New strategies for the synthesis of lactones using peroxymonosulphate salts, ionic liquids and microwave or ultrasound irradiation†
Karolina
Matuszek
a,
Przemysław
Zawadzki
b,
Wojciech
Czardybon
b and
Anna
Chrobok
*a
aSilesian University of Technology, Faculty of Chemistry, Department of Chemical Organic Technology and Petrochemistry, ul. Krzywoustego 4, 44-100 Gliwice, Poland. E-mail: anna.chrobok@polsl.pl; Fax: +48 322371032; Tel: +48 322372917
bSelvita S.A., Park Life Science, Bobrzyńskiego 14, 30-348 Kraków, Poland. E-mail: wojciech.czardybon@selvita.com; Fax: +48 122974701; Tel: +48 122974700
First published on 23rd October 2013
Abstract
A new method for the rapid synthesis of lactones via the one-pot oxidation of alcohols with potassium peroxymonosulphate in an ionic liquid was developed. The use of microwave or ultrasonic irradiation increased the reaction rates significantly. Additionally, new peroxymonosulphate ionic liquids were synthesised and used as effective oxidants in the synthesis of lactones.
Introduction
In recent years, new safety and environmental requirements have led to the design of new reaction systems based on sustainable chemistry principles. Classical processes are being modified to minimise the use and generation of hazardous substances and wastes.1 The use of ionic liquids in organic synthesis provides a good alternative to the use of volatile organic solvents. Ionic liquids possess many interesting properties, including a low vapour pressure, the ability to dissolve organic and inorganic substances, thermal and chemical stability, non-flammability and non-corrosive behaviour. Moreover, they are called “designer solvents” and can act as both catalysts and solvents.2–4Ionic liquids could also potentially be used as oxidants, an application that has not yet been described. One way to synthesise an ionic-liquid oxidant is to introduce a peroxy group into the anion structure, e.g., HSO5−. Commercial sources of potassium peroxymonosulphate, e.g., Oxone®, are stable oxidising agents commonly used in fine chemicals synthesis. The oxidising peroxymonosulphate salt is easy to handle, non-toxic and relatively inexpensive, and it generates non-polluting by-products.5 However, peroxymonosulphate salts have poor solubility in organic solvents, making it difficult to apply effectively in oxidation reactions. To use KHSO5 in oxidation reactions, an aqueous reaction medium must be employed, which can lead to the hydrolysis of unstable products, e.g., lactones or esters. Therefore, in our previous work, alternative methods for the oxidation of ketones or alcohols were presented. The first method was based on the use of an alternative solvent, such as an ionic liquid, to dissolve KHSO5 and eliminate water from the reaction system.6,7 The second method used phase transfer catalysis to avoid the hydrolysis of lactones.8 Y. Wei also presented an interesting approach for the synthesis of aldehydes and ketones. Specifically, a new oxidising agent, tetrabutylammonium peroxymonosulphate ([N4444][HSO5]), was employed in the clean, selective oxidation of alcohols catalysed by a nitroxyl radical, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), in the presence of tetrabutylammonium bromide (TBAB) in an ionic liquid.9 Other work has described the oxidation of primary and secondary alcohols to ketones with KHSO5 in classical solvents, such as dichloromethane or toluene.10 According to the literature, the solubility of the peroxymonosulphate salts and the use of homogenous conditions are critical for the oxidation of ketones to lactones, which is in contrast to the oxidation of alcohols to ketones where homogenous conditions are not required.5 Thus, alcohols can only be oxidised to ketones with KHSO5 in classical solvents. The one-pot, tandem oxidation of alcohols directly to esters or lactones has not been well studied. Only a few reports of this type of oxidation reaction have appeared in the literature.11–14
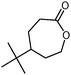
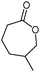
The one-pot oxidation would be especially useful for ε-caprolactone production. Currently, ε-caprolactone is produced from cyclohexanol via a two-step process. From a synthetic and industrial viewpoint, a one-pot procedure would be economically favourable.
Results and discussion
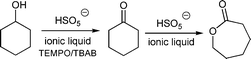
In this work, new strategies for the synthesis of ε-caprolactone were developed. “Greener” chemical syntheses using ionic liquids as solvents for the KHSO5 oxidant were performed using ultrasound and microwave irradiation (Scheme 1). The ionic liquids were also demonstrated to act as oxidising agents. A new class of ionic liquids with peroxymonosulphate anions was synthesised and employed in the model oxidation.Ultrasound or microwave-assisted synthesis of ε-caprolactone using potassium peroxymonosulphate and ionic liquids
We previously utilised [bmim]BF4 as a novel medium for the one-pot, tandem oxidation of cyclohexanol to ε-caprolactone with Oxone® in a TEMPO/TBAB catalytic system with magnetic stirring (Table 1, magnetic stirring).6 Because [bmim]BF4 can dissolve both cyclohexanol and KHSO5, thus resulting in homogeneous conditions, the tandem reaction can be performed.| Mixing procedure | [bmim][BF4]a | |
|---|---|---|
| Reaction time [h] | Yield of ε-caprolactoneb [%] | |
| a Solubility of KHSO5 in IL is equal 0.13 [g ml−1], data from literature.7 b Yield of ε-caprolactone was determined by GC. c Data from literature.6 | ||
| Magnetic stirrer | 8 | 78c |
| Ultrasounds irradiation | 5 | 80 |
| Microwaves irradiation | 0.5 | 85 |
The inability of dichloromethane and other typical organic solvents to dissolve KHSO5 prevents their use in the tandem oxidation of alcohols.
Microwave reactors and ultrasound baths are new alternative heat sources that generate heat evenly throughout the reactor, thus preventing local overheating, which can influence the purity of the reaction product. Furthermore, these methods are very efficient, accelerating many organic reactions.15 The properties of ionic liquids allow them to utilise microwave energy effectively. Ionic species, such as ionic liquids, that consist entirely of ions can absorb microwave energy very efficiently. Another advantage of ionic liquids is their lack of a measurable vapour pressure, resulting in the low risk of an explosion caused by a rapid increase in the solvent vapour pressure.16
The use of ultrasound or microwave irradiation in the one-pot, TEMPO-catalysed oxidation of cyclohexanol in [bmim][BF4] reduced the reaction time (Table 1). TBAB acted as the bromide ion source and reacted with KHSO5 to generate hypobromous acid, which is a stronger oxidant, in situ. The nitroxyl radical was subsequently oxidised to N-oxoammonium bromide.10
Ultrasound irradiation reduced the reaction time for the ε-caprolactone synthesis from 8 h to 5 h and gave a yield of 80%. The microwave reactor led to a significant decrease in the reaction time for the lactone production to 30 min.
The solubility of KHSO5 in the ionic liquid (0.13 g ml−1 for [bmim][BF4])7 is a key parameter for both steps of the oxidation. We discovered a more environmentally friendly ionic liquid based on the dicyanide anion, [bmim][N(CN)2], that could be used in this reaction. The solubility of KHSO5 in this ionic liquid was measured as described elsewhere7 and was also found to be high (0.16 g ml−1). The results for the oxidation of cyclohexanol in [bmim][N(CN)2] are presented in Table 2. In this case, ε-caprolactone was also obtained in high yields in significantly shorter reaction times when ultrasound or microwave irradiation was employed. The results also showed that TBAB could be replaced with 2,2,6,6-tetramethylpiperidine hydrobromide (TMP*HBr).
| Mixing procedure | [bmim][N(CN)2]a | ||
|---|---|---|---|
| Catalyst | Reaction time | Yield of ε-caprolactone [%] | |
| a Solubility of KHSO5 in IL is equal 0.16 [g ml−1]. | |||
| Magnetic stirrer | TEMPO/TBAB | 7 h | 83 |
| TMP*HBr | 7 h | 63 | |
| Ultrasounds irradiation | TEMPO/TBAB | 5 h | 81 |
| TMP*HBr | 5 h | 71 | |
| Microwaves irradiation | TEMPO/TBAB | 10 min | 53 |
| TEMPO/TBAB | 30 min | 83 | |
| TMP*HBr | 10 min | 67 | |
| TMP*HBr | 30 min | 81 | |
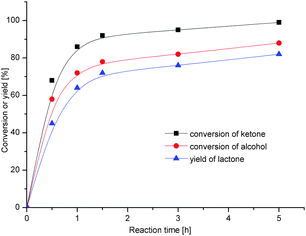
The successful oxidation of alcohols to lactones demonstrated here might lead to a new environmentally friendly protocol for MW- or ultrasound-assisted reactions in ionic liquids. It should be noted that the reactions performed in a microwave or ultrasound bath are completely homogenous with all Oxone® inorganic salts and organic reactants dissolved during the reaction. Fig. 1 shows the progress of the cyclohexanol oxidation in [bmim][N(CN)2] under ultrasound irradiation in terms of the alcohol and intermediate ketone conversions and lactone yield over time. When traditional stirring was used for the same reaction, the partial insolubility of the inorganic salts resulted in longer reaction times.
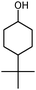
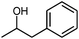
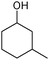
Finally, to determine its practical potential, other cyclic alcohols, including cyclobutanol, 1-phenyl-2-propanol, 3-methyl-cyclohexanol and 4-tert-butylcyclohexanol, were oxidised using the most active reaction system, which consisted of Oxone®, TEMPO/TBAB and [bmim][N(CN)2] in an ultrasound bath. As shown in Table 3, the oxidation reactions proceeded very efficiently; in fact, the cyclic alcohols were readily oxidised to their corresponding lactones in high yields (75–89%) under our mild conditions within relatively short reaction times.
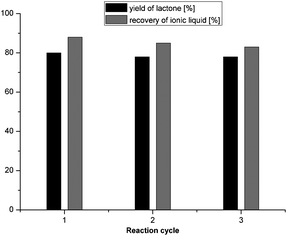
The recyclability of the ionic liquid was tested by oxidising cyclohexanol in the most active reaction system in three successive runs. The results showed that the activity of the ionic liquid in the model reaction was very high but slowly declined for each successive run (Fig. 2).
Synthesis of peroxymonosulphate ionic liquids (PSILs) and their use in the synthesis of ε-caprolactone
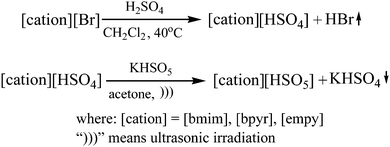
To create homogenous conditions in the reactions involving a peroxymonosulphate oxidation agent, a liquid salt that can act as both an oxidising agent and a solvent can be used. We therefore synthesised ionic liquids with a peroxymonosulphate anion. Only one example of a salt with an organic cation and peroxymonosulphate anion (tetrabutylammonium peroxymono-sulphate [N4444][HSO5]) has been reported in the literature.9 Unfortunately, this salt, which is an effective oxidant for oxidative cleavage reactions, is a solid at room temperature.To lower the melting points of the organic salts used in this work, we treated the following bromides, 1-butyl-3-methylimidazolium [bmim], 1-ethyl-1-methylpyrrolidinium [empy] and 1-butylpyridinium [bpyr], with sulphuric acid (Scheme 2). The produced hydrogen sulphate salts were mixed with Oxone® (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) in acetone under ultrasound irradiation. The waste inorganic salts were then filtered out, and the resulting peroxymonosulphate ionic liquids (PSILs) were concentrated. The active oxygen content ranged from 6.29% to 6.99% (Table 4). The resulting salts were liquids at room temperature. The structures of the new ionic liquids were determined using 1H and 13C NMR. The NMR spectra of the neat ionic liquids exhibited different proton positions for the peroxymonosulphate anions than for hydrogen sulphate.
3) in acetone under ultrasound irradiation. The waste inorganic salts were then filtered out, and the resulting peroxymonosulphate ionic liquids (PSILs) were concentrated. The active oxygen content ranged from 6.29% to 6.99% (Table 4). The resulting salts were liquids at room temperature. The structures of the new ionic liquids were determined using 1H and 13C NMR. The NMR spectra of the neat ionic liquids exhibited different proton positions for the peroxymonosulphate anions than for hydrogen sulphate.
| Peroxymonosulphate ionic liquid (PSIL) | Active oxygen contenta [%] |
T
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) onset
[°C] onset
[°C] |
T
50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) onset
[°C] onset
[°C] |
T dec [°C] |
|---|---|---|---|---|
a Determined by iodometric titration.
b
T
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) onset decomposition of 5% of the sample.
c
T
50% onset decomposition of 5% of the sample.
c
T
50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) onset decomposition of 50% of the sample.
d
T
dec decomposition.
e Multistep decomposition was observed. onset decomposition of 50% of the sample.
d
T
dec decomposition.
e Multistep decomposition was observed.
|
||||
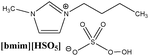
|
Calc.: 6.34 | 97 | 344 | 350 |
| Found: 6.29 | ||||
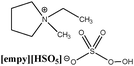
|
Calc.: 7.05 | 78 | 144 | 116, 139, 300e |
| Found: 6.99 | ||||
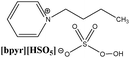
|
Calc.: 6.41 | 90 | 298 | 125, 337e |
| Found: 6.34 | ||||
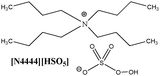
|
Calc.: 4.50 | — | — | — |
| Found: 4.45 | ||||
Additionally, ESI-MS experiments were performed. The peaks found in positive and negative ESI modes confirmed the presence of peroxymonosulphate anions and adequate cations in all PSIL samples. The PSIL decomposition temperatures were measured using thermogravimetric analysis and were in the range of 144–344 °C. All detailed data are presented in the ESI.†
Using 1-butyl-3-methylimidazolium peroxymonosulphate as the oxidant and solvent in the oxidation of cyclohexanol resulted in the efficient synthesis of ε-caprolactone (Table 5). Ultrasound irradiation enabled the high-yield (90%) production of the lactone. Unfortunately, the use of microwave assistance was not successful for this reaction; the reaction system overheated. For comparison, the reaction was also performed using [N4444][HSO5] in dichloromethane, but the reaction was not homogenous, which prevented lactone formation. The use of [bmim][N(CN)2] and ultrasound irradiation for this reaction resulted in a high product yield (78%).
The reaction pathway can be described as proposed in Scheme 3. The initial effect of ionic liquid leads to activation of the carbonyl group. This is followed by a nucleophilic attack of the peroxymonosulphate anion on the carbonyl group and a tetrahedral intermediate (Criege adduct) is formed. The rearrangement of the Criege adduct gives the final lactone and ionic liquid [cation][HSO4].
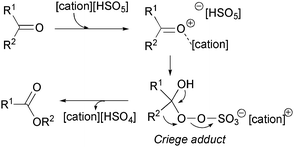 | ||
| Scheme 3 Proposed mechanism of the Baeyer–Villiger oxidation of cyclic ketones with peroxymonosulphate ionic liquids. | ||
Conclusions
In summary, we developed a new general method for lactone synthesis via the one-pot oxidation of alcohols with peroxymonosulphate salts. The addition of ionic liquids to this reaction system facilitated the formation of homogenous conditions in the reactor, which is essential for this tandem reaction, thereby providing a more sustainable approach to lactone synthesis. The synthesis was accelerated by MW/ultrasonic irradiation.Oxone® is a cheap, commercially available oxidant and easily oxidises several functional groups. Our new approach expands the range of its applications. Using the new method, lactones were produced in high yields and with high selectivities. Commonly, application of Oxone® in Baeyer–Villiger reaction requires an aqueous reaction medium, which can lead to the hydrolysis of lactones causing the formation of by-products. This drawback can be avoided by applying ionic liquids proposed in this work. Moreover, peroxymonosulphate ionic liquids do not contain the ballast of inorganic salts (KHSO4 and K2SO4) against Oxone®, thereby resulting in easier work-up and product purification. Further applications of PSILs to other MW/ultrasonic irradiation reactions are currently under study.
Experimental section
General procedure for the synthesis of peroxymonosulphate salts
A solution of 4.2 mmol of a hydrogen sulphate salt ([bmim], [bpyr], [empy]) and 12.6 mmol of Oxone® in 10 ml of anhydrous acetone was placed into a 50 ml two-necked flask. The flask was equipped with a cooler, sealed in a nitrogen atmosphere and placed in an ultrasound bath for 2 h. Next, the mixture was filtered to isolate the rest of the inorganic salts. The filtrate was concentrated under vacuum and dried (10 h, 60 mbar). The resulting peroxymonosulphate salts were pale yellow liquids and were obtained in yields between 90–98%.General procedure for lactone synthesis using microwave or ultrasound irradiation
Cyclohexanol (1 mmol), TEMPO (0.05 mmol), TBAB (0.05 mmol), Oxone® (4 mmol) and [bmim][N(CN)2] or [bmim][BF4] (3 ml) were placed in a round-bottom flask. The reaction mixture was stirred for 10 minutes to 10 h depending on the reaction rate in an oil bath, ultrasound bath or microwave reactor at 40 °C. The reaction progress was monitored by GC. The post-reaction mixture was dissolved in CH2Cl2 and filtered to separate the products from the Oxone® inorganic salts. Next, the filtrate was concentrated. The ionic liquid was then extracted with diethyl ether (6 × 5 ml). After purifying the product by column chromatography with hexane–ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent, the ε-caprolactone yields were 75–80%.
1) as the eluent, the ε-caprolactone yields were 75–80%.
General procedure for the synthesis of ε-caprolactone using peroxymonosulphate salts
Cyclohexanol (1 mmol), TEMPO (0.05 mmol), TBAB (0.05 mmol) and a peroxymonosulphate salt (6 mmol) were placed in a round-bottom flask. The reaction mixture was stirred in an oil or ultrasound bath at 40 °C for 5–7 h depending on the reaction rate. The reaction progress was monitored by GC. After the reaction was completed, the ionic liquid was extracted with diethyl ether (6 × 5 ml). After purifying the product by column chromatography with hexane–ethyl acetate (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent, the ε-caprolactone yields were 75–85%.
1) as the eluent, the ε-caprolactone yields were 75–85%.
Recycling of [bmim][N(CN)2]
The model reaction for the ε-caprolactone synthesis using Oxone® as the oxidant and ultrasound irradiation was chosen for the ionic liquid recycling experiments. After the reaction was completed, the IL was purified for the recycling tests. After filtering the post-reaction mixture and extracting the product with diethyl ether, the IL was concentrated, dried under vacuum (60 °C, 5 h) and reused.Acknowledgements
This work was financed by the Ministry of Science and Higher Education (Grant no. N N209 021739).Notes and references
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1 CrossRef CAS PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2007 Search PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- H. Hussain, I. R. Green and I. Ahmed, Chem. Rev., 2013, 113, 3329 CrossRef CAS PubMed.
- A. Chrobok, Synlett, 2011, 391 CrossRef CAS PubMed.
- A. Chrobok, Tetrahedron, 2010, 66, 6212 CrossRef CAS PubMed.
- S. Baj, A. Chrobok and A. Siewniak, Appl. Catal., A, 2011, 395, 49 CrossRef CAS PubMed.
- C. Zhu, L. Ji and Y. Wei, Catal. Commun., 2010, 11, 1017 CrossRef CAS PubMed.
- C. Bolm, A. S. Magnus and J. P. Hildebrand, Org. Lett., 2000, 2, 1173 CrossRef CAS.
- L. Balbinot, U. Schuchardt, C. Vera and J. Sepúlveda, Catal. Commun., 2008, 9, 1878 CrossRef CAS PubMed.
- M. L. Morin-Fox and M. A. Lipton, Tetrahedron Lett., 1992, 33, 5699 CrossRef CAS.
- J. A. Cella, J. P. McGrath, J. A. Kelley, O. El Soukkary and L. Hilpert, J. Org. Chem., 1977, 42, 2077 CrossRef CAS.
- O. Fukuda, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 2001, 42, 3479 CrossRef CAS.
- R. S. Varma, Green Chem. Lett. Rev., 2007, 1, 37 CrossRef CAS.
- S. Toma, R. Sebesta and M. Meciarova, Curr. Org. Chem., 2011, 15, 2257 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and NMR spectra. See DOI: 10.1039/c3nj01170d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |