Crooked Ag2Te nanowires with rough surfaces: facile microwave-assisted solution synthesis, growth mechanism, and electrical performances†
Jian
Pei
ab,
Gang
Chen
*a,
Dechang
Jia
*b,
Yaoguang
Yu
a,
Jingxue
Sun
a,
Haiming
Xu
a and
Zhuangzhuang
Qiu
a
aDepartment of Chemistry, Harbin Institute of Technology, Harbin, 150001, P. R. China. E-mail: gchen@hit.edu.cn
bInstitute for Advanced Ceramics, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: dcjia@hit.edu.cn
First published on 4th November 2013
Abstract
Crooked Ag2Te nanowires with rough surfaces have been successfully fabricated via a one-pot microwave-assisted solution process in a short time. A possible formation mechanism related to the oriented attachment crystal growth mechanism and the incomplete Ostwald ripening process is presented. The obtained Ag2Te shows a maximum power factor of about 13.4 × 10−4 W m−1 K−2.
The problem of energy and environment is becoming more and more serious. Among the various energies, heat energy is one of the energies with the lowest utilization rate. The vast majority of heat energy is discharged into the environment as waste heat or exhaust heat rather than reused for some practical purpose. How to put the existing waste heat to better use is a hot issue to relieve the energy crisis. Thermoelectric (TE) generators, capable of converting waste heat sources to electricity through the Seebeck effect, have been considered as one of the potential methods to solve the energy crisis.1 The efficiency of a TE generator is characterized by the dimensionless figure of merit, defined as ZT = (S2σ/κ)T, where S, σ, κ and T are the Seebeck coefficient, the electrical conductivity, the thermal conductivity and the absolute temperature, respectively.2 Unfortunately, the poor conversion efficiency of the TE makes the generators unsuitable for wide applications and limits their contribution to the waste heat recovery at present. Therefore, great efforts have been made to enhance the ZT by enhancing the power factor (S2σ) or lowering the thermal conductivity (κ), among which remarkable progress has recently been achieved with an exceptional ZT in the alloy TE materials through band structure engineering, hierarchical architecturing, fabrication of superlattices or quantum dots, and nanostructuring.3 Theoretical and experimental explorations demonstrated that significant improvement in TE efficiency could be achieved in nanostructured systems.4 Especially, one-dimensional (1D) nanostructures received much concern owing to the fact that they can efficiently transport electrical carriers and are suitable for building films with crystal orientation.
As a potential TE material, silver telluride (Ag2Te) has attracted increasing attention due to its interesting and useful characteristics.5 Ag2Te exhibits two phases: the low-temperature monoclinic structure (β-Ag2Te) and the high-temperature face-centered cubic structure (α-Ag2Te).6 Both phases of Ag2Te are suitable as thermoelectric materials. Inspired by the increasing interest in and demand for 1D nanostructured materials and nanodevices, a number of methods for the synthesis of 1D Ag2Te nanomaterials have been developed. For example, Ag2Te nanowires were obtained in porous anodic alumina membranes by a cathodic electrolysis method.6b Ag2Te nanorods and fibers were prepared by room-temperature chemical routes in which Te nanorods and fibers were used as the reactive self-sacrificing templates.5a,7 Ag2Te nanotubes were synthesized by a hydrothermal method using AgNO3 and Na2TeO4 as precursors.5b Ag2Te nanowires were formed by a composite-hydroxide-mediated approach at temperatures of 180–225 °C.8 Laterally epitaxial single crystalline Ag2Te nanowires were formed on sapphire substrates by the vapor transport method.9 However, most objects of these syntheses require hard templates, complex procedures or long reaction times. As an efficient and environmentally friendly method, the microwave-assisted solution method has attracted more and more attention, which is especially suitable for the preparation of nanoscale materials. Compared with traditional methods, it possesses a great advantage of remarkable reaction rate enhancement which accelerates the growth of crystals and shortens the reaction time from hours to minutes. Several TE materials have been synthesized via microwave-assisted solution methods,10 and recently Ag2Se dendrites and hierarchical Bi2Se3 microrods have been prepared using this method by our group.11 However, to the best of our knowledge, fabrication of Ag2Te nanowires by a microwave-assisted solution method has not yet been investigated.
Herein, we use this method for one-pot preparation of Ag2Te nanowires in an ethylene glycol solution without using any surfactant or template in a short time. The possible growth mechanism has been proposed on the basis of a series of field emission scanning electron microscope (FESEM) observations of the products collected at different time intervals. Moreover, the electrical performances are evaluated ranging from 300–525 K.
The composition and phase purity of the sample prepared at 190 °C for 15 min in the presence of an EG solution were initially examined by the X-ray powder diffraction (XRD) pattern, as shown in Fig. 1a. All detectable diffraction peaks can be readily indexed to the monoclinic phase of Ag2Te, and no peaks of impurities (such as Ag, Ag2O) are detected. The unit cell constants calculated from the diffraction peaks are a = 0.8091, b = 0.8995, and c = 0.7962, which are consistent with the standard literature values (JCPDS Card No. 034-0142). Fig. 1b shows the typical microstructure of the Ag2Te product, which clearly reveals that the product consists of a simple and uniform crooked wire-like structure. The average diameter and length of the wires can be calculated to be 150–300 nm and 3–8 μm, respectively. When observed carefully, the surface of each wire is found to be rough, which is composed of numerous nanocrystals. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) provide further insight into the details of the wire-like Ag2Te. The TEM analysis reveals that the nanowires are rough, crooked and composed of numerous nanoparticles (Fig. 1c), which is consistent with the FESEM observations. A representative HRTEM image is recorded at the edge of a single nanoparticle marked by an ellipse. The clear lattice fringe and the corresponding fast Fourier transformation (FFT) pattern indicate the single crystalline nature of the single nanoparticle (Fig. 1d and inset). The plane spacing is 0.686 nm, which corresponds to the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) planes of monoclinic Ag2Te.
01) planes of monoclinic Ag2Te.
 | ||
| Fig. 1 (a) XRD pattern, (b) FESEM image, (c) TEM image, (d) HRTEM image and its FFT pattern (inset) taken at the edge of a single nanoparticle. | ||
The above characterization confirms that Ag2Te nanowires have been successfully prepared. Then how do the wires with rough surfaces form? In our previous work, Ag2Se dendrites and nanoparticles were fabricated via this method and the growth mechanism related to a self-template in situ growth process was systematically discussed.11a In the present work, it seems that a similar template growth mechanism can be explained by the formation of Ag2Te nanowires, just as discussed by Zuo or Qian's group.5a,7 To understand the actual formation mechanism of the as-prepared Ag2Te nanowires, the samples collected at different stages of the microwave-heat reaction were investigated, and the FESEM images and XRD patterns of the typical products obtained at 190 °C for 3, 5, and 10 min are displayed in Fig. 2. As illustrated in Fig. 2a, numerous irregular nanoparticles with slight aggregation are obtained in the early stage (3 min). Upon increasing the reaction time to 5 min, short nanorods as well as a few nanoparticles appeared. The TEM image indicates that the nanorods are rough and crooked (Fig. S1, ESI†). Finally, upon extending the reaction time to 10 min, Ag2Te nanowires with rough surfaces are obtained. Fig. 2d shows the XRD patterns of the above three samples. Upon increasing the reaction time, the initially formed Ag will transform into Ag2Te gradually. During the microwave heating process, the solution colour changed from white to yellowish-brown, and then to dark. According to the above discussions, the reaction process in the present system can be explained as four stages: (1) ethylene glycol is dehydrated into acetaldehyde,12 which is accelerated under alkaline conditions. (2) Ag+ ions are first reduced to elemental Ag by the aldehyde group of acetaldehyde. (3) Then TeO32− ions are reduced to elemental Te at higher temperature. (4) The newly formed Te quickly reacts with Ag resulting in the formation of the Ag2Te product. The reaction process can be formulated as follows:
| 2HOCH2CH2OH → CH3CHO + 2H2O | (1) |
| 2CH3CHO + 2Ag+ → 2Ag + CH3COCOCH3 | (2) |
| 2CH3CHO + 2TeO32− → 2Te + CH3COCOCH3 | (3) |
| 2Ag + Te → Ag2Te | (4) |
It is noticeable that the reaction temperature may significantly affect the phase composition and morphology of the final products. To ascertain the effects of temperature, the products obtained at 130 °C and 160 °C are characterized by FESEM and XRD. At a lower reaction temperature (130 °C), large irregular blocks mixed with some pencil-shaped rods are found (Fig. S2a, ESI†). The corresponding XRD pattern (Fig. S3, ESI†) shows that almost only elemental Ag is obtained, indicating that TeO32− ions are difficult to be reduced at this temperature. By increasing the temperature to 160 °C, non-uniform crooked rods mixed with some blocks are obtained (Fig. S2b, ESI†). In addition to elemental Ag, a new phase of Ag7Te4 can also be found (Fig. S3, ESI†), indicating that single Ag2Te cannot be obtained at a temperature of 160 °C under the present work conditions.
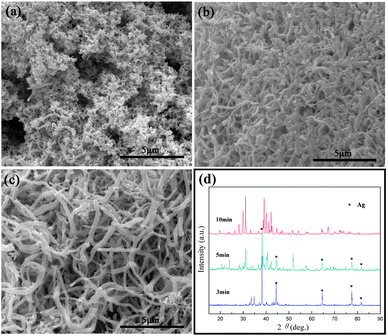 | ||
| Fig. 2 FESEM images of the samples obtained at different time intervals: (a) 3 min, (b) 5 min and (c) 10 min, and (d) corresponding XRD patterns of the above samples. | ||
On the basis of the above experimental results, the growth mechanism of Ag2Te nanowires with a rough surface structure in the present work is quite different from the early conjecture of the template growth mechanism. Then, a possible formation mechanism was proposed, as illustrated in Fig. 3. In this formation process, time is the key controlling factor. In the initial stage, crystalline Ag nuclei are generated in the supersaturated solution and simultaneously grew into nanoparticles. After additional reaction time (the temperature improved), crystalline Te nuclei appear in pace with the reduction of TeO32− ions. The fresh Te quickly reacts with Ag to form Ag2Te, and crystalline Ag2Te start to self-assemble together (Fig. S4, ESI†) and spontaneously grow into nanorods in order to minimize the surface energy through the process known as oriented attachment. The driving force is possibly derived from the dipole-field of a layered crystal structure of monoclinic Ag2Te (Fig. S5, ESI†). As the reaction proceeds, the nanorods continue to grow and the crooked nanowires are finally generated through the incomplete Ostwald ripening process (short ripening time).
 | ||
| Fig. 3 Schematic illustration of the proposed formation mechanism for Ag2Te nanowires with rough surfaces. | ||
To elucidate the electrical performance of the sample, the relative density of the as-synthesized specimen was first calculated to be 83.8% using Archimedes' method. The electrical conductivity (σ) of the obtained sample has been investigated at temperatures ranging from 300–525 K (Fig. 4a). The values of σ decrease with increasing temperature, showing a typical electrical conductivity of the metal for the entire measuring temperature range. From 375 to 473 K, the σ(T) curve greatly decreases with an increase in temperature. According to the studies reported previously,2a,13 there is a structure phase transition at around 410 K for the Ag2Te alloy, in which the structure of Ag2Te changes from β-Ag2Te to α-Ag2Te, resulting in a great decrease in carrier concentration.
 | ||
| Fig. 4 (a) Electrical conductivities, (b) Seebeck coefficients and (c) power factors of the sample measured at the temperature range from 300 K to 525 K. | ||
Fig. 4b presents the temperature dependence of the Seebeck coefficient (S) for the obtained Ag2Te sample. The negative sign S in the whole measured temperature indicates that the sample is an n-type material. The absolute values of S increase with increasing temperature, which is contrary to the change of the measured σ(T) curve because the Seebeck coefficient is strongly dependent on electrical conductivity.14 There is also an abrupt increase in the S(T) curve from 375 to 475 K, which can be ascribed to the structural phase transition of Ag2Te. The highest absolute Seebeck coefficient for measured temperature spots is 139.9 mV K−1, which is higher than the reported values (130 mV K−1 and 110 mV K−1, respectively13b,15). The enhancement of the Seebeck coefficient for the as-prepared sample is thought to be related to the electron energy filtering effect, which is characterized as the separation of higher energy electrons from lower energy electrons and the selective scattering of electrons when the size of the nanostructures is less than the electron mean free path. The crooked nanowires are composed of numerous nanoparticles (Fig. 1c), resulting in a higher Seebeck coefficient obtained in this study.
The corresponding temperature dependence of the power factor P (P = S2σ) of the above specimen is given in Fig. 4c. The values of P show a trend of initial increase followed by a decrease with the increase of temperature. The maximum value of P reaches 13.4 × 10−4 W m−1 K−2 at 450 K, which is quite sizable for Ag2Te materials. Among the few papers that reported the power factors of silver telluride nanostructures, the power factor of Ag2Te nanowires with rough surfaces obtained in the present work is obviously higher than some previously reported data, for instance, the maximum P value of 9.01 × 10−4 W m−1 K−2 for Ag2Te hollow microspheres and 5.99 × 10−4 W m−1 K−2 at 340 K for the α-phase.16 Such an increase of the power factor can be a promising route to achieve highly efficient TE energy conversion devices. In addition, the existence of the nanostructure should efficiently reduce the lattice thermal conductivity due to effective phonon scattering at the interface between boundaries of the nanograins in the rough surfaces of the nanowires.17 Thus, the enhancement of the ZT value can be expected.
In summary, 1D Ag2Te nanowires with rough surfaces have been fabricated by a facile one-pot microwave-assisted solution route in a short time. It was found that the microwave irradiation time and the temperature are the key controlling factors of the morphology of the product. Through a series of time-dependent morphological evolution studies, the possible reaction process and the formation mechanism of crooked Ag2Te nanowires with rough surfaces are proposed, which involves the oriented attachment crystal growth mechanism and the incomplete Ostwald ripening process. Moreover, the electrical conductivity and the Seebeck coefficient of the bulk specimen were investigated as a function of temperature. The fabricated nanostructure of Ag2Te showed sizable electrical performances in the range of 300–525 K, and the maximum power factor (13.4 × 10−4 W m−1 K−2) can be obtained at 450 K. This work reports for the first time that crooked Ag2Te nanowires with rough surfaces can be synthesized by a facile, one-step microwave-heating process, which may be a promising approach for the fabrication of highly efficient TE materials.
Experimental section
In the typical synthetic procedure, 1 mmol of AgNO3, 0.5 mmol of Na2TeO3 and 0.2 g of NaOH were dissolved in 45 mL of EG to form a homogeneous solution. Then the solution was poured into a 50 mL round-bottomed flask and subsequently microwave-heated rapidly to 190 °C and maintained at this temperature for 15 min. After microwave heating, the solution was cooled naturally and the precipitate was collected, centrifuged and washed with distilled water three times and with ethanol once, then dried at 60 °C in a vacuum.This work was financially supported by projects of the Natural Science Foundation of China (21201050 and 21271055), the Natural Science Foundation of Heilongjiang Province (ZD201011), the China Postdoctoral Science Foundation (2012M510086) and the China Postdoctoral Science Special Foundation (2013T60365).
Notes and references
- (a) G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105–114 CrossRef CAS PubMed; (b) J. Baxter, G. Chen, D. Danielson, Z. Bian, M. S. Dresselhaus, A. G. Fedorov, T. S. Fisher, C. W. Jones, E. Maginn, U. Kortshagen, A. Manthiram, A. Nozik, D. R. Rolison, T. Sands, L. Shi, D. Sholl and Y. Wu, Energy Environ. Sci., 2009, 2, 559–588 RSC.
- (a) R. Venkatasubramanian, E. Siivola, T. Colpitts and B. O'Quinn, Nature, 2001, 413, 597–602 CrossRef CAS PubMed; (b) J. H. Yang, H. L. Yip and A. K. Y. Jen, Adv. Energy Mater., 2013, 3, 549–565 CrossRef CAS.
- (a) Y. Z. Pei, H. Wang and G. J. Snyder, Adv. Mater., 2012, 24, 6125–6135 CrossRef CAS PubMed; (b) K. Biswas, J. He, I. D. Blum, C. Wu, T. Hogan, D. N. Seidman, V. P. Dravid and M. G. Kanatzidis, Nature, 2012, 489, 414–418 CrossRef CAS PubMed; (c) Y. Z. Pei, X. Shi, A. LaLonde, H. Wang, L. Chen and G. J. Snyder, Nature, 2011, 473, 66–69 CrossRef CAS PubMed; (d) J. P. Heremans, V. Jovovic, E. S. Toberer, A. Saramat, K. Kurosaki, A. Charoenphakdee, S. Yamanaka and G. J. Snyder, Science, 2008, 321, 554–557 CrossRef CAS PubMed; (e) K. F. Hsu, S. Loo, F. Guo, W. Chen, J. S. Dyck, C. Uher, T. Hogan, E. K. Polychroniadis and M. G. Kanatzidis, Science, 2004, 303, 818–821 CrossRef CAS PubMed.
- (a) J. R. Szczech, J. M. Higgins and S. Jin, J. Mater. Chem., 2011, 21, 4037–4055 RSC; (b) P. Vaqueiro and A. V. Powel, J. Mater. Chem., 2010, 20, 9577–9584 RSC.
- (a) P. F. Zuo, S. Y. Zhang, B. K. Jin, Y. P. Tian and J. X. Yang, J. Phys. Chem. C, 2008, 112, 14825–14829 CrossRef CAS; (b) A. M. Qin, Y. P. Fang, P. F. Tao, J. Y. Zhang and C. Y. Su, Inorg. Chem., 2007, 46, 7403–7409 CrossRef CAS PubMed.
- (a) R. Dalven and R. Gill, J. Appl. Phys., 1967, 38, 753–756 CrossRef CAS; (b) R. Z. Chen, D. S. Xu, G. L. Guo and L. L. Gui, J. Mater. Chem., 2002, 12, 2435–2438 RSC.
- L. Mu, J. X. Wan, D. K. Ma, R. Zhang, W. C. Yu and Y. T. Qian, Chem. Lett., 2005, 52–53 CrossRef CAS.
- F. Y. Li, C. G. Hu, Y. F. Xiong, B. Y. Wan, W. Yan and M. C. Zhang, J. Phys. Chem. C, 2008, 112, 16130–16133 CAS.
- J. In, Y. Yoo, J. Kim, K. Seo, H. Kim, H. Ihee, S. Oh and B. Kim, Nano Lett., 2010, 10(11), 4501–4504 CrossRef CAS PubMed.
- (a) G. H. Dong, Y. J. Zhu and L. D. Chen, J. Mater. Chem., 2010, 20, 1976–1981 RSC; (b) B. Zhou, Y. Ji, Y. F. Yang, X. H. Li and J. J. Zhu, Cryst. Growth Des., 2008, 8, 4394–4397 CrossRef CAS; (c) R. Harpeness and A. Gedanken, New J. Chem., 2003, 27, 1191–1193 RSC; (d) Y. Zhang, Z. P. Qiao and X. M. Chen, J. Mater. Chem., 2002, 12, 2747–2748 RSC.
- (a) J. Pei, G. Chen, D. C. Jia, R. C. Jin, H. M Xu and D. H. Chen, New J. Chem., 2013, 37, 323–328 RSC; (b) H. M. Xu, G. Chen, R. C. Jin, J. Pei and Y. Wang, CrystEngComm, 2013, 15, 1618–1625 RSC.
- F. Fievet, J. P. Lagier, B. Blin, B. Beaudoin and M. Fiflarz, Solid State Ionics, 1989, 32/33, 198–205 CrossRef.
- (a) F. Xiao, G. Chen, Q. Wang, L. Wang, J. Pei and N. Zhou, J. Solid State Chem., 2010, 183, 2382–2388 CrossRef CAS PubMed; (b) P. Gnanadurai, N. Soundararajan and C. E. Sooriamoorthy, Vacuum, 2002, 67, 275–284 CrossRef CAS.
- (a) M. Zebarjadi, B. Liao, K. Esfarjani, M. Dresselhaus and G. Chen, Adv. Mater., 2013, 25, 1577–1582 CrossRef CAS PubMed; (b) G. Joshi, X. Yan, H. Z. Wang, W. S. Liu, G. Chen and Z. F. Ren, Adv. Energy Mater., 2011, 1, 643–647 CrossRef CAS.
- V. D. Das and D. Karunakaran, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 2036–2041 CrossRef CAS.
- (a) G. H. Dong and Y. J. Zhu, CrystEngComm, 2012, 14, 1805–1811 RSC; (b) M. Fujikane, K. Kurosaki, H. Muta and S. Yamanaka, J. Alloys Compd., 2005, 393, 299–301 CrossRef CAS PubMed.
- (a) A. Hochbaum, R. Chen, R. D. Delgado, W. Liang, E. C. Garnett, M. Najarian, A. Marumdar and P. Yang, Nature, 2008, 451, 163–167 CrossRef CAS PubMed; (b) T. Markussen, A. P. Jauho and M. Brandbyge, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 035415 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nj01303k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |
