Homochiral 3D lanthanide camphorates with high thermal stability†
Ming-Ling
Sun
,
Xin
Zhang
,
Yuan-Yuan
Huang
,
Qi-Pu
Lin
,
Ye-Yan
Qin
and
Yuan-Gen
Yao
*
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: yyg@fjirsm.ac.cn; Fax: +86-591-83714946; Tel: +86-591-83711523
First published on 3rd October 2013
Abstract
Three isomorphous homochiral 3D lanthanide coordination polymers ([Ln(HCOO)(D-cam)]n, where Ln = Dy (1), Ho (2), Er (3)), featuring sra topology have been constructed from triple-strand helical {Ln–O}n rod SBUs and chiral D-cam spacers. Thermogravimetric analyses show the remarkable thermal stabilities of all three rigid lanthanide camphorate frameworks up to 450 °C.
Recently, homochiral metal–organic framework (MOF) materials have attracted a lot of interest due to their chirality and potential applications in catalysis, selective separation, drug delivery, nonlinear optical properties and so on.1,2 In light of the importance of homochiral MOF materials, there have been tremendous research efforts devoted to its development.3 Among the most utilized means of designing homochiral MOFs, readily available homochiral ligands occupy a prominent position in that they can endow their desirable chirality to homochiral materials during construction.4
As a correspondingly inexpensive ligand source, D-camphoric acid has proved its effectiveness as a chiral building block for the syntheses of homochiral MOFs materials with many transition metals.5,6 On the other hand, many enantiomerically pure lanthanide complexes with diverse structures and high thermal stabilities have been prepared but homochiral lanthanide camphorates have rarely been reported.7,8 In our previous investigation, multifunctional homochiral lanthanide camphorates with mixed achiral terephthalate ligands were explored.8a Herein, we report three isomorphous 3D homochiral lanthanide camphorates based on a unique D-camphoric acid ligand as further development.
Three isostructural 3D homochiral rigid frameworks, [Ln(HCOO)(D-cam)]n (Ln = Dy (1), Ho (2), Er (3); D-cam = D-camphoric acid) constructed from triple-strand helical {Ln–O}n rod SBUs and chiral D-cam spacers have been synthesised. During syntheses under high temperature and pressure, DMF undergoes hydrolyzation to generate the formate ligand.9 But when the formic acid and dimethylamine were matched in a proper molar ratio or a formic acid and DMF mix was used as a starting material, no crystals were obtained. Based on these facts, we speculate that DMF is a significant contributing factor which not only behaves as the source of the formate ligand but also provides favourable experimental conditions. Many attempts to synthesize their counterparts with other lanthanide camphorates failed probably due to the effect of lanthanide contraction. Complexes 1–3 are air-stable and insoluble in water and common organic solvents. The formation of these complexes was confirmed using single-crystal X-ray diffraction, IR spectroscopy and elemental analysis. Powder X-ray diffraction (PXRD) studies confirm the pure phase of the complexes 1–3.
X-ray structural analyses reveal that complexes 1–3 are isomorphous,‡ which is further confirmed by their similar PXRD patterns. Therefore, only the structure of 1 will be discussed in detail as a representative. The asymmetric unit of 1 contains one Dy3+, one D-cam ligand and one formate ligand. The fundamental unit of 1 is shown in Fig. 1. The coordination environment of the Dy3+ ion is hepta-coordinated and can be described as pentagonal bipyramid geometry. The equatorial plane is defined by five carboxylate oxygen atoms (O2B, O6B, O1, O6 and O5), which are from two different D-cam ligands and two different formate ligands. The axial sites are occupied by two carboxylate oxygen atoms (O3C and O4A) from two different D-cam ligands. In this framework, each D-cam ligand links four different Dy(III) ions using its two carboxylate groups in a uniform bis-monodentate mode, and each formate ligand connects two different Dy(III) ions in a 2.21 mode (Scheme S1, ESI†). This is noteworthy as the use of a 2.21 mode for the formate ligand, functioning as an anchor to link two metal ions, has rarely been reported before.10 The Dy–O bond lengths are in the range of 2.226(5)–2.462(4) Å (Table S1, ESI†), comparable to similar Dy(III) coordination polymers previously reported.11 The Dy3+ centers are bridged by the carboxylate groups of D-cam ligands and formate ligands into infinite 1D triple-strand helical rod-shaped secondary building units (SBUs) (Fig. 2a). Then, each 1D helical {Dy–O}n rod SBU is further linked to four neighboring symmetry-related analogs via chiral D-cam spacers, giving rise to a 3D rod-packing architecture (Fig. 2d). The methyl groups on the ring of the D-cam ligands protrude into the void space of the 3D framework preventing the inclusion of any guest molecules.
Topological analysis was also used to simplify this complicated 3D framework. The SBU consists of an infinite {Dy–O}n chain with DyO7 pentagonal bipyramids sharing opposite corners, in which the nearest Dy⋯Dy separation is 4.1949 Å. The carboxylate C atoms are at the vertices of each helical {Dy–O}n rod SBU. Joining these carboxylate C atoms together results in a 1D zigzag ladder (Fig. 2b). If each chiral D-cam spacer is reduced into a linker (Fig. 2c), these ladders are further linked together, giving rise to a 3D network with sra topology (Fig. 2e). The sra topology of 1 is similar to that of MIL-47,12 except that the V(III) atoms in MIL-47 are changed to Dy(III) atoms in 1 and the formate ligands in 1 replace negative μ2-OH groups in MIL-47. Finally, owing to the intrinsic bending of the D-cam building unit, the 3D sra topological network deviates somewhat from the ideal sra topological network of MIL-47.
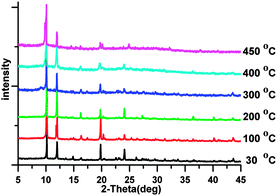
It is of note that thermal stability is one of the pivotal concerns in MOF materials. According to the reported literature,7 isostructural lanthanide complexes show similar thermal behaviors and generally undergo two steps of weight loss relating to the removal of guest molecules or small terminal coordinated molecules and the organic moiety, respectively. The second weight loss, namely the whole framework collapse, indicates that the organic moiety plays a more important role in stabilizing the structure. In comparison, no noticeable mass losses were detected for these lanthanide camphorates from room temperature to 450 °C, as shown in thermogravimetric analyses (TGA) (Fig. S2, ESI†). Above 450 °C, one step of weight loss occurred in continuous descending stages which were ascribed to the decomposition of the formate group and the camphorate species, meanwhile, the whole framework began to collapse. As shown in Fig. 3, variable-temperature powder X-ray diffraction (PXRD) of 1 was carried out to evaluate its potential application. It is noteworthy to point out that the PXRD patterns of 1 after calcination at elevated temperature in the range of 100–450 °C agrees well with the calculated version, indicating that its crystal lattice remains intact at 450 °C.
The variable-temperature magnetic susceptibilities of 1–3 were measured in the temperature range of 2–300 K with a magnetic field strength of 1000 Oe (Fig. S3, ESI†). All three compounds exhibit similar temperature-dependent magnetic curves. At 300 K, the corrected χmT values of 1, 2 and 3 per formula unit are cooling down, the χmT values decrease gradually and finally reach an abrupt drop in values to 5.90, 8.86 and 4.15 cm3 K mol−1 at 2 K. Fitting the data (χm−1vs. T) according to the Curie–Weiss law gave Weiss constants (θ) of −4.38, −2.39 and −7.16 K, respectively for compounds 1, 2 and 3 in the range between 2 and 300 K. Magnetic behaviors such as the decrease in χmT and the negative values of θ, typical for lanthanide compounds, are primarily due to the populations of the Stark levels, thermal depopulation of the free-ion excited states or the splitting of the Ln(III) ion ligand fields as a result of strong spin–orbital coupling. These are partly attributed to the possible antiferromagnetic intradimer coupling.13
In summary, three homochiral isomorphous lanthanide camphorates were successfully synthesized through solvothermal reactions. These complexes possessing sra topology have been constructed from triple-strand helical {Ln–O}n rod SBUs and chiral D-cam spacers to generate 3D rod-packing architectures. Thermogravimetric analyses show that the three compounds exhibit high thermal stabilities up to 450 °C. Weak antiferromagnetic properties have also been investigated. These studies may provide a useful insight into the synthesis of new chiral lanthanide materials and work on related research is underway in our laboratory.
Experimental
A mixture of 0.5 mmol Ln2O3 (Dy2O3 0.187 g, Ho2O3 0.189 g, Er2O3, 0.191 g), 0.5 mmol D(+)-camphoric acid (0.100 g), HClO4 (0.385 mmol, pH ≈ 2) and DMF/H2O (2 mL/6 mL) was stirred in air for 0.5 h, and then sealed in a 23 mL Parr Teflon-lined stainless-steel bomb at 150 °C for 96 h. It was then slowly cooled to room temperature. Prismatic crystals of 1 (white), 2 (light-yellow) and 3 (pink) were recovered by filtration, washed with distilled water and dried in air, respectively (yield: 42%, 39% and 35% based on Ln2O3). Elemental analyses calc. for C11H15DyO6 (1) (M = 405.73): C, 32.56; H, 3.73. Found: C, 33.07; H, 3.29%. IR bands (cm−1) for 1: 3428.72, 2977.42, 1631.81, 1535.05, 1422.92, 1369.56, 1305.48, 810.00. Elemental analyses calc. for C11H15HoO6 (2) (M = 408.16): C, 32.37; H, 3.70. Found: C, 32.82; H, 3.16%. IR bands (cm−1) for 2: 3444.85, 2976.74, 1630.81, 1539.15, 1422.32, 1369.73, 1305.19, 808.97. Elemental analyses calc. for C11H15ErO6 (3) (M = 410.49): C, 32.19; H, 3.68. Found: C, 32.70; H, 3.18%. IR bands (cm−1) for 3: 3417.23, 2977.33, 1633.82, 1536.13, 1423.52, 1369.38, 1304.00, 811.8.This work was supported by 973 Program of China (2011CBA00505), National Key Technology R&D Program (2012BAE06B08), the Chinese Academy of Sciences (KJCX2-YW-H30, KGCX-YW-222 and KJCX2-YW-M10) and the Science Foundation of Fujian Province (2009HZ005-1 and 2006l12005).
Notes and references
- (a) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS PubMed; (b) C. J. Kepert, T. J. Prior and M. J. Rosseinsky, J. Am. Chem. Soc., 2000, 122, 5158 CrossRef CAS; (c) I. Destoop, E. Ghijsens, K. Katayama, K. Tahara, K. S. Mali, Y. Tobe and S. De Feyter, J. Am. Chem. Soc., 2012, 134, 19568 CrossRef CAS PubMed.
- (a) C. Livage, N. Guillou, P. Rabu, P. Pattison, J. Marrot and G. Ferey, Chem. Commun., 2009, 4551 RSC; (b) C. D. Wu and W. B. Lin, Angew. Chem., Int. Ed., 2005, 44, 1958 CrossRef CAS PubMed; (c) G. C. Xu, W. Zhang, X. M. Ma, Y. H. Chen, L. Zhang, H. L. Cai, Z. M. Wang, R. G. Xiong and S. Gao, J. Am. Chem. Soc., 2011, 133, 14948 CrossRef CAS PubMed.
