Application of a new interface for rapid optimisation of bio-catalysed processes: proteolytic digestion and an enzyme-catalysed transesterification as examples
Lauren M.
Stencel
ab and
Nicholas E.
Leadbeater
*ac
aDepartment of Chemistry, University of Connecticut, 55 North Eagleville Road, Storrs, CT 06269, USA. E-mail: nicholas.leadbeater@uconn.edu; Fax: +1 860 486 2981; Tel: +1 860 486 5076
bDepartment of Chemistry, Amherst College, Amherst, MA 01002, USA
cDepartment of Community Medicine & Health Care, University of Connecticut Health Center, The Exchange, 263 Farmington Ave, Farmington, CT 06030, USA
First published on 29th October 2013
Abstract
The results of an evaluation of the iChemExplorer for the study of bio-catalysed processes are reported. The iChemExplorer comprises of a specially-designed sample tray and a control unit, the former of which replaces a traditional tray in an HPLC autosampler assembly. It can be heated and reaction mixtures can be agitated. The system has been used to study the trypsin digestion of insulin chain B, cytochrome c and bovine serum albumin as well as the lipase-catalysed transesterification reaction between ethyl benzoate and 1-butanol.
Introduction
Biomolecules play key roles in chemical reactions.1 Examples are the use of enzymes for performing the selective digestion of proteins into smaller polypeptide fragments2 or for preparative organic chemistry.3 There are a number of tools used for the analysis of the reaction products,4 one of them being high performance liquid chromatography (HPLC).5 As well as a stand-alone instrument, HPLC can also be used in tandem with other apparatus.6 One such peripheral tool that has recently become available is the iChemExplorer (Fig. 1).7 It is comprised of a specially-designed sample tray and a control unit. When installed, this sample tray replaces a traditional one in the HPLC autosampler assembly, with space for 60 vials. The sample tray can be heated to temperatures up to 150 °C with an in-block thermocouple providing direct temperature feedback control. It is also possible to agitate reaction mixtures by means of magnetic stirrer bars placed in individual vials. The control unit sits directly below the autosampler and the software that runs the system loads to the PC controlling the HPLC. The software has three tabs for set-up and analysis for the reaction: iHeat, which regulates the temperature for the reaction and has the ability to be set at one temperature or changed during the reaction; iSample, which allows sequences to be created and run by the HPLC; and iGraph, which is where the data can be analysed on the screen or exported to Excel for further processing. The system can be programed to take samples at pre-defined time periods and the small sample volume saves precious starting materials while also maintaining near-constant reaction concentration. Samples can either be directly injected onto the HPLC column or alternatively transferred to a vial on the sample tray containing solvent to allow for dilution before injection. The iChemExplorer has been used with success in preparative chemistry to monitor a reaction in real-time8 and also in pharmaceutical pH stress testing for the elucidation of degradation products and pathways.9 It occurred to us that this simple, inexpensive, yet innovative alternative to other tools may be valuable for studying enzyme-catalysed protein digestion and organic chemistry. We present our findings here.Results and discussion
We decided to focus on trypsin digestion of proteins and a lipase-catalysed transesterification reaction. The goal for trypsin digestion was to begin with a small protein and prove the iChemExplorer could be used to optimise reaction conditions, such as temperature and ratio of enzyme to substrate. Then, the scope would be broadened to a larger protein. To this end, we decided to start with insulin chain B and move to cytochrome c. From an enzyme catalysis standpoint, we wanted to focus on the reaction of ethyl benzoate and butanol to yield butyl benzoate catalysed by an immobilized lipase from Candida antarctica. This transformation tends to proceed rather slowly and as a result, chemists often try varying numerous parameters such as temperature, solvent, enzyme to substrate ratio, and stoichiometry of substrates in order to optimise conditions. The iChemExplorer seemed ideally suited for this application.One of our first objectives was to find a method for comparison of the outcome of different trials for each reaction. Using the HPLC chromatograms, the relative percent digestion or conversion could be calculated. First, the starting materials and products were individually run through the HPLC so that retention time data could be collected. Next, HPLC conditions were optimised for each class of reaction so that a clear separation of the signals was achieved. Relative conversion data could then be obtained by one of two methods, the application of which we had shown in a previous study using microwave heating.10 For transesterification reactions, the value for the area of the product peak was divided by that of the product plus unreacted starting material. In the case of the proteolytic digests, the value for the area of the signals from the cleaved fragments was divided by that of the sum of all product peaks plus unreacted starting material.
Proteolytic digestion
Insulin chain B, with a sequence of 30 amino acids, has three cleavage points possible upon digestion with trypsin.11 In the HPLC chromatogram, one peak is seen at 15.6 min corresponding to the undigested protein [BFVNQHLCoxGSHLVEALYLVCoxGERGFFYTPKA], and two peaks for digested fragments (14.1 min [FVNQHLCoxGSHLVEALYLVCoxGER] and 11.6 min [GFFYTPK]). Starting with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 ratio of trypsin to insulin chain B, the effect of varying the temperature on the rate of digestion was probed. Proteolytic digests were performed at room temperature (26 °C), 40 °C, and 50 °C and the degree of digestion monitored every 10 min using the iChemExplorer. As shown in Fig. 2, at room temperature and 40 °C the digestion reached completion within 1 h, there not being significant difference in relative rate between the two. At 50 °C, the degree of digestion reached a plateau at 35%. This can be attributed to the denaturation and precipitation of the insulin chain B at the elevated temperature.
25 ratio of trypsin to insulin chain B, the effect of varying the temperature on the rate of digestion was probed. Proteolytic digests were performed at room temperature (26 °C), 40 °C, and 50 °C and the degree of digestion monitored every 10 min using the iChemExplorer. As shown in Fig. 2, at room temperature and 40 °C the digestion reached completion within 1 h, there not being significant difference in relative rate between the two. At 50 °C, the degree of digestion reached a plateau at 35%. This can be attributed to the denaturation and precipitation of the insulin chain B at the elevated temperature.
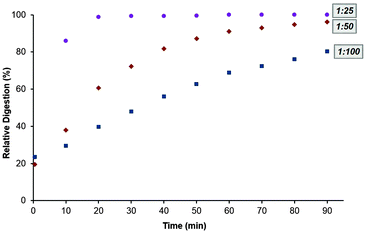
The effect of varying the protease to protein ratio on the rate of digestion was next probed. Keeping the temperature constant at 26 °C, the digests were performed at trypsin to insulin chain B ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1
25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and 1
50, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (Fig. 3). These ratios were chosen such that if autolysis of trypsin occurs, the resultant fragments are in too low a concentration to be detected. As could be expected, the greatest initial rate of digestion was observed when using the highest ratio of protease to protein (1
100 (Fig. 3). These ratios were chosen such that if autolysis of trypsin occurs, the resultant fragments are in too low a concentration to be detected. As could be expected, the greatest initial rate of digestion was observed when using the highest ratio of protease to protein (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) although, by the 80 minute mark, the level of digestion at the lower 1
25) although, by the 80 minute mark, the level of digestion at the lower 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio was comparable to that at 1
50 ratio was comparable to that at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.
25.
Our attention turned next to cytochrome c (cyt c). This has a sequence of 103 amino acids with 20 cleavage fragments possible upon trypsin digestion.11 Our study again began by probing the effects of temperature on the proteolytic digestion using a protease to protein ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. Compared to insulin chain B, cyt c is significantly more complex. Since it is a full protein, it contains multiple degrees of structural integrity to maintain its structure and therefore may not necessarily denature or aggregate at the same temperature. With this digestion, there was little variation across the range from room temperature to 50 °C (Fig. 4). That said, the digest at 40 °C did perform slightly better, and thus was chosen as the optimum temperature for the next step; study of the effects of varying the protease to protein ratio. The digest was assayed at protease to protein ratios of 1
25. Compared to insulin chain B, cyt c is significantly more complex. Since it is a full protein, it contains multiple degrees of structural integrity to maintain its structure and therefore may not necessarily denature or aggregate at the same temperature. With this digestion, there was little variation across the range from room temperature to 50 °C (Fig. 4). That said, the digest at 40 °C did perform slightly better, and thus was chosen as the optimum temperature for the next step; study of the effects of varying the protease to protein ratio. The digest was assayed at protease to protein ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 1
5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, the former not surprisingly being optimal (Fig. 5). Unlike the case of insulin chain B, a lower protease to protein ratio does not result in a comparable level of digestion over longer times.
25, the former not surprisingly being optimal (Fig. 5). Unlike the case of insulin chain B, a lower protease to protein ratio does not result in a comparable level of digestion over longer times.
The final protein we decided to study was bovine serum albumen (BSA) which comprises of 583 amino acids with 74 cleavage sites.11 This leads to many more fragments than the previous proteins analysed and did pose a problem for analysis. Rather than obtain quantitative data, we were only able to monitor digestion from more of a qualitative perspective, watching disappearance of the signal for the whole protein. As before, we varied the temperature at which the digestion was performed, using a protease to protein ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, observing in this case a significantly greater degree of digestion at 50 °C than at the other (lower) temperatures studied (Fig. 6). Operating at 50 °C, we varied the protease to protein ratio, 1
25, observing in this case a significantly greater degree of digestion at 50 °C than at the other (lower) temperatures studied (Fig. 6). Operating at 50 °C, we varied the protease to protein ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 proving optimal (Fig. 7).
10 proving optimal (Fig. 7).
Having shown we were able to monitor proteolytic digests in real-time using the iChemExplorer, and at the same time seeing the limitation of the method when using larger protein substrates, we decided to turn our attention to the other reaction for study; the lipase-catalysed transesterification.
Lipase-catalysed transesterification
With a shift towards environmentally friendly protocols, chemists are increasingly turning to biocatalysts for performing reactions.3,12 Due to their broad substrate scope, ease of use and the mild reaction conditions required, hydrolases have seen particular application and within the hydrolase family, lipases are the most frequently used.13 They can catalyse reactions as diverse as kinetic resolution of racemic amines and the regioselective transesterification of polyfunctional substrates. When employing an enzyme in synthetic chemistry, there are a number of parameters that need to be optimised, these including solvent, temperature and enzyme loading. In our mind, the iChemExplorer seemed like a valuable tool that could be used to accelerate the optimisation process since it can monitor multiple reactions at once in an automated fashion. We decided to probe the transesterification reaction between ethyl benzoate and 1-butanol catalysed by immobilized lipase from Candida antarctica (Scheme 1). We selected four parameters for study: quantity of enzyme used, temperature of the reaction, solvent, and relative stoichiometry of the reagents.As the first parameter for study, we selected to vary the enzyme loading. Using loadings of 50 mg and 75 mg, the reaction was run at 60 °C for 24 h using butanol both as reagent and solvent. Samples were taken every 15 min for 12 h and then once after 24 h (Fig. 8). Although the initial rate of reaction was similar, the conversion to butyl benzoate over 24 h using 50 mg of lipase was significantly lower than that using 75 mg of the enzyme. However, even when using the higher enzyme loading we obtained only about a 40% conversion to product.
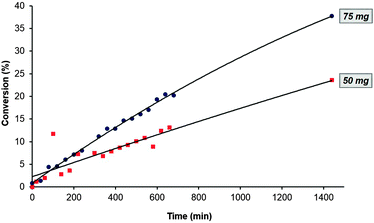 | ||
Fig. 8 Conversion of ethyl benzoate to butyl benzoate as a function of enzyme loading:  50 mg, 50 mg,  75 mg. 75 mg. | ||
A literature search revealed that small chain alcohols can inhibit lipase activity, so it was plausible that the excess butanol used in our reaction was inhibiting the enzyme activity.14 Another source stated that this was not the case and that lipases show an increase in activity when using excess butanol.15 To probe this further, the reaction was performed in another solvent. Butanol was originally chosen as the substrate and as solvent because it does not show up when using UV detection, allowing only the substrate and product to be seen in the HPLC chromatogram. Decane is another solvent that meets these requirements. The reaction was performed with decane and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ethyl benzoate to n-butanol ratio at 60 °C for 4 hours, employing 75 mg of enzyme (Fig. 9). Since the conversion to product was increased from about 10% in that time frame to about 40%, decane proved to be a better solvent for the reaction as compared to butanol. Also from this result, it does appear that butanol has an inhibitory effect on the activity of the lipase in this reaction.
1.5 ethyl benzoate to n-butanol ratio at 60 °C for 4 hours, employing 75 mg of enzyme (Fig. 9). Since the conversion to product was increased from about 10% in that time frame to about 40%, decane proved to be a better solvent for the reaction as compared to butanol. Also from this result, it does appear that butanol has an inhibitory effect on the activity of the lipase in this reaction.
We next probed the effects of changing the relative stoichiometry of the starting materials on the outcome of the reaction. Our initial trials were performed at an ethyl benzoate to butanol ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. We ran the reaction at a higher molar ratio (1
1.5. We ran the reaction at a higher molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and a lower ratio (1
2) and a lower ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2), finding that the latter gave the best conversion to butyl benzoate after 240 min when operating at 60 °C in decane as the solvent and using 75 mg of enzyme (Fig. 10).
1.2), finding that the latter gave the best conversion to butyl benzoate after 240 min when operating at 60 °C in decane as the solvent and using 75 mg of enzyme (Fig. 10).
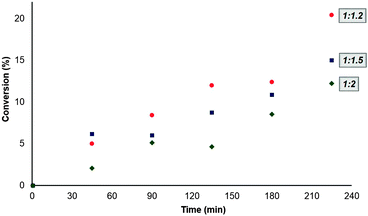 | ||
Fig. 10 Conversion of ethyl benzoate to butyl benzoate as a function of ethyl benzoate to butanol stoichiometry:  1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, 1.2,  1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1.5,  1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. 2. | ||
We finally monitored the conversion of the reaction as a function of temperature across the range from 40–70 °C, operating back at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio of ethyl benzoate to butanol, in decane as the solvent, and using 75 mg of enzyme. After 4 h, the reaction performed at 70 °C showed a significantly greater conversion than those at lower temperatures (Fig. 11). This shows that the immobilized lipase works even at elevated temperatures. This is not unprecedented, previous literature showing that enzyme localization on a surface helps prevent thermal damage and hence the diminution of the catalytic activity.16
1.5 molar ratio of ethyl benzoate to butanol, in decane as the solvent, and using 75 mg of enzyme. After 4 h, the reaction performed at 70 °C showed a significantly greater conversion than those at lower temperatures (Fig. 11). This shows that the immobilized lipase works even at elevated temperatures. This is not unprecedented, previous literature showing that enzyme localization on a surface helps prevent thermal damage and hence the diminution of the catalytic activity.16
 | ||
Fig. 11 Conversion of ethyl benzoate to butyl benzoate as a function of temperature 40 °C,  50 °C, 50 °C,  60 °C, and 60 °C, and  70 °C. 70 °C. | ||
Conclusions
The results presented in this study show the applicability of the iChemExplorer to the monitoring of bio-catalysed processes. Both proteolytic digestions and a lipase-catalysed transesterification were studied and, in both cases, rapid reaction scouting was possible using the apparatus. Putting the work in context, other techniques have been used for monitoring proteolytic digestions,17 most involving mass spectroscopy in some way.18,19 An alternative approach is to use multichannel devices to perform multiple digestions at once.20 These techniques are undoubtedly very powerful and allow the user to obtain a wealth of information in a short period of time. For these reasons they are clearly more data rich than our approach. They are, however, significantly more costly than the methodology presented here. For transesterification reactions, chemists have used in situ probes such as the now ubiquitous ReactIR system,21 as well as ex situ methods such as NMR spectroscopy.22 Overall, for both classes of reaction studied here, we believe our approach offers a viable alternative to those already employed and that the potential for application in other chemical processes is promising.Experimental
General experimental
For proteolytic digestions, ammonium bicarbonate, albumin bovine serum, insulin chain B oxidized from bovine insulin and cytochrome c from horse heart were purchased from Sigma. TPCK trypsin from bovine pancreas was purchased from Pierce Protein Biology Products. For lipase transesterification reactions, 1-butanol, decane, acetonitrile, and ethyl benzoate were all purchased from Sigma Aldrich. Lipase acrylic resin from Candida antarctica (≥5000 U g−1, recombinant, expressed in Aspergillus niger) was purchased from Sigma. All chemicals were used without further purification. HPLC analysis was performed using a Hewlett Packard HPLC Series 1100 HPLC utilizing the iChemExplorer. The software for the iChemExplorer communicates with the ChemStation software of the HPLC. The software creates Excel workbooks with reaction conditions, chromatographs and peak profile for each vial and tabulates the data for further analysis.General experimental procedures
Digestions at varied temperatures. Two stock solutions were prepared, one of protein (1 mg of protein in 1 mL of 75 mM ammonium bicarbonate), and a second of trypsin (1 mg of trypsin in 1 mL of 75 mM ammonium bicarbonate). The trypsin solution was then diluted 25-fold. To a 2 mL capacity HPLC vial, an aliquot of the protein solution (150 μL), 75 mM ammonium bicarbonate (300 μL), and a stir bar were added. To this, an aliquot of the trypsin solution (150 μL) was added and the HPLC run started. Trials were performed at room temperature (26 °C), 40 °C, and 50 °C. Solvent A for the HPLC procedure was 0.1% trifluoroacetic acid in water and solvent B was 0.07% trifluoroacetic acid in acetonitrile. Samples were monitored by HPLC every 6 min for 90 min with a linear gradient from 5–75% in solvent B for the insulin chain B, every 10 min for 90 min with a linear gradient from 10–50% in solvent B for the cytochrome c, and every 17 min for 160 min with a linear gradient from 5–50% in solvent B for the BSA. Each run had a 2 min post time and 5 μL of sample was injected. An Agilent Poroshell 300SB-C18 column was used for analysis and monitoring performed using UV detection at 210 nm. From the chromatogram, the percentage of digested protein was determined by measuring the area of all the cleaved fragments and dividing it by that of the sum of all product peaks plus unreacted starting material. Owing to the fact that BSA when digested produced a large number of signals, the relative percentage of BSA was determined in a slightly modified way. One fragment peak of BSA in a clearly defined part of the HPLC trace (7.3 min) was used and the relative extent of digest was determined by ratio in this to the peak of undigested BSA (15.2 min).
Digestion at varied protease to protein ratios. Two stock solutions were prepared as in the case of the temperature studies. The trypsin solution was then diluted appropriately in order to obtain a variety of protease to protein ratios. To a 2 mL HPLC vial, an aliquot of a protein solution (150 μL), 75 mM ammonium bicarbonate (300 μL), and a stir bar were added. To this, an aliquot of the trypsin solution (150 μL) was added and the HPLC run started. The proteins used in this study were insulin chain B, cytochrome c, and BSA. Trials were performed at the optimum temperature for the digestion. Protease to protein ratios for insulin chain B were 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1
25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and 1
50, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, runs being performed at room temperature. Protease to protein ratios for cytochrome c were 1
100, runs being performed at room temperature. Protease to protein ratios for cytochrome c were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 with runs being performed at 40 °C. Protease to protein ratios for albumin bovine serum were 1
25 with runs being performed at 40 °C. Protease to protein ratios for albumin bovine serum were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1
25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and 1
50, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 with runs being performed at 50 °C. HPLC analysis was performed using analogous methodology to that for the temperature studies.
100 with runs being performed at 50 °C. HPLC analysis was performed using analogous methodology to that for the temperature studies.
Varying the enzyme loading for the reaction of ethyl benzoate and 1-butanol. In a 2 mL capacity HPLC vial, lipase (50 or 75 mg) and 1-butanol (1 mL) and were added. When the HPLC was started, ethyl benzoate (29 μL, 0.2 mmol) was added to the reaction vial. The trials were performed at 60 °C. A 15 μL sample was automatically transferred from the reaction vial to a vial containing acetonitrile (1 mL). The contents of the transfer vial were stirred for 1 min before analysis by HPLC. Solvent A for the HPLC procedure was 0.1% trifluoroacetic acid in water and solvent B was 0.07% TFA in acetonitrile. Samples were analysed every 15 min with a 2 min post time with a 5 μL injection onto a Waters XTerra RP18 column with a gradient of 5–95% in solvent B. The entire procedure of transferring an aliquot of the reaction mixture into a fresh vial containing pure acetonitrile and analysis was repeated every 15 min. The relative percentage of butyl benzoate was calculated by dividing the butyl benzoate peak (9.0 min) by the total area of butyl benzoate and ethyl benzoate (7.2 min) and multiplied by 100.
Varying the solvent in the reaction of ethyl benzoate and 1-butanol. In a 2 mL capacity HPLC vial, lipase (50 or 75 mg), decane (1 mL) and ethyl benzoate (29 μL, 0.2 mmol) were added. When the HPLC was started, 1-butanol (27 μL, 0.3 mmol) was added to the reaction vial. The trials were performed at 60 °C. A 15 μL sample was automatically transferred from the reaction vial to a vial containing acetonitrile (1 mL). The contents of the transfer vial were stirred for 1 min before analysis by HPLC. The chromatography and data analysis were performed in an analogous method to that use in the case of enzyme loading trials.
Varying the stoichiometry for the reaction of ethyl benzoate and 1-butanol. In a 2 mL capacity HPLC vial, lipase (75 mg), decane (1 mL) and ethyl benzoate (29 μL, 0.2 mmol) were added. When the HPLC was started, 1-butanol was added to the reaction vial. The amount of 1-butanol was varied to give different ratios of ethyl benzoate to butanol (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, 1
1.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The trials were performed at 60 °C. A 15 μL sample was automatically transferred from the reaction vial to a vial containing acetonitrile (1 mL). The contents of the transfer vial were stirred for 1 min before analysis by HPLC. The chromatography and data analysis were performed in an analogous method to that use in the case of enzyme loading trials.
2). The trials were performed at 60 °C. A 15 μL sample was automatically transferred from the reaction vial to a vial containing acetonitrile (1 mL). The contents of the transfer vial were stirred for 1 min before analysis by HPLC. The chromatography and data analysis were performed in an analogous method to that use in the case of enzyme loading trials.
Varying the temperature for the reaction of ethyl benzoate and 1-butanol. In a 2 mL capacity HPLC vial, lipase (75 mg), decane (1 mL) and ethyl benzoate (29 μL, 0.2 mmol) were combined. When the HPLC was started, 1-butanol (27 μL, 0.3 mmol) was added to the reaction vial. Trials were performed at 40–70 °C. A 15 μL sample was automatically transferred from the reaction vial to a vial containing acetonitrile (1 mL). The contents of the transfer vial were stirred for 1 min before analysis by HPLC. The chromatography and data analysis were performed in an analogous method to that use in the case of enzyme loading trials.
Acknowledgements
Reaction Analytics Inc. is thanked for access to an iChemExplorer system and funding from the National Science Foundation (CAREER award CHE-0847262) is acknowledged. Michael Lopez of Reaction Analytics Inc. is thanked for training and equipment support.Notes and references
- S. P. Bhutani, Chemistry of Biomolecules, CRC Press, 2010 Search PubMed.
- (a) A. Sickmann, Practical Proteomics, Wiley, 2013 Search PubMed; (b) N. C. Mishra, Introduction to Proteomics: Principles and Applications, Wiley, 2011 Search PubMed.
- Enzyme Catalysis in Organic Synthesis, ed. K. Drauz, H. Gröger and O. May, Wiley-VCH, 2012 Search PubMed.
- See, for example: J. L. Capelo, R. Carreira, M. Diniz, L. Fernandes, M. Galesio, C. Lodeiro, H. M. Santos and G. Vale, Anal. Chim. Acta, 2009, 650, 151 CrossRef CAS PubMed.
- V. R. Meyer, Practical High-Performance Liquid Chromatography, Wiley, 2010 Search PubMed.
- See, for example: (a) M. Nobilis, J. Mikusek, B. Szotakova, R. Jirasko, M. Holcapek, C. Chamseddin, T. Jira, R. Kucera, J. Kunes and M. Pour, J. Pharm. Biomed. Anal., 2013, 80, 164 CrossRef CAS PubMed; (b) L. Henneman, A. van Crutchen, S. Denis, M. Amolins, A. Placzek, R. Gibbs, W. Kulik and H. Waterham, Anal. Biochem., 2008, 383, 18 CrossRef CAS PubMed; (c) S. Shi, H. Zhou, Y. Zhang, X. Jiang, X. Chen and K. Huang, Trends in Analytical Chemistry, 2009, 28, 865 CrossRef CAS PubMed.
- iChemExplorer; Reaction Analytics Inc., Wilmington, DE, www.ichemexplorer.com.
- S. B. Kedia and M. B. Mitchell, Org. Process Res. Dev., 2009, 13, 420 CrossRef CAS.
- F. Qiu, L. Du, A. Soman, C. Jankovsky and C. Li, J. Pharm. Biomed. Anal., 2013, 76, 219 CrossRef CAS PubMed.
- L. M. Stencel, C. M. Kormos, K. B. Avery and N. E. Leadbeater, Org. Biomol. Chem., 2009, 7, 2452 CAS.
- There are a number of online tools for predicting potential cleavage sites cleaved by proteases or chemicals in a given protein sequence. See. for example http://web.expasy.org/peptide_cutter/ (Accessed September 30, 2013).
- K. Faber, Biotransformations in Organic Chemistry: A Textbook, Springer, 6th edn, 2011 Search PubMed.
- For an introduction, see: (a) V. Gotor-Fernández and V. Vicente, Use of Lipases in Organic Synthesis, in Industrial Enzymes: Structure, Function and Applications, ed. J. Polaina, A. P. MacCabe, Springer, 2007, pp. 301–315 Search PubMed; (b) R. Sharma, Y. Chisti and U. C. Banerjee, Biotechnol. Adv., 2001, 19, 627 CrossRef CAS.
- J. Chen and W. Wu, J. Biosci. Bioeng., 2003, 95, 466 CAS.
- G. Yadav and P. Lathi, J. Mol. Catal. B: Enzym., 2005, 32, 107 CrossRef CAS PubMed.
- See, for example: J. C. Cruz, K. Wurges, M. Kramer, P. H. Pfromm, M. E. Rezac and P. Czermak, Methods Mol. Biol., 2011, 743, 147 CAS.
- Quantitative Proteome Analysis: Methods and Applications, ed. K. Imai and S. L. F. Yau, CRC Press, Boca Raton, FL, 2013 Search PubMed.
- For reviews, see: (a) J. R. Yates, C. I. Ruse and A. Nakorchevsky, Annu. Rev. Biomed. Eng., 2009, 11, 49 CrossRef CAS PubMed; (b) X. Han, A. Aslanian and J. R. Yates, Curr. Opin. Chem. Biol., 2008, 12, 483–490 CrossRef CAS PubMed.
- For specific examples, see: (a) P. Picotti, M. Clément-Ziza, H. Lam, D. S. Campbell, A. Schmidt, E. W. Deutsch, H. Röst, Z. Sun, O. Rinner, L. Reiter, Q. Shen, J. J. Michaelson, A. Frei, S. Alberti, U. Kusebauch, B. Wollscheid, R. L. Moritz, A. Beyer and R. Aebersold, Nature, 2013, 494, 266 CrossRef CAS PubMed; (b) V. Sinha, G. T. Wijewickrama, R. E. P. Chandrasena, H. Xu, P. D. Edirisinghe, I. T. Schiefer and G. R. J. Thatcher, ACS Chem. Biol., 2013, 5, 667 CrossRef PubMed; (c) Y. Y. Zhong, S. J. Hyung and B. T. Ruotolo, Expert Rev. Proteomics, 2012, 9, 47 CrossRef CAS PubMed.
- See, for example: (a) M. Salim, S. L. McArthur, S. Vaidyanathan and P. C. Wright, Mol. Biosyst., 2011, 7, 101 RSC; (b) S. L. Faley, M. C. Copland, J. Reboud and J. M. Cooper, Biomicrofluidics, 2011, 2, 024106 CrossRef PubMed; (c) J. Lee, S. A. Soper and K. K. Murray, J. Mass Spectrom., 2009, 44, 579 CrossRef CAS PubMed.
- ReactIR™ is a system manufactured by Mettler-Toledo International Inc.
- For examples in synthetic chemistry, see: (a) C. F. Carter, H. Lange, S. V. Ley, I. R. Baxendale, B. Wittkamp, J. G. Goode and N. L. Gaunt, Org. Process Res. Dev., 2010, 14, 393 CrossRef CAS; (b) M. D. Argentine, T. M. Braden, J. Czarnik, E. W. Conder, S. E. Dunlap, J. W. Fennell, M. A. LaPack, R. R. Rothhaar, R. B. Scherer, C. R. Schmid, J. T. Vicenzi, J. G. Wei and J. A. Werner, Org. Process Res. Dev., 2010, 14, 131 Search PubMed; (c) J. N. Payette and H. Yamamoto, J. Am. Chem. Soc., 2008, 130, 12276 CrossRef CAS PubMed; (d) S. E. Denmark, S. M. Pham, R. A. Stavenger, X. P. Su, K. T. Wong and Y. Nishigaichi, J. Org. Chem., 2006, 71, 3904 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |