, USA
First published on 26th September 2013
Abstract
We present the influence of a substrate on the properties of well-dispersed individual ZnO nanorods (NRs) with different diameters, especially on the photoluminescence (PL) properties. The studied ZnO NRs were partially supported by the quartz substrate and partially suspended in air. Continuous redshift and intensity decrease of the near band-edge emission (NBE) were observed along the suspended segment of the ZnO NRs due to the increasing temperature under UV laser excitation, suggesting that the presence of the substrate can effectively enhance the heat-sinking capability of ZnO NRs. Based on the PL measurements on individual suspended ZnO NRs with diameters from 86 nm to 2.35 μm, the redshift of NBE along the suspended segment was more obvious for ZnO NRs with a smaller diameter, indicating that the thermal conductive ability increases as diameter increases. Additionally, by combining the experimental and simulation results, we found that the presence of the substrate also quenched the whispering gallery modes (WGMs) of the ZnO NRs with a diameter above about 350 nm due to the symmetry breaking induced by the quartz substrate which has a larger refractive index compared with air. Our studies confirm that the substrate significantly influences the properties of ZnO NRs. To fully utilize the potential properties of nanomaterials for applications in nanodevices, the substrate-induced effects should be considered thoughtfully.
Introduction
For decades, nanomaterials have been intensively investigated for their remarkable properties, and they are expected to play important roles in applications of photonic, electronic, optoelectronic, and piezoelectric devices and so on.1–5 However, the dielectric environment critically influences the properties of nanomaterials. It has been reported that the optical and electrical properties of metal nanomaterials, carbon nanotubes (CNTs) and semiconductor NRs can be significantly influenced by the dielectric environment.6–18 For example, the surface plasmon resonant frequency of gold nanomaterials6 and the propagating surface plasmons on Ag NRs7 can be largely tuned by changing the bulk surrounding medium and by surface coating of dielectric layers with different thicknesses. Except for the noble metal nanomaterials, single-walled carbon nanotubes (SWCNTs), which have been intensively studied, it has also been reported that when in contact with a substrate, the PL and Raman signals from the SWCNTs can be quenched or tuned a lot.8,9 At the same time, the electrical transport characteristics of SWCNTs can be significantly influenced by the substrate.10,11 Besides, the dielectric environment was found to influence the semiconductor nanostructures as well. Especially, for ZnO NRs which are considered to be the potential material as the key unit in nanodevices,19–23 lots of work has been carried out to investigate the surface modification effect on their optical properties.12–18 It has been reported that great enhancement of NBE and quenching of the deep level emission (DLE) in ZnO NRs can be observed after surface coating of polymers, oxides or metals.12–18 Given above, the dielectric environment including the bulk surrounding medium and the substrate can significantly influence the properties of nanomaterials and hence the nanodevices. In order to exclude the influences induced by the substrate, studies focused on the suspended one-dimensional nanomaterials have been carried out.8,24–27Nevertheless, for individual ZnO NRs, little is known about how the substrate influences their properties. Since almost all the single ZnO NR-based nanodevices under investigation are substrate-supported, the interactions between the substrate and ZnO NRs will inevitably be introduced, and hence, the properties of the ZnO NRs will be significantly influenced. Accordingly, the performance of the nanodevices may not behave what we expect. Besides, the presence of the substrate may impede the investigation of the intrinsic properties of ZnO NRs. Thus, it is quite important to investigate the substrate-induced effects for individual ZnO NRs. In this work, we designed a simple system in which well-dispersed individual ZnO NRs were partially supported by the substrate and partially suspended in air, then we systematically investigated the optical properties of individual suspended ZnO NRs with different diameters. Combined with the axially directed position-dependent, excitation power-dependent and temperature-dependent PL measurements, the optical properties of the supported and suspended segments of the same ZnO NRs were studied. We found that the quartz substrate could enhance the heat-sinking capability but simultaneously could quench the WGMs of ZnO NRs. The possible mechanisms were fully discussed. We believe that the systematic investigation of substrate-induced effects on ZnO NRs with different diameters in this paper will provide a basic understanding and inspiration for substrate-supported applications of other nanostructures.
Experimental details
Fabrication of individual suspended ZnO NRs with different diameters
The ZnO NR arrays with different diameters from 80 nm to 2.5 μm were grown on silicon substrates by chemical vapor deposition (CVD) at 910 °C with Au films as the catalyst.15,28 The diameter of the ZnO NR array was mainly controlled by the chemical activity of the carbon powder which was used in the source materials.15 The as-grown ZnO NRs were first dispersed in alcohol solution. And then, the solution containing the ZnO NRs was dropped onto the quartz substrate. Thus, well dispersed individual ZnO NRs can be found on the substrate. Subsequently, the substrate was cut into several small pieces, and consequently, we found that on the edge of the substrate, a number of individual ZnO NRs with different diameters partially lay on the substrate and partially suspended in air.Characterization
The morphology of the samples was characterized by scanning electron microscopy (SEM, Hitachi S4800 and FEI Quanta 600) and optical microscopy. The PL spectra were obtained using a confocal microscopic optical system (JY-Horiba LabRAM HR800) with sub-μm spatial resolution at different temperatures which were controlled using a Linkam cryostat in the range of 80–673 K. A 325 nm He–Cd laser was used to excite the samples with a spot size of 2 μm through the optical lens which was vertical to the substrate. The emission signal was collected from the same position where the laser beam was focused through the same optical lens. To study the substrate-induced effects, a series of PL spectra were collected step by step along the axial direction of the ZnO NR from the supported segment to the suspended segment. For comparison, two different incident power densities were chosen. The higher power density (16 kW cm−2) named as D1 acted as the local heat source for the ZnO NR. The lower power density was about 1/10 of D1 and named as D2.Results and discussion
Morphology
Fig. 1(a) schematically illustrates the side view of the structure considered in this paper. d represents the distance between the edge of the substrate and the position where the PL spectrum is collected. Fig. 1(b) shows a typical top-view optical image of a suspended ZnO NR which is partially supported by the substrate and partially suspended in air with a diameter of about 355 nm. The corresponding SEM image is shown in Fig. 1(c). The arrows represent the positions where the PL spectra were collected. Fig. 1(d)–(g) show the typical SEM images of individual suspended ZnO NRs with diameters of about 86 nm, 750 nm, 1.9 μm and 2.35 μm, respectively. The insets show the corresponding magnified SEM images of the edge region. It can be seen that the ZnO NRs have flat and smooth side surfaces, and the ZnO NRs with large diameters exhibit well-defined hexagonal cross-sections with sharp surface edges. The corresponding optical images of these NRs are shown in Fig. S1,† from which we can easily distinguish the supported part from the suspended part. It shows that the strong interaction between the substrate and the ZnO NR can strongly support the long suspended ZnO NR, even though the contact area is extremely small.Thermal-induced redshift and intensity decrease of NBE on the suspended segment
In order to get a general idea how the substrate influences the optical properties of ZnO NRs, we started our research with carrying out the axially directed position-dependent PL measurements along a suspended ZnO NR with a diameter of about 355 nm (shown in Fig. 1(c)). Fig. 2(a) shows the corresponding PL spectra. Positions 1–4 locate on the supported segment, and position 5 locates on the edge of the substrate, while 6–10 locate on the suspended segment. All the PL spectra shown in Fig. 2(a) were measured under the same excitation conditions (D1) at room temperature (RT). There is no obvious change from lines 1 to 4, no matter the shape, intensity or position of the NBE peaks, that is, the PL spectra collected from the substrate-supported ZnO NR segment show very similar spectral signatures. However, once entering the suspended segment, the PL spectra exhibit dramatic changes as d increases from 5 to 10. Continuous redshift and intensity decrease of NBE can be observed. Besides, the shape of the peaks varies obviously from lines 4 to 6. In order to compare the peak position and shape more clearly, we normalized all the PL spectra in Fig. 2(a) and demonstrated the intensity-normalized PL spectra in Fig. 2(b). From lines 1 to 10, the NBE redshifts by a total of 21.8 nm (∼180 meV). As a contrast, we carried out the position-dependent PL measurements on a ZnO NR with a similar diameter totally lying on the quartz substrate under D1. Fig. S2(a) and (b)† exhibit the corresponding SEM image and the PL spectra, indicating that the variation of the position does not induce any noticeable change of the NBE peaks. Accordingly, it can be concluded that the dramatic changes from lines 5 to 10 in Fig. 2(a) are mainly attributed to the absence of the substrate.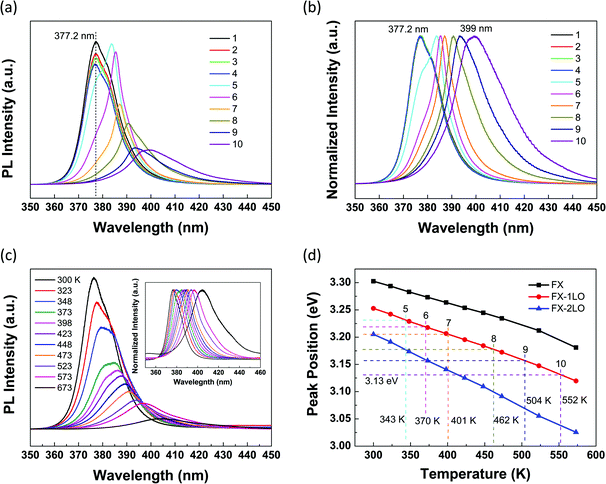 | ||
| Fig. 2 (a) PL spectra along the suspended ZnO NR shown in corresponding colors for each position in Fig. 1(c). All the PL spectra were obtained under D1. (b) Intensity-normalized PL spectra of (a). (c) Temperature-dependent PL spectra obtained from position 2 between 300 and 673 K. The inset shows the intensity-normalized PL spectra. (d) The energy of FX, FX-1LO and FX-2LO obtained from fitting as a function of temperature. The dashed lines mark the energy of FX-1LO and the corresponding temperatures at the suspended positions from 5 to 10. | ||
To figure out the physical mechanism of these spectral changes, we carried out the excitation power-dependent and temperature-dependent PL measurements. First, in order to exclude the laser-induced temperature increase, we decreased the power density of the laser beam to D2. Fig. S3† shows the corresponding intensity-normalized PL spectra. Lines 1–4 exhibit the same signature, which is very similar to that in Fig. 2(b), but the peak position of NBE is about 1.2 nm smaller than that obtained under D1. The redshift from lines 5 to 10 is not as obvious as that in Fig. 2(b). The above results are considered being attributed to the lower local temperature caused by the lower power density of D2. In addition, the shape of lines 4 and 5 still varies dramatically. Our results suggest that the continuous redshift and intensity decrease of NBE with d increase in Fig. 2(a) can be mainly attributed to the increase in local temperature induced by the laser excitation under D1, but the dramatic shape difference between lines 4 and 5 may be induced by other factors which will be discussed later.
To further prove our assumption, we carried out the temperature-dependent PL measurements on the same ZnO NR shown in Fig. 1(c). Since the PL spectra of 1–4 exhibit the same signature, we chose position 2 as an example to be measured at different temperatures between 300 and 673 K. Fig. 2(c) shows the corresponding PL spectra. Obviously, continuous redshift and intensity decrease of NBE can be observed as temperature increases. The intensity-normalized PL spectra are shown in the inset in Fig. 2(c). From 300 to 673 K, the peak position of NBE redshifts by a total of 26.4 nm (∼214 meV). The tendency of the spectral changes is the same as that shown in Fig. 2(a) and (b). Thus, it is considered that the temperature increase is the main reason for the dramatic changes of the peak position and intensity in Fig. 2(a).
The continuous redshift and intensity decrease of NBE with temperature increase can be explained by the following discussion. Due to the temperature-induced lattice dilatation and electron–lattice interaction, the band gap energy Eg decreases with temperature increase, complying with the Varshni formula,29
| Eg(T) = Eg(0) − αT2/(T + β) | (1) |
| EFX(T) = Eg(T) − 60 meV | (2) |
In order to identify the local temperatures at the suspended positions from 5 to 10, we carried out the process of peak fittings. First, to find the relationship between the energy of FX, FX-nLO and the temperature, the PL spectra obtained at each temperature in Fig. 2(c) were well fitted by three Lorentzian peaks (FX, FX-1LO and FX-2LO). Fig. S4† shows a typical fitting result for the PL spectrum obtained at 473 K. From the fitting results, we found that all the peaks redshift obviously as temperature increases. Besides, the relative intensity of FX decreases and FX-1LO increases obviously with temperature increase. And the FX-1LO emission predominates the NBE above around 373 K. While for the FX-2LO, the relative intensity increases slowly. Fig. 2(d) shows the energy positions of FX and FX-nLO as a function of temperature, and all exhibit approximately linear dependences on the temperature. Based on the fitting results, the FX-1LO almost predominates the NBE in the whole temperature range and the fitting error of the peak position is the smallest. Thus, the fitting result of FX-1LO in Fig. 2(d) is most reliable, and hence, we chose the red line in Fig. 2(d) as the standard for temperature identification. After a similar process of peak fittings, the PL spectra of 5–10 in Fig. 2(a) can also be fitted. Then, the energy of FX-1LO in each spectrum can be obtained and the local temperatures at the suspended positions can be deduced as the dashed lines show in Fig. 2(d). For example, for line 10 in Fig. 2(a), the energy of FX-1LO from fitting is about 3.13 eV, corresponding to the temperature of 552 K. It can be seen that the temperature increases dramatically at the suspended positions as d increases under D1, indicating that the heat-sinking capability of the ZnO NR reduces remarkably as d increases.
The mechanisms of heat transfer on both the supported and suspended segments can be explained as follows. For the supported segment, the local heat induced by the laser excitation is dissipated through the following approaches: the thermal conductance of the ZnO NR in the axial direction, the thermal contact conductance between the ZnO NR and the quartz substrate, the thermal convection between the ZnO NR and the air around, and the thermal radiation of the ZnO NR. While for the suspended segment, the surface area exposed to air increases, promoting the thermal convection, but the direct thermal contact conductance between the ZnO NR and the substrate exists no more. However, the thermal conductivity of air is about two to three orders of magnitude lower than that of the quartz crystal. Thus, the heat at the suspended segment cannot be dissipated effectively through air, and accordingly, the local temperature in this region is higher than the supported segment under the same excitation conditions. It can be seen that the thermal contact conductance plays a very important role in heat transfer, consistent with the results reported before.37 Furthermore, at the suspended positions, the effect of the substrate on transporting the local heat becomes weaker as d increases and thus the local temperature becomes higher. It is easy to imagine that as long as the suspended length is long enough, the effect of the substrate will disappear and the NBE will stop redshifting at certain positions. It is interesting that this phenomenon was observed in an 86 nm ZnO NR with a suspended length of 16 μm in our study, which will be discussed below.
Diameter-dependent thermal conductive ability of ZnO NRs
After studying the substrate-induced thermal effects on the properties of individual ZnO NRs, we carried out the axially directed position-dependent PL measurements on individual suspended ZnO NRs with different diameters shown in Fig. 1(d)–(g) to investigate the diameter-dependent thermal conductive ability. Notice that all the PL spectra were collected under D1. Fig. 3(a)–(d) show the position-dependent PL spectra of these four individual suspended ZnO NRs with diameters of about 86 nm, 750 nm, 1.9 μm and 2.35 μm, respectively. The measuring lengths of the suspended segment are about 16 μm, 18 μm, 12 μm and 20 μm respectively, as the arrows show in Fig. 1(d)–(g). Dashed lines are utilized in Fig. 3(a)–(d) to mark the peak positions of line 1. In Fig. 3(a), we can see that for the 86 nm ZnO NR, the NBE peak shifts by a total of 26.6 nm (∼216 meV). The series of peaks in Fig. 3(b)–(d) are related to the multi-mode behavior of the whispering gallery (WG) resonator which has a hexagonal cross-section and a large radius.38 In the case of the 750 nm ZnO NR, the average redshift for each peak is about 2.6 nm from lines 1 to 13 (Fig. 3(b)), which is much smaller than that in the ZnO NRs with smaller diameters of 86 nm and 355 nm. As the diameter increases, the average redshift further reduces, which is about 1.6 nm for the 2.35 μm NR. While for the 1.9 μm ZnO NR, the redshift is almost unobservable, which could be mainly attributed to the large diameter and the large contact area between the ZnO NR and the substrate on the supported segment. It is clear that the redshift of the NBE peak is more pronounced for the ZnO NR with a smaller diameter. As the diameter increases, the redshift becomes unnoticeable. Even though the lengths of suspended segments for various ZnO NRs studied here are slightly different, we can see from the above results that they will not influence the whole trend. It can be inferred that for a ZnO NR with a larger diameter, the local temperature at the suspended positions should be lower than that with a smaller diameter, which implies that the thermal conductive ability of the ZnO NR decreases as diameter decreases.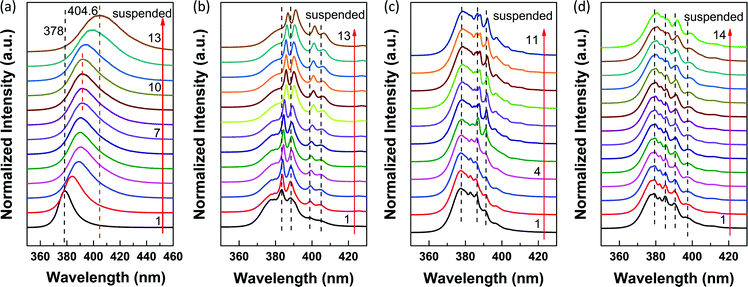 | ||
| Fig. 3 (a–d) Position-dependent PL spectra of the suspended ZnO NRs with diameters of about 86 nm, 750 nm, 1.9 μm and 2.35 μm, respectively. The corresponding SEM images are shown in Fig. 1(d)–(g). | ||
For semiconductors, the thermal conductivity consists of both lattice and electronic contributions. Since the carrier concentration in ZnO is normally less than 1019 cm−3,39,40 the calculated electronic thermal conductivity is lower than 10−3 W cm−1 K−1 which is negligible compared with the lattice portion,39,40 and thus only lattice contributes to the thermal conductivity. In other words, the phonons predominate the thermal conduction in ZnO. Both theoretical and experimental results have been obtained showing that the thermal conductivity decreases as the size of the nanostructures decreases.26,27,41–44 As the diameter of the ZnO NR decreases, the phonon interactions increase due to the size confinement, which causes the increase of thermal resistance and decrease of heat conduction.41 At the same time, the surface-to-volume ratio increases. The relatively large fraction of surface atoms enhances the surface scattering of phonons and decreases the phonon mean free path, which results in a lower conductivity.42 Besides, as the size decreases, the surface roughness becomes relatively large, which induces the less probability of specularity scattering and the more probability of diffusive scattering, further lowering the conductivity.41 Thus, it can be confirmed that the ZnO NR with a larger diameter has a better thermal conductivity and the local temperatures at the suspended positions are lower, contributing to the smaller redshift of NBE peaks as d increases. In addition, it has been reported that the thermal resistance decreases at the NR/substrate interface as the diameter of the NR increases.37,45 Thus, for the ZnO NR with a larger diameter, the local heat at the suspended segment can be more effectively conducted to the substrate, further decreasing the redshift of NBE peaks.
Following this conclusion, the ZnO NR with a diameter of 86 nm should have bad thermal conductivity. In Fig. 3(a), the peak position does not redshift continuously but remains unchanged for a short distance, which is seldom seen for ZnO NRs with large diameters. The observed phenomenon is attributed to the bad thermal conductivity and the long suspended length of this NR. Due to the bad thermal conductivity, the heat at the suspended positions cannot be conducted to the substrate effectively through the thermal conductance in the axial direction. When d reaches a critical value (dc), the effect of the substrate will disappear. We can imagine that dc increases as the diameter of the ZnO NR increases. Above dc, for a certain region, the local heat can be conducted to the lower-temperature region towards both directions along the c-axis of the ZnO NR. In this region, the temperature remains unchanged, such as lines 7–10 in Fig. 3(a). When the suspended position gets closer to the end side in air, the local heat can only be conducted effectively towards the substrate-direction along the c-axis. Thus, the local temperature becomes higher, contributing to the further redshift of NBE.
Substrate-induced quenching of the WGMs
Except for the intensity and peak position, we can see from Fig. 2(b) and S3† that the shape of the NBE peaks was also influenced significantly by the quartz substrate. From lines 4 to 6, the lineshape exhibits dramatic changes. In order to figure out the reason for the notable difference induced by the substrate, we carried out the temperature-dependent PL measurements between 80 and 290 K at 3 different positions along this NR with a diameter of 355 nm. Position 1 locates on the supported segment, and position 2 locates on the edge of the substrate, while position 3 locates on the suspended segment, as shown in Fig. S5(a).†Fig. 4(a) shows the corresponding PL spectra obtained at 80 K. For lines 2 and 3, a new peak (marked as N) arises at the lower energy side of the donor bound excitons (D0X), suggesting that the presence of the substrate quenches this peak at position 1. Additionally, all the peaks redshift and the relative intensity of the new peak gradually increases from 1 to 3, which is attributed to the higher temperature at the suspended positions. Fig. 4(b) and (c) show the temperature-dependent PL spectra of positions 1 and 3 from 80 to 290 K. The dashed lines mark the evolution of corresponding peaks. As temperature increases, the FX emission shifts to the lower energy. Simultaneously, D0X thermally dissociate into FX gradually, and totally dissociate above approximately 150 K, leading to stronger FX emission. In Fig. 4(b), above 150 K, the FX emission continues redshifting and merges with FX-1LO at 290 K due to the line broadening of each of these peaks. In Fig. 4(c), the evolution of the new peak is marked by the red dashed line. As temperature increases, the relative intensity of this new peak increases and it merges with the FX emission gradually. Due to the higher local temperature at position 3, the energy of FX is lower compared to that at position 1, and thus, the FX emission redshifts more seriously. Besides, as we have discussed above, the FX-1LO emission tends to predominate the NBE peak as temperature increases. Thus, the FX and FX-1LO merge at the lower energy side and the relative intensity of FX-1LO is higher in Fig. 4(c) at 290 K. The peak at the higher energy side in Fig. 4(c) at 290 K corresponds to the merge of the new peak and the FX emission. In brief, the new peak and the higher local temperature at the suspended positions contribute to the shape differences between lines 4 and 6 shown in Fig. 2(b) and S3.† The new peak arising at positions 2 and 3 is considered to be corresponding to the WGM in the hexagonal ZnO NR cavity in which multi-mode behavior resulting from WG resonance occurred.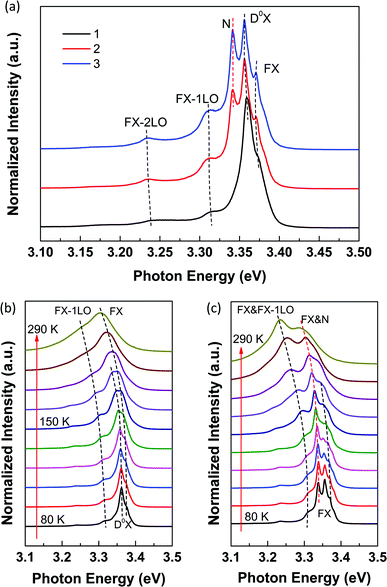 | ||
| Fig. 4 (a) PL spectra of 3 different positions obtained at 80 K. The corresponding SEM image is shown in Fig. S5(a).† The diameter of this NR is about 355 nm. Temperature-dependent PL spectra of positions 1 (b) and 3 (c) respectively between 80 and 290 K. | ||
To systematically investigate the substrate-induced effects on the WG resonance occurred in the ZnO NR cavity, we carried out massive PL measurements on a series of individual suspended ZnO NRs with various diameters ranging from 250 nm to 2 μm. Similar to Fig. 4(a), 3 to 4 different positions along the ZnO NRs were measured at 80 K. Position 1 is on the supported segment, and position 2 is on the edge of the substrate, while positions 3 and 4 are on the suspended segment. The obtained PL spectra of the ZnO NRs with different diameters are shown in Fig. 5. Fig. 5(a) shows the typical PL spectra of a suspended ZnO NR with a diameter below about 350 nm. Obviously, the signatures of the PL spectra obtained from these 3 positions are very similar. No new peaks can be observed at positions 2 and 3. While for ZnO NRs with diameters around 350 nm, a new peak arises at positions 2 and 3, as we can see from Fig. 4(a). With increasing diameter, more peaks arise and become more pronounced at the suspended positions. Fig. 5(b) shows the position-dependent PL spectra of a ZnO NR with a diameter of about 1.9 μm. The corresponding SEM image is shown in Fig. S5(b).† From the results above, we found that 350 nm was a critical diameter. Above around 350 nm, the quenching of WGMs in the ZnO NR occurs at the supported segment. While below 350 nm, WGMs cannot be observed even at the suspended segment in the PL spectral region. It suggests that for the ZnO NR around 350 nm the multi-mode behavior occurs in the UV region, but the presence of the substrate quenches the WGMs to a certain degree. Similar quenching results have been observed at 80 K in the ZnO NR with a diameter of about 1 μm after surface coating of the Al2O3 layer.15
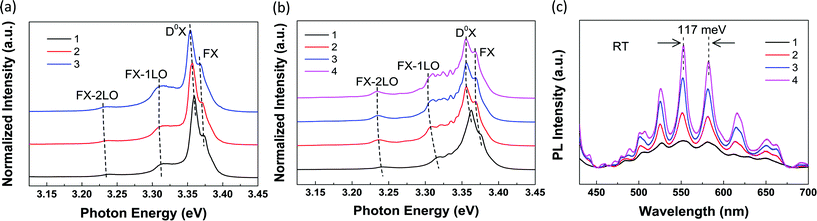 | ||
| Fig. 5 (a) PL spectra of 3 different positions along a ZnO NR with a diameter typically below about 350 nm at 80 K. (b) PL spectra of 4 different positions along a ZnO NR with a diameter of about 1.9 μm obtained at 80 K. The corresponding SEM image is shown in Fig. S5(b).† (c) Position-dependent PL spectra of the ZnO NR shown in Fig. S5(b)† in the visible region obtained at RT. | ||
Furthermore, we carried out the position-dependent PL measurements on the ZnO NR shown in Fig. S5(b)† at RT. A similar quenching phenomenon was also observed in the visible region. Fig. 5(c) shows the corresponding PL spectra. It can be seen that dramatic changes occur from lines 1 to 4. At position 1, the PL spectrum exhibits slight waves. On entering the suspended segment, the peaks become clearer and the intensity increases dramatically from 1 to 4. At position 4, the PL spectrum exhibits sharp and distinct oscillating peaks. The energy difference ΔE between two adjacent sharp oscillating peaks varies slightly around 117 meV, as shown in Fig. 5(c). The WGMs of the hexagonal cavity follow the equation:46
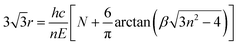 | (3) |
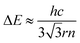 | (4) |
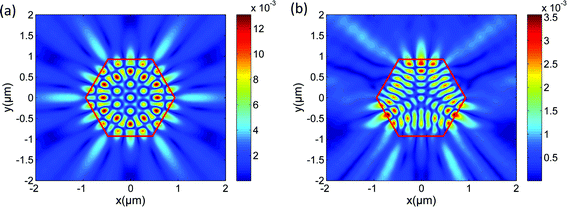
Further, to clarify the mechanism of the increasing cavity loss on the substrate, a 2D-FDTD method was utilized to simulate the resonant modes existing in the hexagonal ZnO NR cavity. The intensity patterns of the resonant modes were plotted. Fig. 6(a) and (b) show the typical intensity patterns at the same resonant frequency in the hexagonal cavity as surrounded by air and lying on the substrate. Based on our simulation results, the mode profile exhibits WGM type resonance which has a hexagonal symmetry within the cavity when surrounded by air, as shown in Fig. 6(a). However, when the ZnO NR lies on the substrate, the mode profile does not exhibit WGM type resonance any more but quasi-WGM type resonance which has a triangular symmetry,48,49 and simultaneously, the intensity decreases dramatically, as we can see from Fig. 6(b).
The change of the mode profile can be attributed to the symmetry breaking induced by the quartz substrate. Based on Sellmeier's dispersion functions for ZnO and SiO2,47 the refractive index of ZnO varies around 2.2 and the refractive index of SiO2 varies around 1.55 in the whole PL spectral region. The refractive index of air is considered to be a constant equals to 1, which is smaller than that of the quartz substrate. According to Snell's law, the calculated critical angle of the total internal reflection (TIR) θc is about 27° when surrounded by air, but increases to 45° at the NR/substrate interface. When surrounded by air, the dielectric environment around the NR is homogeneous and symmetrical. For the WGM resonance, the circulating ray is reflected by the six boundary surfaces of the cavity with an incident angle of 60° which is much larger than 27°. Thus, the WGM type circulating ray can be well confined and is stable in the cavity. While for the ZnO NR lying on the quartz substrate which has a larger refractive index than air, the surrounding dielectric environment is inhomogeneous and asymmetrical. If the WGM-like circulating ray strikes the NR/substrate interface, the ray will leak through the substrate seriously and the ray orbit may change due to the symmetry breaking, and consequently, this kind of WGM-like resonance is unstable in the hexagonal cavity on the substrate. Thus, the ray will choose a more stable orbit to circulate within the cavity. For the quasi-WGM resonance, the circulating ray is reflected by only three boundary surfaces of the cavity, which can avoid striking the NR/substrate interface. Compared with the WGM resonance, the quasi-WGM resonance is more stable in the NR cavity lying on the substrate. Accordingly, the mode profile changes. But for the quasi-WGM resonance, the incident angle is around 30° which is quite close to 27°, and consequently, more light can leak out of the cavity through these three boundary surfaces.48 Thus, though the quasi-WGM resonance is more stable, it is less confined within the cavity than the WGM resonance when surrounded by air. Accordingly, the cavity loss is higher for the NR on the substrate compared with the NR suspended in air. And thus, the intensity in the cavity decreases obviously, as we can see from Fig. 6(b).
Here, it can be confirmed that the presence of the substrate can quench the WGMs in ZnO NRs, which will inevitably influence the performance of the substrate-supported ZnO NR-based nanodevices or impede the investigation of the intrinsic optical properties of ZnO NRs.
Conclusions
In this paper, we carried out PL spectroscopy on individual ZnO NRs that were partially supported by the substrate and partially suspended in air to verify the substrate-induced effects on the properties of ZnO NRs. Besides, we carried out PL measurements on individual suspended ZnO NRs with different diameters. According to our results, the NBE exhibits continuous redshift and intensity decrease due to the increasing local temperature along the suspended ZnO NR segment under UV laser excitation, suggesting that the substrate can effectively conduct the heat from the ZnO NR. Additionally, we found that the thermal conductive ability increases as the diameter of the ZnO NR increases. Though the quartz substrate can enhance the heat-sinking capability of ZnO NRs, simultaneously, it can quench the WGMs of ZnO NRs with diameters above around 350 nm, which impedes the investigation of the intrinsic properties of ZnO NRs. Thus, how to rationally and effectively choose the substrate or the surrounding media needs thoughtful consideration, especially in substrate-supported applications of ZnO NRs in photonic and optoelectrical nanodevices.Acknowledgements
We would like to thank Xiaorui Tian, Deng Pan, Siyun Liu and Benli Wang for discussion. This work is supported by the National Basic Research Program of China (Grant no. 2012CB932302), the National Natural Science Foundation of China (51172271 and 90921012), and Beijing Municipal Education Commission (Grant no. YB20108000101).Notes and references
- D. J. Sirbuly, M. Law, H. Q. Yan and P. D. Yang, J. Phys. Chem. B, 2005, 109, 15190–15213 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. C, 2007, 111, 2834–2860 CAS.
- Z. L. Wang, Nano Today, 2010, 5, 540–552 CrossRef CAS.
- G. Z. Shen, P. Chen, K. Ryu and C. W. Zhou, J. Mater. Chem., 2009, 19, 828–839 RSC.
- C. Novo, A. M. Funston, I. Pastoriza-Santos, L. M. Liz-Marzan and P. Mulvaney, J. Phys. Chem. C, 2008, 112, 3–7 CAS.
- H. Wei, S. P. Zhang, X. R. Tian and H. X. Xu, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4494–4499 CrossRef CAS PubMed.
- J. Lefebvre, Y. Homma and P. Finnie, Phys. Rev. Lett., 2003, 90, 217401 CrossRef CAS PubMed.
- M. Steiner, M. Freitag, J. C. Tsang, V. Perebeinos, A. A. Bol, A. V. Failla and P. Avouris, Appl. Phys. A: Mater. Sci. Process., 2009, 96, 271–282 CrossRef CAS.
- P. Avouris, Z. H. Chen and V. Perebeinos, Nat. Nanotechnol., 2007, 2, 605–615 CrossRef CAS PubMed.
- P. Avouris, M. Freitag and V. Perebeinos, Nat. Photonics, 2008, 2, 341–350 CrossRef CAS.
- J.-P. Richters, T. Voss, L. Wischmeier, I. Rückmann and J. Gutowski, Appl. Phys. Lett., 2008, 92, 011103 CrossRef.
- K. W. Liu, R. Chen, G. Z. Xing, T. Wu and H. D. Sun, Appl. Phys. Lett., 2010, 96, 023111 CrossRef.
- J. P. Richters, T. Voss, D. S. Kim, R. Scholz and M. Zacharias, Nanotechnology, 2008, 19, 305202 CrossRef PubMed.
- D. Zhao, X. X. Zhang, H. B. Dong, L. J. Yang, Q. S. Zeng, J. Z. Li, L. Cai, X. Zhang, P. S. Luan, Q. Zhang, M. Tu, S. Wang, W. Y. Zhou and S. S. Xie, Nanoscale, 2013, 5, 4443–4448 RSC.
- X. X. Zhang, L. H. Zhang, G. Q. Yan, J. Shen, M. Gao, J. Z. Li, H. B. Dong, D. Zhao, L. Cai, Q. Chen, W. Y. Zhou and S. S. Xie, J. Nanosci. Nanotechnol., 2012, 12, 1082–1086 Search PubMed.
- C. W. Cheng, E. J. Sie, B. Liu, C. H. A. Huan, T. C. Sum, H. D. Sun and H. J. Fan, Appl. Phys. Lett., 2010, 96, 071107 CrossRef.
- X. J. Zhang, P. W. Wang, X. Z. Zhang, J. Xu, Y. Y. Zhu and D. P. Yu, Nano Res., 2009, 2, 47–53 CrossRef CAS.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, 1897–1899 CrossRef CAS PubMed.
- D. J. Sirbuly, M. Law, P. Pauzauskie, H. Yan, A. V. Maslov, K. Knutsen, C. Z. Ning, R. J. Saykally and P. Yang, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7800–7805 CrossRef CAS PubMed.
- M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. Yang, Nat. Mater., 2005, 4, 455–459 CrossRef CAS PubMed.
- J. X. Wang, X. W. Sun, A. Wei, Y. Lei, X. P. Cai, C. M. Li and Z. L. Dong, Appl. Phys. Lett., 2006, 88, 233106 CrossRef.
- Z. L. Wang and J. Song, Science, 2006, 312, 242–246 CrossRef CAS PubMed.
- K. H. Liu, W. L. Wang, M. H. Wu, F. J. Xiao, X. P. Hong, S. Aloni, X. D. Bai, E. G. Wang and F. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 113404 CrossRef.
- M. Gao, W. L. Li, Y. Liu, Q. Li, Q. Chen and L. M. Peng, Appl. Phys. Lett., 2008, 92, 113112 CrossRef.
- D. Li, Y. Wu, P. Kim, L. Shi, P. Yang and A. Majumdar, Appl. Phys. Lett., 2003, 83, 2934 CrossRef CAS.
- C. T. Bui, R. Xie, M. Zheng, Q. Zhang, C. H. Sow, B. Li and J. T. Thong, Small, 2012, 8, 738–745 CrossRef CAS PubMed.
- D. F. Liu, Y. J. Xiang, Z. X. Zhang, J. X. Wang, Y. Gao, L. Song, L. F. Liu, X. Y. Dou, X. W. Zhao, S. D. Luo, C. Y. Wang, W. Y. Zhou, G. Wang and S. S. Xie, Nanotechnology, 2005, 16, 2665–2669 CrossRef CAS.
- Y. P. Varshni, Physica, 1967, 34, 149 CrossRef CAS.
- L. J. Wang and N. C. Giles, J. Appl. Phys., 2003, 94, 973 CrossRef CAS.
- B. Q. Cao, W. P. Cai and H. B. Zeng, Appl. Phys. Lett., 2006, 88, 161101 CrossRef.
- G. T. Dang, H. Kanbe, T. Kawaharamura and M. Taniwaki, J. Appl. Phys., 2011, 110, 083508 CrossRef.
- X. H. Zhang, S. J. Chua, A. M. Yong, H. Y. Yang, S. P. Lau, S. F. Yu, X. W. Sun, L. Miao, M. Tanemura and S. Tanemura, Appl. Phys. Lett., 2007, 90, 013107 CrossRef.
- C. Boemare, T. Monteiro, M. J. Soares, J. G. Guilherme and E. Alves, Physica B, 2001, 985–988 CrossRef CAS.
- W. Shan, W. Walukiewicz, J. W. Ager, K. M. Yu, H. B. Yuan, H. P. Xin, G. Cantwell and J. J. Song, Appl. Phys. Lett., 2005, 86, 191911 CrossRef.
- D. Sentosa, B. Liu, L. M. Wong, Y. V. Lim, T. I. Wong, Y. L. Foo, H. D. Sun and S. J. Wang, J. Cryst. Growth, 2011, 319, 8–12 CrossRef CAS.
- L. H. Zhang, X. X. Zhang, J. L. Lai, S. Wang, G. Zhang, Z. X. Wang, S. M. Hou and M. Gao, J. Phys. Chem. C, 2011, 115, 8283–8287 CAS.
- J. Wiersig, Phys. Rev. A: At., Mol., Opt. Phys., 2003, 67, 023807 CrossRef.
- D. I. Florescu, L. G. Mourokh, F. H. Pollak, D. C. Look, G. Cantwell and X. Li, J. Appl. Phys., 2002, 91, 890 CrossRef CAS.
- U. Ozgur, X. Gu, S. Chevtchenko, J. Spradlin, S. J. Cho, H. Morkoc, F. H. Pollak, H. O. Everitt, B. Nemeth and J. E. Nause, J. Electron. Mater., 2006, 35, 550–555 CrossRef CAS.
- L. Liang and B. Li, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 153303 CrossRef.
- A. J. Kulkarni and M. Zhou, Appl. Phys. Lett., 2006, 88, 141921 CrossRef.
- X. Lü, W. Z. Shen and J. H. Chu, J. Appl. Phys., 2002, 91, 1542 CrossRef.
- Z. Wang, X. Zu, F. Gao, W. J. Weber and J.-P. Crocombette, Appl. Phys. Lett., 2007, 90, 161923 CrossRef.
- R. Prasher, Nano Lett., 2005, 5, 2155–2159 CrossRef CAS PubMed.
- T. Nobis, E. Kaidashev, A. Rahm, M. Lorenz and M. Grundmann, Phys. Rev. Lett., 2004, 93, 103903 CrossRef PubMed.
- M. Bass, E. W. V. Stryland, D. R. Williams and W. L. Wolfe, Handbook of Optics, McGraw-Hill, 1995, vol. 2, pp. 33.66–33.67 Search PubMed.
- D. Wang, H. W. Seo, C. C. Tin, M. J. Bozack, J. R. Williams, M. Park and Y. Tzeng, J. Appl. Phys., 2006, 99, 093112 CrossRef.
- D. S. Yu, Y. J. Chen, B. J. Li, X. D. Chen, M. Q. Zhang, F. L. Zhao and S. Ren, Appl. Phys. Lett., 2007, 91, 091116 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nr04300b |
| This journal is © The Royal Society of Chemistry 2014 |