- (a) L. Q. Ma, C. Abney and W. B. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC; (b) O. R. Evans and W. B. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS PubMed; (c) L. Q. Ma and W. B. Lin, J. Am. Chem. Soc., 2008, 130, 13834 CrossRef CAS PubMed; (d) H. Yang, F. Wang, Y. Kang, T. H. Li and J. Zhang, Chem. Commun., 2012, 48, 9424 RSC.
- (a) B. Kesanli and W. B. Lin, Coord. Chem. Rev., 2003, 246, 305 CrossRef CAS PubMed; (b) X. Q. Lu, Y. Q. Qiao, J. R. He, M. Pan, B. S. Kang and C. Y. Su, Cryst. Growth Des., 2006, 6, 1910 CrossRef; (c) H. L. Ngo and W. B. Lin, J. Am. Chem. Soc., 2002, 124, 14298 CrossRef CAS PubMed; (d) Y. Cui, S. J. Lee and W. B. Lin, J. Am. Chem. Soc., 2003, 125, 6014 CrossRef CAS PubMed.
- (a) M. H. Zeng, B. Wang, X. Y. Wang, W. X. Zhang, X. M. Chen and S. Gao, Inorg. Chem., 2006, 45, 7069 CrossRef CAS PubMed; (b) D. N. Dybtsev, M. P. Yutkin, E. V. Peresypkina, A. V. Virovets, C. Serre, G. Ferey and V. P. Fedin, Inorg. Chem., 2007, 46, 6843 CrossRef CAS PubMed; (c) X. Q. Liang, D. P. Li, X. H. Zhou, Y. Sui, Y. Z. Li, J. L. Zuo and X. Z. You, Cryst. Growth Des., 2009, 9, 4872 CrossRef CAS; (d) J. Y. Wu, S. M. Huang and M. H. Chiang, CrystEngComm, 2010, 12, 3909 RSC.
- (a) J. Zhang and X. H. Bu, Angew. Chem., Int. Ed., 2007, 46, 6115 CrossRef CAS PubMed; (b) J. Zhang, Y. G. Yao and X. H. Bu, Chem. Mater., 2007, 19, 5083 CrossRef CAS; (c) S. M. Chen, J. Zhang and X. H. Bu, Inorg. Chem., 2008, 47, 5567 CrossRef CAS PubMed; (d) J. Zhang, S. M. Chen, T. Wu, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2008, 130, 12882 CrossRef CAS PubMed; (e) J. Zhang and X. H. Bu, Chem. Commun., 2009, 206 RSC.
- (a) J. W. Dai and M. L. Tong, CrystEngComm, 2012, 14, 2124 RSC; (b) J. C. Ma, Y. Y. Liu, J. Yang, Y. Y. Liu and J. F. Ma, CrystEngComm, 2011, 13, 3498 RSC; (c) Z. Amghouz, S. Garcia-Granda, J. R. Garcia, A. Clearfield and R. Valiente, Cryst. Growth Des., 2011, 11, 5289 CrossRef CAS; (d) Y. Han, X. Li, L. Li, C. Ma, Z. Shen, Y. Song and X. You, Inorg. Chem., 2010, 49, 10781 CrossRef CAS PubMed.
- (a) M. L. Sun, J. Zhang, Q. P. Lin, P. X. Yin and Y. G. Yao, Inorg. Chem., 2010, 49, 9257 CrossRef CAS PubMed; (b) Y. M. Song, H. X. Huang, G. M. Sun, Z. W. Liao, M. B. Luo, S. J. Liu and F. Luo, CrystEngComm, 2011, 13, 6827 RSC; (c) D. B. Dang, B. An, Y. Bai, G. S. Zheng and J. Y. Niu, Chem. Commun., 2013, 49, 2243 RSC.
- (a) J. Juillard, Pure Appl. Chem., 1977, 49, 885 CrossRef; (b) G. Huang, P. Yang, N. Wang, J.-Z. Wu and Y. Yu, Inorg. Chim. Acta, 2012, 384, 333 CrossRef CAS PubMed; (c) A. D. Burrows, K. Cassar, R. M. W. Friend, M. F. Mahon, S. P. Rigby and J. E. Warren, CrystEngComm, 2005, 7, 548 RSC.
- (a) D. N. Dybtsev, M. P. Yutkin, E. V. Peresypkina, A. V. Virovets, C. Serre, G. Ferey and V. P. Fedin, Inorg. Chem., 2007, 46, 6843 CrossRef CAS PubMed; (b) X. Y. Wang, H. Y. Wei, Z. M. Wang, Z. D. Chen and S. Gao, Inorg. Chem., 2005, 44, 572 CrossRef CAS PubMed; (c) W.-G. Lu, D.-C. Zhong, L. Jiang and T.-B. Lu, Cryst. Growth Des., 2012, 12, 3675 CrossRef CAS.
- (a) L. G. Westin, M. Kritikos and A. Caneschi, Chem. Commun., 2003, 1012 RSC; (b) A. Thirumurugan and S. Natarajan, Cryst. Growth Des., 2006, 6, 983 CrossRef CAS; (c) H. S. Wang, B. Zhao, B. Zhai, W. Shi, P. Cheng, D. Z. Liao and S. P. Yan, Cryst. Growth Des., 2007, 7, 1851 CrossRef CAS; (d) L. F. Chen, J. Zhang, G. Q. Ren, Z. J. Li, Y. Y. Qin, P. X. Yin, J. K. Cheng and Y. G. Yao, CrystEngComm, 2008, 10, 1088 RSC.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. L. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504 CrossRef CAS PubMed.
- (a) B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, C. H. Yan and G. X. Xu, Angew. Chem., Int. Ed., 2000, 39, 3644 CrossRef CAS; (b) W. H. Fang, L. Cheng, L. Huang and G. Y. Yang, Inorg. Chem., 2013, 52, 6 CrossRef CAS PubMed; (c) X. J. Kong, Y. Wu, L. S. Long, L. S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6918 CrossRef CAS PubMed; (d) Y. L. Wang, Y. Y. Gao, Y. Ma, Q. L. Wang, L. C. Li and D. Z. Liao, CrystEngComm, 2012, 14, 4706 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, additional tables and figures, PXRD, TG curve, the plots of temperature dependence of the magnetic susceptibilities. CCDC 740834–740836. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3nj00981e |
| ‡ Crystal data for 1: C11H15DyO6, orthorhombic, P2(1)2(1)2(1), a = 8.152(3) Å, b = 10.886(4) Å, c = 14.782(5) Å, V = 1311.8(8) Å3, Z = 4, Dc = 2.054 g cm−3, Flack parameter = 0.01(2), F(000) = 780, T = 293(2) K. The final R1 = 0.0293, wR2 = 0.0754 and S = 1.068 for 2783 observed reflections with I > 2.0σ(I). Crystal data for 2: C11H15HoO6, orthorhombic, P2(1)2(1)2(1), a = 8.1824(3) Å, b = 10.8837(5) Å, c = 14.7894(9) Å, V = 1317.07(1) Å3, Z = 4, Dc = 2.058 g cm−3, Flack parameter = 0.06(2), F(000) = 784, T = 293(2) K. The final R1 = 0.0320, wR2 = 0.0747 and S = 1.054 for 2798 observed reflections with I > 2.0σ(I). Crystal data for 3: C11H15ErO6, orthorhombic, P2(1)2(1)2(1), a = 8.1178(5) Å, b = 10.9087(7) Å, c = 14.7493(1) Å, V = 1306.12(2) Å3, Z = 4, Dc = 2.088 g cm−3, Flack parameter = 0.01(3), F(000) = 788, T = 293(2) K. The final R1 = 0.0352, wR2 = 0.0844 and S = 1.173 for 2775 observed reflections with I > 2.0σ(I). |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |