Chitin nanowhiskers as alternative antimicrobial controlled release carriers†‡
María Emilia
Villanueva
,
Ana
Salinas
,
Luis Eduardo
Díaz
and
Guillermo Javier
Copello
*
Cátedra de Química Analítica Instrumental, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires (UBA), IQUIMEFA (UBA-CONICET), Junín 956, C1113AAD Buenos Aires, Argentina. E-mail: gcopello@ffyb.uba.ar; Fax: +54 11 49648254; Tel: +54 11 49648254
First published on 29th October 2014
Abstract
Antimicrobial finishings protect users from pathogenic and odor-generating microorganisms, which are of medical and hygiene concerns. Controlled release is a useful approach to obtain antimicrobial finishings in several materials because it provides a gradual and persistent antibiotic release from the surface into the surroundings. Such a property has been taken into account in this work, using chitin nano-whiskers (CNWs) as carriers of methylparaben to prepare durable antimicrobial cotton textiles. This durability has been endowed with fixing CNWs in a silicon oxide matrix. Antimicrobial activity has been determined using Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Acinetobacter baumanii and Salmonella choleraesuis. Treated textiles have shown antimicrobial activity with laundering durability up to 20 washing cycles. Methylparaben leaching from the textile has been assessed by liquid chromatography showing a methylparaben controlled release which could be responsible for the obtained antimicrobial laundry durability. Textile mechanical properties have not been altered by the finishing.
Introduction
In the hospital environment, the issue of infections associated with the material surface is of overriding relevance.1,2 When the microbial adhesion to surfaces is followed by bacterial growth and colonization, the outcome is the formation of a compact biofilm matrix which protects the underlying bacteria from the action of antibiotics and host defense mechanisms.3–5 Currently, there is an increasing demand for antimicrobial materials that do not allow microbes to adhere and proliferate on material surfaces.6Textiles, especially those made from natural fibers, are an excellent medium for the growth of microorganisms when the basic requirements such as nutrients, moisture, oxygen and appropriate temperature are present.7 Clothing and other textile materials may act as carriers for microorganisms like pathogenic or odor-generating bacteria. When they are in contact with the human body, they offer an ideal environment for microbial growth, providing oxygen, water and appropriate temperature, as well as nutrients from spillages and body exudates.8 Textile substrates find various applications apart from the conventional apparel usage, such as masks, hospital covers and surgical gowns.12 Therefore, there is a great demand for developing antimicrobial finishes of textiles to control the growth of microorganisms, preventing the textile from the deterioration of strength, quality, staining, odors and health concerns. Moreover, the use of nanotechnology in the textile industry has increased rapidly as a result of its unique properties which can produce multifunctional fibers with variable functions and applications such as antimicrobial, UV protection, self-cleaning, etc.10
There are various chemical possibilities that should be considered in the production of antimicrobial textiles. In practice, the antimicrobial effect is obtained through the application of specific chemical products during the finishing stage or through the incorporation of these substances into textile materials during the spinning or dipping process.11 Different synthetic agents have been widely studied for antimicrobial textile finishing. Compounds like triclosan, metals and their salts, organo-metallics, polymerizable quaternary ammonium salts and encapsulated nanoparticle agents embedded into polymer matrices of various compositions have been used for the antimicrobial finishing of textile products. On the other hand, natural agents such as chitosan, sericin from silk and natural polyphenols have also been used for the same purpose.12–14
When an antimicrobial effect is achieved, protection for both the user and the textile itself is granted.11 Besides, the antimicrobial finishing should be resistant to laundering. This is the greatest challenge as textile products are subjected to repeated washing cycles during their lifespan.9 When an antibiotic agent is used to get an antimicrobial finishing its controlled release provides the best laundry durability as the active agent is physically embedded in the structure of the fiber and released slowly during use.9,15
Over the last few years, many strategies have been proposed to design a coating that can prevent infections.16–18 One is the development of nanometric carriers loaded with antimicrobial compounds immobilized over a surface in order to achieve a controlled release system. The benefit of working with a nanoscale material is the high surface-to-volume ratio that allows a higher drug loading. Recently, diverse biodegradable nanoparticles including polysaccharide nanocrystals have been explored for drug controlled release.19,20
Chitin, a polysaccharide present in shellfish, insects, exoskeletons of crustaceans and arthropods, and microorganisms, is the second most abundant structural biopolymer.12,21 It has the potential to be converted into individual nano-fibrils, called chitin nanowhiskers (CNWs), by downsizing processes. CNWs have drawn attention in various applications due to their properties like nanosized dimensions, high surface area, high absorptivity, biodegradability, nontoxicity, renewability, low density and easy modification. CNWs are currently being studied and used as reinforcing additives for high performance environmentally friendly biodegradable nanocomposite materials, as biomedical composites for drug/gene delivery or nanoscaffolds in tissue engineering.20 Taking into account physicochemical characteristics of CNWs, an antimicrobial controlled release system can be obtained using CNWs as drug carriers.
Methylparaben is one among a homologous series of parabens (including methyl, ethyl, butyl, heptyl and benzyl parabens), used singly or in combination to exert the intended antimicrobial effect. These substances can have multiple biological effects, but it is generally considered that their inhibitory effects on membrane transport and mitochondrial function processes are key to their actions.22 The parabens have a broad spectrum of antimicrobial activities, are safe to use (i.e. relatively non-irritating, non-sensitizing, and of low toxicity), are stable over the pH range, and are sufficiently soluble in water to achieve the effective concentration in the aqueous phase.22 Methylparaben is widely used as an antimicrobial preservative in cosmetics, food products and pharmaceutical formulations. It may be used either alone, in combination with other parabens, or with other antimicrobial agents. In cosmetics, methylparaben is the most frequently used antimicrobial preservative.23
The aim of this work was to develop a process for the antimicrobial finishing of cotton textiles with laundry durability. The antimicrobial treatment was performed by treating cotton textile with CNWs loaded with the well-known preservative methylparaben. CNWs were fixed in a silicon oxide matrix in order to achieve a methylparaben controlled release considering hydrophobic characteristics of both CNWs and the preservative. The antimicrobial activity was assessed against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Acinetobacter baumanii and Salmonella choleraesuis. Methylparaben leaching after washing and the fabric tensile strength after treatment were also analyzed.
Experimental
Reagents and materials
Cotton fabric was acquired from a local store and cut into pieces of 1 cm × 1 cm; tetraethoxysilane (TEOS), Chitin from crab shells (DA: 92%; Mr ≈ 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and methylparaben were purchased from Sigma (St Louis, MO, USA). All other reagents were of analytical grade.
000) and methylparaben were purchased from Sigma (St Louis, MO, USA). All other reagents were of analytical grade.
Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 29213 were gently provided by the Microbial Culture Collection of Facultad de Farmacia y Bioquímica (CCM 29), University of Buenos Aires and Escherichia coli wild type, Acinetobacter baumanii wild type and Salmonella choleraesuis wild type were isolated from a hospital environment. All microorganisms were grown at 35 °C for 24 h on Luria Bertani (LB) medium (Britania, BA, Argentina).
Chitin nanowhisker preparation
The CNW suspension was prepared as described elsewhere.24 Briefly, chitin was treated with 3 N HCl at 100 °C for 90 min under vigorous stirring. The ratio of 3 N HCl to chitin was 100 mL g−1. After treatment, the suspension was diluted with distilled water, followed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. This process was repeated three times. The suspension was then transferred to a dialysis bag and dialyzed against deionized water up to neutral pH. The CNW suspension was sealed and preserved by storing in a refrigerator at 4 °C.
000 rpm for 5 min. This process was repeated three times. The suspension was then transferred to a dialysis bag and dialyzed against deionized water up to neutral pH. The CNW suspension was sealed and preserved by storing in a refrigerator at 4 °C.
Methylparaben adsorption
This assay was performed in order to evaluate the pH at which methylparaben maximum adsorption was achieved. CNW suspension, 1 mL (0.012 g mL−1), was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 rpm for 5 min. After discarding the supernatant, 1 mL of 0.003% w/v methylparaben in 50 mM phosphate solutions (pH 4, 5, 6, 7 and 8) was added and left with continuous stirring under ambient conditions. After 18 h, centrifugation at 14
600 rpm for 5 min. After discarding the supernatant, 1 mL of 0.003% w/v methylparaben in 50 mM phosphate solutions (pH 4, 5, 6, 7 and 8) was added and left with continuous stirring under ambient conditions. After 18 h, centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 rpm for 5 min was carried out. The remnant methylparaben in the supernatant was measured spectrophotometrically at 255 nm.
600 rpm for 5 min was carried out. The remnant methylparaben in the supernatant was measured spectrophotometrically at 255 nm.
The results were expressed as % adsorption by eqn (1)
| % adsorption = 100 − (R × 100/C) | (1) |
Cotton fabric coating
A 0.03% methylparaben solution was prepared in 50 mM pH 7.5 phosphate solutions. 6 mL of 0.012 g mL−1 CNW suspension was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and 10 mL of the methylparaben solution was added to the pellet and left at room temperature for 18 h. The pH of the solution was chosen according to the results obtained in the methylparaben adsorption assay (Section 2.3; ESI 1†).
000 rpm for 5 min and 10 mL of the methylparaben solution was added to the pellet and left at room temperature for 18 h. The pH of the solution was chosen according to the results obtained in the methylparaben adsorption assay (Section 2.3; ESI 1†).
A TEOS sol was prepared by sonicating a mixture of 1 mL of TEOS, 0.06 mL of 0.05 M HCl and 0.2 mL of deionized water for 15 min at 20 °C. The coating solvent mixture was prepared by making a 20-fold dilution of the TEOS sol in a mixture of acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (75
water (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25).25
25).25
The cotton samples were impregnated in the methylparaben adsorbed CNW suspension for 2 min and dried under ambient conditions; these samples were named FMW (fabric–methylparaben–CNW). FMW samples were immersed in the TEOS coating solvent mixture described above for 15 s and dried under ambient conditions. The obtained samples were named FMWT.
Controls for the antimicrobial and methylparaben elution assays were prepared as follows. Fabric samples were immersed in the 0.03% methylparaben solution prepared in 50 mM pH 7.5 phosphate solutions for 2 min and then dried under ambient conditions; these samples were named FM. FM samples were immersed in the TEOS coating solvent mixture described above for 15 s and then dried under ambient conditions. The obtained samples were named FMT.
Microscopic characterization
Scanning Electron Microscopy (SEM) images of freeze-dried and gold coated samples were taken using a FEI Quanta 200 microscope. The morphology of the chitin whiskers was analyzed using a transmission electron microscope (TEM, Zeiss 109).Tensile test
This assay was performed on uncoated fabric, FMT and FMWT samples. The test was carried out according to standard IRAM-INTI CIT G 7509-1:200326 in an Instron CRE dynamometer. Briefly, tensile tests were carried out on two sets of the test specimen (quantity 5 per set): one in the warp direction and the other in the weft direction of the material. These were individually supported by clamping of the specimen and then extended at the constant rate of 100 mm min−1 until they ruptured.The maximum tensile strength and elongation at break at that rate were recorded.
Washing procedure
All the treated fabrics were subjected to 5, 10, 15 and 20 consecutive launderings in the presence of a 0.5% nonionic detergent. Each washing cycle lasted 15 min and consisted of laundering under ambient conditions, water-rinsing and squeezing. Then the antibacterial activity and the methylparaben elution were measured.Test for antimicrobial activity and efficacy
Antimicrobial activity assays were performed in all samples every 5 washing cycles up to 20 cycles. Antimicrobial activity was performed according to a modified assay from Japanese Industrial Standards (JIS) Z 280127 and assessed using P. aeruginosa ATCC 27853 and S. aureus ATCC 29213. The two kinds of microorganisms were grown in LB medium for 24 h. The preparation of the inoculum was performed by diluting LB broth with purified water to a 500fold volume and the concentration was adjusted to 105 cfu mL−1.The treated (FM, FMT, FMW, and FMWT) and untreated fabric samples were sterilized, exposed to the diluted cultures and incubated for 24 h at 35 °C and above 90% of humidity. The test samples were placed in a sterilized bag using sterilized forceps to ensure the bacterial survival. Subsequently, they were left in a 2,3,5-triphenyltetrazolium chloride (TTC) solution at 35 °C. After 24 h, they were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min, the supernatants were discarded and 1 mL of acetone was added to the pellet. Finally, after shaking, the absorbance was measured spectrophotometrically at 485 nm.
000 rpm for 5 min, the supernatants were discarded and 1 mL of acetone was added to the pellet. Finally, after shaking, the absorbance was measured spectrophotometrically at 485 nm.
TTC is a live/dead colorant that is reduced to 1,3,5-triphenylformazan in the presence of living bacteria. This method serves as an indicating system for the determination of the viability of microorganisms and can be used on surfaces. Formazan absorbance is directly proportional to the amount of living bacteria.28,29 The linear range of 1,3,5-triphenylformazan absorbance versus log cfu mL−1 calculated by the agar plate culture method has been assessed27 for every bacteria and used in the linear working range.
The results were expressed as the percent reduction of formazan absorbance (Reduction (%)) by eqn (2).
| Reduction (%) = [(C − A)/C] × 100 | (2) |
This assay was also repeated with FMWT fabric washed 20 times against E. coli, A. baumanii, and S. choleraesuis to evaluate the antimicrobial spectrum. A control of untreated fabric was carried out with each cell culture.
Diffusion tests in LB agar were performed to evaluate the antibacterial activity of the samples31 against P. aeruginosa and S. aureus as models of Gram negative and Gram positive bacteria, respectively. In this method, colonies of the previously mentioned bacteria obtained from an overnight culture were suspended in LB broth and the concentration was adjusted to 105 cfu mL−1. 200 μL of this suspension was spread on LB agar plates. The fabric samples were then placed on the inoculated medium and the plates were kept for incubation for 24 h at 37 °C. The zones of inhibition were observed the next day.
All experimental conditions were conducted in triplicate, each time using a fresh cell suspension.
Methylparaben leaching quantification after washing
To evaluate the methylparaben leaching, the non-washed and washed samples were left in the physiological solution for 24 h. The methylparaben quantification in the supernatants was performed by HPLC (Spectra series P100 pump, Thermo separation products, Virginia, USA) in an isocratic mode. The mobile phase was acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pH 3.5 0.2 M phosphate buffer (40
pH 3.5 0.2 M phosphate buffer (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), the column was a C18 Germini-NX (Phenomenex CA, USA, 4.6 mm × 15 cm, 5 μm particle size) the flow rate was 1 mL min−1, and the detection was carried out spectrophotometrically at 255 nm.32
60), the column was a C18 Germini-NX (Phenomenex CA, USA, 4.6 mm × 15 cm, 5 μm particle size) the flow rate was 1 mL min−1, and the detection was carried out spectrophotometrically at 255 nm.32
Statistics
All quantitative results were obtained from triplicate samples. Data were expressed as means ± SD. Statistical analysis was carried out using a Two-way ANOVA test and a Bonferroni post-test. A value of p < 0.05 was considered to be statistically significant.Results and discussions
Chitin nanowhisker characterization by TEM, fabric SEM images and the tensile strength test
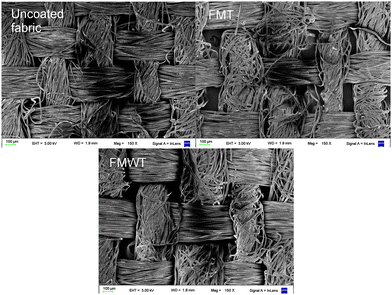
As can be observed in TEM images (ESI 1†) the CNWs were successfully obtained with a width of 10–15 nm. The macroscopic observations of the fabrics showed no differences between coated and uncoated fabrics. This was confirmed by TEM and the tensile strength test. SEM images (Fig. 1) did not show major topographical variations within the samples indicating that the coatings did not disturb the microscopic structure. This was expected due to CNW dimensions.Maximum tensile strength and elongation at break of samples is shown in Table 1. No significant difference (p > 0.05) was found between the samples in the elongation at break assay. However, maximum tensile strength was slightly different (p < 0.05) between samples. The maximal value was obtained for FMWT and the minimal for untreated fabric. Nevertheless, this variation only accounted for approximately 10%. These results indicated that mechanical properties of the samples were improved even on a small scale after the impregnation of nanowhiskers and further coating. This reinforcement effect provided by CNWs has also been reported elsewhere for other materials.33
| Sample | Direction | Elongation at break [%] | Maximum tensile strength [N cm−1] |
|---|---|---|---|
| Uncoated Fabric | Weft | 16.2 ± 3.5 | 84 ± 2.0 |
| Warp | 15.3 ± 2.5 | 118 ± 1.9 | |
| FMT | Weft | 16.2 ± 4.1 | 92 ± 3.4 |
| Warp | 15.6 ± 2.7 | 128 ± 0.9 | |
| FMWT | Weft | 16.9 ± 4.8 | 96 ± 4.1 |
| Warp | 15.5 ± 3.2 | 133 ± 2.0 | |
Methylparaben adsorption
The methylparaben adsorption at different pH was studied and is shown in the ESI 2.† As can be seen, the adsorption tended to increase with pH. This behavior may be due to the presence of charged groups at low pH. Chitin is a polysaccharide consisting of a chain of β-(1 → 4)-linked 2-acetamido-2-deoxy-D-glucopyranose (N-acetyl-D-glucosamine).29 It is neutral throughout the pH range studied. Yet, the degree of acetylation of chitin was 92%; thus, the presence of glucosamine residues in the structure of the CNW could cause the pH dependence. When pH increased, the NH2 groups present in glucosamine residues became unprotonated and the hydrophobic interaction between methylparaben and CNW increased.Antimicrobial efficacy
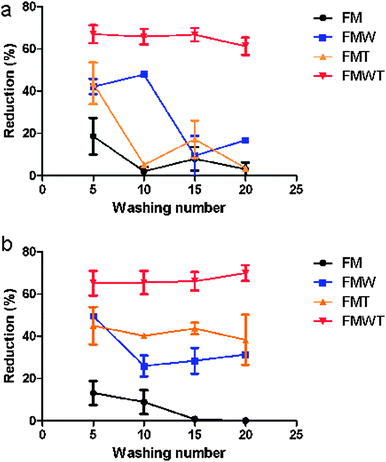
Results on the antibacterial activity of treated fabrics versus the number of washings against S. aureus and P. aeruginosa are presented in Fig. 2. As expected, no bacterial reduction was found on the unfinished cotton sample. This value was considered for 100% bacterial growth.A high reduction of both bacteria occurred on the unwashed FM, FMT, FMW and FMWT cotton samples (data not shown). This antimicrobial activity came from an excess of methylparaben deposited in the fabric yarns. The excess of methylparaben was lost within the first washing cycles. In order to analyze the laundry durability of the antimicrobial finishing, the presented results refer to samples that have been washed for at least 5 cycles.
The antimicrobial activity against S. aureus and P. aeruginosa provided by methylparaben could be conserved for at least 20 washings in FMWT. The Reduction % of bacteria was around 70% for both bacteria and there was no significant difference between every washing cycle (p > 0.05). In contrast, in the controls (FM, FMW, and FMT) antimicrobial efficacy showed a negative trend with less accurate results, probably accounting for a heterogenic stability of the finishing. Besides, the Reduction % of bacteria was significantly lower than that in FMWT (p < 0.05) with few exceptions. In the case of FMW exposed to 5 and 10 washing cycles and FMT washed 5 cycles, no difference could be found between these surfaces and FMWT in P. aeruginosa, Reduction % (p > 0.05). Also, no difference was seen in the Reduction % of S. aureus between FMWT and FMW washed 5 times and FMT washed 10 cycles (p > 0.05).
Control samples (FM, FMT and FMW) did not get as good performance as FMWT because of multiple factors. In the case of FMW the CNW may have been randomly lost within the washing cycles probably because they are not fixated in the fabric. In FM and FMT the interaction between methylparaben and the surface was not as homogeneous as in FMWT because of the lack of a significant hydrophobic interaction with the drug. The data exposed above may suggest that both the CNW and the TEOS coating are necessary to get laundry durability.
Agar diffusion test
The results described above were confirmed by the agar diffusion test. This assay was performed according to Standard SNV 195920-1992.2934 When an inhibition zone is observed both close and under the fabric, the antibacterial properties are defined as ‘‘good.’’ When growth inhibition area is observed under the sample only, the antibacterial properties are defined as ‘‘sufficient.’’ If the sample is totally covered by the bacteria as well as the area under the fabric, the antibacterial properties are defined as ‘‘not sufficient.’’Although no diffusion was observed, the antimicrobial activity was found sufficient in all the cases where FMWT was tested: 5, 10, 15 and 20 washing cycles and against P. aeruginosa and S. aureus. (ESI 3†). The lack of a diffusion zone may be due to low methylparaben solubility.
Antimicrobial spectrum
A general problem of antimicrobial finishes is their selective effect. Some are efficient against Gram-positive and other against Gram-negative bacteria.35 FMWT fabrics had shown antimicrobial activity against P. aeruginosa and S. aureus, Gram-negative and Gram-positive bacteria, respectively. Nevertheless, FMWT submitted to 20 washing cycles was tested against wild type species isolated from a hospital environment: E. coli, A. baumanii and S. choleraesuis.The Reduction % found was between 46 and 89 for the assessed microorganisms (Fig. 3). These results reinforced the benefits of this coating confirming its antimicrobial spectrum and its laundry durability.
Methylparaben leaching quantification after washing
As can be seen in Fig. 4, the eluated methylparaben leaching was in agreement with the observed antimicrobial activity. It can be seen that methylparaben concentration was less than 4.5 μg g−1 after 10 washing cycles in the samples not coated with TEOS (FM and FMW). In contrast, those coated (FMT and FMWT) got a better performance, suggesting that TEOS coating played an important role in the attachment to the fabric of methylparaben and CNWs. However, in FMT, methylparaben concentration decreased after every washing cycle, reaching less than 4.5 μg g−1 in the twentieth. This suggested that TEOS coating delayed the preservative release, but did not guarantee laundry durability.In FMWT, the concentration of methylparaben in the supernatant was around 10 μg g−1 after every washing cycle, suggesting a methylparaben controlled release from the CNW. This controlled released may be due to the hydrophobic characteristics of both materials. The methylparaben loaded CNW was adsorbed on the fabric before the TEOS coating was performed, so the TEOS polymeric network acted by interpenetrating CNW in the fabric. Besides, a possible reason for this superior stability might be the existence of silicon oxide polymer crosslinked structures, both on the inner and outer surfaces of the cotton fiber, which protected the coatings from excessive mechanical effects of the washing cycles.36 Thus, a gradual and persistent methylparaben release from the textile into the surroundings was achieved in the presence of moisture, where the methylparaben acted as an antimicrobial agent.
The antimicrobial efficiency depends directly on the concentration,35 thus, the fact that methylparaben concentration reached a plateau of 10 μg g−1 in FMWT suggested that this coated fabric may resist a higher number of washings retaining its antibacterial activity for a longer use.
Conclusions
The results of this work confirmed the advantages of CNWs as antibiotic carriers allowing a controlled release from textile to obtain an antibacterial surface. In the fabrics that had been exposed to methylparaben loaded CNWs and coated with TEOS (FMWT) long lasting antibacterial activity and laundering durability were achieved. SEM analysis showed the morphological characteristics of the different samples, showing no major differences between treated and untreated samples. Tensile strength tests showed no difference between treatments in elongation at break assay and little difference in the maximum tensile strength test, showing that the coating did not introduce major changes in mechanical properties. The methylparaben leaching concentration was assessed by HPLC and showed that in FMWT, the leaching concentration was almost the same after every washing cycle suggesting that the release was controlled in concordance with the antimicrobial assays. On the other hand, in control surfaces (FM, FMT and FMW) the antimicrobial activity and methylparaben leaching concentration decreased as a function of the washing number.The antimicrobial efficiency of the treated surfaces against different bacterial species was confirmed. This suggested that this coating may have a broad antimicrobial spectrum for at least 20 washing cycles, confirming its versatility and applicability.
Taking into account the above mentioned results, this prototype would be a proper system to develop an antimicrobial finishing in other surfaces. Moreover, the combination of CNWs loaded with a hydrophobic drug and then coated with a TEOS polymeric network could be of interest, because other different drugs, such as contraceptives or glucocorticoids could be loaded onto the CNW to get a different effect.
Acknowledgements
A.S. is grateful for her undergraduate fellowship granted by CIN. M.E.V. is grateful for her doctoral fellowship granted by CONICET. This work was supported under grants from Universidad de Buenos Aires (UBACYT 20020100100919; UBACYT 20020110200131) and from ANPCYT (PICT-2008-1783). The authors would like to thank J. Nesterzak for his technical assistance and Dr M. D'Aquino for very useful discussions.Notes and references
- C. Gasquères, G. Schneider, R. Nusko, G. Maier, E. Dingeldein and A. Eliezer, Surf. Coat. Technol., 2012, 206, 3410–3414 CrossRef PubMed.
- R. M. Donlan, Clin. Infect. Dis., 2001, 33, 1387–1392 CrossRef CAS PubMed.
- W. M. Dunne, Clin. Microbiol. Rev., 2002, 15, 155–166 CrossRef CAS.
- N. Poulter, M. Donaldson, G. Mulley, L. Duque, N. Waterfield, A. G. Shard, S. Spencer, A. T. A. Jenkins and A. L. Johnson, New J. Chem., 2011, 35, 1477–1484 RSC.
- S. A. Ansari, M. M. Khan, M. Omaish Ansari, J. Lee and M. H. Cho, New J. Chem., 2014, 38, 2462–2469 RSC.
- A. Travan, E. Marsich, I. Donati, M. Benincasa, M. Giazzon, L. Felisari and S. Paoletti, Acta Biomater., 2011, 7, 337–346 CrossRef CAS PubMed.
- W. Su, S. S. Wei, S. Q. Hu and J. X. Tang, J. Text. Inst., 2011, 102, 150–156 CrossRef CAS.
- V. R. G. Dev, J. Venugopal, S. Sudha, G. Deepika and S. Ramakrishna, Carbohydr. Polym., 2009, 75, 646–650 CrossRef CAS PubMed.
- Y. Gao and R. Cranston, Text. Res. J., 2008, 78, 60–72 CrossRef CAS PubMed.
- H. Barani, New J. Chem., 2014, 38, 4365–4370 RSC.
- S. S. Jakub Wiener, Antimicrobial agents, InTech, 2012 Search PubMed.
- A. M. El-Shafei, M. M. G. Fouda, D. Knittel and E. Schollmeyer, J. Appl. Polym. Sci., 2008, 110, 1289–1296 CrossRef CAS.
- B. Simoncic and B. Tomsic, Text. Res. J., 2010, 80, 1721–1737 CrossRef CAS PubMed.
- T. Öktem, Color. Technol., 2003, 119, 241–246 Search PubMed.
- E. S. Abdel-Halim, F. A. Abdel-Mohdy, M. M. G. Fouda, S. M. El-Sawy, I. A. Hamdy and S. S. Al-Deyab, Carbohydr. Polym., 2011, 86, 1389–1394 CrossRef CAS PubMed.
- G. J. Copello, S. Teves, J. Degrossi, M. D’Aquino, M. F. Desimone and L. E. Diaz, J. Ind. Microbiol. Biotechnol., 2006, 33, 343–348 CrossRef CAS PubMed.
- K. Zawadzka, K. Kadziola, A. Felczak, N. Wronska, I. Piwonski, A. Kisielewska and K. Lisowska, New J. Chem., 2014, 38, 3275–3281 RSC.
- X. Ren, L. Kou, H. B. Kocer, C. Zhu, S. D. Worley, R. M. Broughton and T. S. Huang, Colloids Surf., A, 2008, 317, 711–716 CrossRef CAS PubMed.
- R. Dash and A. J. Ragauskas, RSC Adv., 2012, 2, 3403–3409 RSC.
- M. M. V. Ostafe and A. Negrulescu, Rev. Adv. Mater. Sci., 2012, 30, 225–242 Search PubMed.
- Y. Lu, L. Weng and L. Zhang, Biomacromolecules, 2004, 5, 1046–1051 CrossRef CAS PubMed.
- M. G. Soni, S. L. Taylor, N. A. Greenberg and G. A. Burdock, Food Chem. Toxicol., 2002, 40, 1335–1373 CrossRef CAS.
- F. A. Andersen, Int. J. Toxicol., 1984, 3, 147–209 CrossRef.
- J. Sriupayo, P. Supaphol, J. Blackwell and R. Rujiravanit, Carbohydr. Polym., 2005, 62, 130–136 CrossRef CAS PubMed.
- G. J. Copello, M. C. De Marzi, M. F. Desimone, E. L. Malchiodi and L. E. Díaz, J. Immunol. Methods, 2008, 335, 65–70 CrossRef CAS PubMed.
- IRAM-INTI CIT G 7509-1:2003, 2003.
- JIS 2801:2000, 2000.
- M. M. G. Fouda, R. Wittke, D. Knittel and E. Schollmeyer, Int. J. Diabetes Mellitus, 2009, 1, 61–64 CrossRef PubMed.
- T. Textor, M. M. G. Fouda and B. Mahltig, Appl. Surf. Sci., 2010, 256, 2337–2342 CrossRef CAS PubMed.
- M. Orhan, D. Kut and C. Gunesoglu, J. Appl. Polym. Sci., 2009, 111, 1344–1352 CrossRef CAS.
- M. Pollini, F. Paladini, A. Licciulli, A. Maffezzoli, L. Nicolais and A. Sannino, J. Appl. Polym. Sci., 2012, 125, 2239–2244 CrossRef CAS.
- S. Ho Kang and H. Kim, J. Pharm. Biomed. Anal, 1997, 15, 1359–1364 CrossRef.
- Standard SNV 195920-1992.
- W. D. Schindler, P. J. Hauser and E. Textile Institute Manchester, Chemical Finishing of Textiles, CRC, 2004 Search PubMed.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, J. Appl. Polym. Sci., 2012, 124, 4230–4238 CrossRef CAS.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, J. Appl. Polym. Sci., 2012, 124, 4230–4238 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nj01522c |
| ‡ In memoriam of Professor Luis Eduardo Díaz. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |