Efficient iodine-catalyzed one pot synthesis of highly functionalised pyrazoles in water
Madhulika
Srivastava
a,
Pratibha
Rai
a,
Jaya
Singh
b and
Jagdamba
Singh
*a
aEnvironmentally Benign Lab, Department of Chemistry, University of Allahabad, 211002 India. E-mail: dr.jdsau@gmail.com; Tel: +91 9415218507
bL R PG College, Sahibabad, UP, India
First published on 18th November 2013
Abstract
An efficient one pot multi-component sequential reaction of phenyl hydrazine, malanonitrile and a diverse range of aldehydes to afford highly functionalised pyrazoles is reported. The reaction proceeds in water using molecular iodine as a catalyst, with no by-product formation, and involves simple operation requiring no column chromatography. Thus, this protocol fulfils all of the criteria that define green chemistry.
Increasingly, chemists are looking for one-pot multi-component reactions (MCRs) in order to make desired compounds.1a MCRs have many significant advantages such as their time efficiency1b and reaction step economy,1c due to the simultaneous formation of two or more bonds,1d resulting overall in a better chemical yield than a corresponding multi-step synthesis. The conventional multi-step synthesis of molecules involves more than one step, including the purification of compounds after each individual step,1e which leads to two main disadvantages: synthetic inefficiency and the production of large quantities of waste.
Significant attention has been paid to MCRs in the aqueous phase,2a primarily because of the high synthetic efficiency of these protocols and also their inherent safety,2b due to the special physical and chemical properties of water such as its high dielectric constant, its ability to dissolve a large number of different chemical compounds by means of hydrogen bonding2c, nonflammability,2d cost effectiveness,2e high availability,2f ease of handling, and most important of all, the non toxic nature of water,2g making it synonymous with the term “green solvent”.1e These properties of water are highly beneficial compared to those of organic solvents.
An important aspect of the reaction is the choice of catalyst. A literature survey shows that many catalysts involve metals which are toxic,3a and detrimental to both health and the environment. Some catalysts are very difficult to remove from the reaction mixture while others are very expensive.3b We have searched for a catalyst which is metal free, non-toxic and mild.3c Such catalysts have always attracted the attention of the synthetic community, and one such catalyst is elemental iodine. Several research groups have implemented various strategies for the synthesis of a desired product which are catalysed by molecular iodine. For example, Kulathu I. Sathiyanarayanan et al. synthesised 1-((phenylthio)(phenyl)methyl)pyrrolidin-2-one4a with the use of iodine. B. V. Subba Reddy and co-workers have reported the synthesis of naphthopyranopyrimidines4b catalysed by iodine under solvent free conditions. Javad Mokhtari et al. synthesised spiro[indoline-3,40-pyrrolo[1,2-a]quinoxalin]-2-one4c in the presence of iodine, which not only enhanced the yield of the product but also considerably reduced the reaction time.
With these three aspects of green synthesis in mind,5 we have searched for a nucleus which shows outstanding performance in the fields of pharmaceutical chemistry6 and agriculture.7 Of the nitrogen heterocycles, the pyrazole moiety is a versatile lead molecule in pharmaceutical development. It also has a wide range of biological activities such as antimicrobial,8a antitumor,8b anti-inflammatory8c antiviral,9a analgesic,9b antidiabetic,9c anti-obesity,10a and anticonvulsant activities.10b In addition, pyrazole derivatives are used as inhibitors of HIV-1 reverse transcriptase.10a Moreover, pyrazole oxime11a ethers have proven to be very important, as they show antitumor and cytotoxic activity. Some pyrazole derivatives show biological activities, for example, P38 MAP kinase,11b COX-2,11c,detc., and pyranopyrazoles show antihypoglycemic activity.11e
Rimonabant,10b a pyrazole moiety also known as SR141716, is used as an appetite suppressant in the treatment of obesity. Similarly, viagra12 is used as an antihypertensive for the treatment of pulmonary arterial hypertension. Celebrex13 is a potential drug used in the treatment of arthritis. Pyrazole derivatives are also used as anti-diabetic drugs, for example, 5-methyl pyrazole-3-carboxylic acid.14 Moreover, CDPPB acts as a positive allosteric modulator in scientific research while Deracoxib15 is a non-steroidal veterinary medicine used to treat osteoarthritis in dogs.
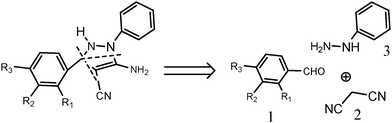
In light of our recent work on organic reactions in water15 and considering the well known broad spectrum qualities of pyrazoles, we were motivated to devise a new protocol for the synthesis of pyrazole derivatives from easily available and simple starting materials: aldehyde (1), malanonitrile (2) and phenylhydrazine (3), affording densely functionalized pyrazoles16 (Scheme 1).
Reagents (1), (2), and (3) were mixed in an equimolar ratio in 50 ml water. The reaction reached completion in 4 hours, giving the product as a solid which contained some by-products as well. To overcome this shortcoming, we performed the experiment in a sequential manner,17 based on a conclusion drawn from our previous work15 which proved that the addition of reagents in a sequential manner provides the product in excellent yield, with a corresponding reduction in the amount of by-products. So we again attempted to achieve the desired product in high yield with excellent purity. We took 50 ml of water, to which was added reagent 1 followed by 2. After 2 min when the reaction mixture had turned white, reagent 3 was added. Only one spot appeared on a TLC plate after 2 h 30 min. Thus, this minute procedural change led to an increase in the product yield and a reduction in the overall reaction time. The product formation takes place via a Knoevenagel reaction followed by a Michael addition, intermolecular cyclisation and oxidation, as depicted in Scheme 2.
We performed a series of these reactions in this sequential manner, over a varied range of temperatures (Table 1). When the reaction was carried out at 40 °C, the product was formed in 50 min. At 60 °C the product formation took place in only 20 min, but when the temperature was increased to 80 °C and above, the product was obtained as a gummy residue.
Due to the insufficient yields obtained in these reactions, we catalysed the reaction using a Lewis acid, which is electron deficient and polarises the carbonyl group of the aldehyde. We carried out the reaction using various types of Lewis acids and discovered that molecular iodine was the best among all of the traditional Lewis acids (Table 2, entry 1). A considerable improvement was observed in the reaction with the use of molecular iodine and the yield of the product was raised to 92%. 20 mol% iodine (Table 3, entry 3) was an ideal concentration for the catalysis of the reaction. When we used 15 mol% and 10 mol% iodine (Table 3, entries 1 and 2), the yield of the obtained product was low, while further increasing the amount of iodine beyond 20 mol% did not affect the yield at all. Thus iodine catalyses the reaction at each step.18 Not only was the yield of the product increased, but the rate of reaction was increased as well, resulting in a shorter reaction time. Iodine is one of the most abundant elements, and its toxicity is relativity low in comparison to that of catalysts in which metal is present.
| Entry | Lewis acid (20 mol%) | Yield (%) |
|---|---|---|
| 1 | I2 | 92 |
| 2 | InCl3 | 88 |
| 3 | Fecl3 | 85 |
| 4 | SnCl2 | 84 |
| 5 | ZnCl2 | 83 |
| Entry | Iodine (mol%) | Yield (%) |
|---|---|---|
| 1 | 10 | 85 |
| 2 | 15 | 88 |
| 3 | 20 | 92 |
| 4 | 30 | 92 |
| 5 | 40 | 92 |
At 60 °C, alcoholic solvents and water gave approximately the same yield (Table 4, entries 1–3). For non polar solvents such as toluene, the product was obtained in negligible yield (Table 3, entry 4). Water conducts heat more readily than any other liquid except mercury. Due to the desired parameters of green chemistry, we chose water as the solvent for the synthesis of the desired product.
In order to gain insight into the role of the electronic effect on the reaction, the reaction was performed with a range of aldehydes possessing different electron withdrawing and electron donating substituents. It was found that an aldehyde with an electron withdrawing substituent (Table 5, entries 4g and 4h) provided a better result in comparison to those having an electron donating substituent. In contrast, a lower yield was obtained with phenyl hydrazine having an electron withdrawing group and vice versa. Thus we concluded that the synthesis could best be carried out at 60 °C with malanonitrile 2, phenylhydrazine 3, a variety of aldehydes, and 20 mol% iodine, giving the respective highly functionalised pyrazoles in excellent yields of 85–94% (Table 5).
| Entry | Ra | 2 | 3 | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a R is a substituent on the aldehyde. b Yield of isolated, purified product. | |||||
| 4a | 4-OCH3 | 2 | 3 | 6 | 92 |
| 4b | H | 2 | 3 | 7 | 91 |
| 4c | 2-Cl | 2 | 3 | 5 | 92 |
| 4d | 4-Cl | 2 | 3 | 5 | 93 |
| 4e | 2-OCH3 | 2 | 3 | 7 | 90 |
| 4f | 3-4-Di methoxy | 2 | 3 | 8 | 88 |
| 4g | 3-NO2 | 2 | 3 | 4 | 93 |
| 4h | 4-NO2 | 2 | 3 | 4 | 94 |
| 4i | 4-F | 2 | 3 | 5 | 92 |
| 4j | 4-N-Dimethyl | 2 | 3 | 6 | 87 |
| 4k | 4-CH3 | 2 | 3 | 7 | 86 |
| 4l | 1-OH | 2 | 3 | 8 | 87 |
| 4m | 3-CN | 2 | 3 | 5 | 88 |
| 4n | 4-OCH3 | 2 | 3(4Cl) | 6 | 91 |
To summarise, we have efficiently synthesised a series of pyrazole derivatives in good yields using an eco-friendly approach. The adopted route has many significant features including the use of water as a solvent and iodine as a catalyst, a short reaction time and simple handling. The most important aspect of the adopted method is that it requires no purification of the desired product. This method achieves all of the criteria that define green chemistry, reducing waste, material hazard, risk and cost.
General methods
A. General information
Reagents were obtained from commercial suppliers, and used without further purification unless otherwise specified by a reference. All reactions were performed using oven-dried glassware. Organic solutions were concentrated using a Buchi rotary evaporator. TLC was performed using silica gel GF254 (Merck) plates. Melting points were determined by the open glass capillary method and are uncorrected. IR spectra were recorded in KBr on a Perkin-Elmer 993 IR spectrophotometer, and 1H NMR spectra were recorded on a Bruker AVII 400 spectrometer in CDCl3 using TMS as an internal reference with chemical shift values being reported in ppm. All coupling constants (J) are reported in Hertz (Hz). 13C NMR spectra were recorded on the same instrument at 100 MHz in CDCl3 and TMS was used as an internal reference. Mass (EI) spectra were recorded on a JEOL D-300 mass spectrometer. Elemental analyses were performed using a Coleman automatic carbon, hydrogen and nitrogen analyzer.B. General procedure for the synthesis of pyrazoles (4a to 4n)
50 ml water was transferred into a round bottom flask, to which was added 20 mol% iodine, aldehyde (1 mmol) and malanonitrile (1 mmol). Phenyl hydrazine was then added (1 mmol). After completion of the reaction in the appropriate time (Table 5), the reaction mixture was diluted with water (with a small amount of Na2S2O3). The solid crude products were collected by filtration, washed with water and dried. Thus, the isolated product was obtained, and was tested by TLC producing a single spot on the TLC plate (silica gel coated aluminium plates, Merk). Products 5c (semi solid) and 5i (liquid) were extracted with ethyl acetate (3 times). After completion of the reaction (monitored by TLC), the combined organic phases were dried over anhydrous Na2SO4, filtered and evaporated to yield the final pure product. All resulting solid products (except 5i and 5c) were recrystallised from hot ethanol.Acknowledgements
We sincerely thank SAIF, Punjab University, Chandigarh, for providing micro-analyses and spectra. The authors are also thankful to UGC, New Delhi, for the award of Junior Research Fellowship (JRF).Notes and references
- (a) A. M. Zonouz, I. Eskandari and H. R. Khavasi, Tetrahedron Lett., 2012, 53, 5519 CrossRef CAS PubMed; (b) L. Wen, Z. Li, M. Li and H. Cao, Green Chem., 2012, 14, 707 RSC; (c) H. Chen and D. Shi, Tetrahedron, 2011, 67, 5686 CrossRef CAS PubMed; (d) A. T. Khan, M. Lal, S. Ali and M. Khan, Tetrahedron Lett., 2011, 52, 5327 CrossRef CAS PubMed; (e) Y. Gu, Green Chem., 2012, 14, 2091 RSC.
- (a) Z. N. Tisseh, M. Dabiri, M. Nobahar, H. R. Khavasi and A. Bazgir, Tetrahedron, 2012, 68, 1769 CrossRef CAS PubMed; (b) Z. P. Demko and K. Barry Sharpless, J. Org. Chem., 2001, 66, 7945 CrossRef CAS PubMed; (c) J. Pinto, V. L. M. Silva, A. M. G. Silva, A. M. S. Silva, J. C. S. Costa, L. B. M. N. F. Santos, R. Enes, J. A. S. Cavaleiro, A. A. M. O. S. Vicented and J. A. C. Teixeira, Green Chem., 2013, 15, 970 RSC; (d) Y. He, X. Zhang, L. Cui, J. Wang and X. Fan, Green Chem., 2012, 14, 3429 RSC; (e) N. E. Leadbeater, Chem. Commun., 2005, 2881 RSC; (f) Garima, V. P. Srivastava and L. D. S. Yadav, Green Chem., 2010, 12, 1460 RSC; (g) R. Patel, V. P. Srivastava and L. D. S. Yadav, Synthesis, 2011, 1261 CAS.
- (a) X. Wang and J. W. Canary, Bioconjugate Chem., 2012, 23, 2329 CrossRef CAS PubMed; (b) P. Pelphrey, J. Hansen and H. M. L. Davies, Chem. Sci., 2010, 1, 254 RSC; (c) F. Bigi, S. Carloni, L. Ferrari, R. Maggi, Al. Mazzacani and G. Sartori, Tetrahedron Lett., 2001, 42, 5203 CrossRef CAS.
- (a) G. Ramachandran, N. S. Karthikeyan, P. Giridharanc and K. I. Sathiyanarayanan, Org. Biomol. Chem., 2012, 10, 5343 RSC; (b) K. P. Kumar, S. Satyanarayana, P. L. Reddy, G. Narasimhulu, N. Ravirala and B. V. Subba Reddy, Tetrahedron Lett., 2012, 53, 1738 CrossRef PubMed; (c) A. Alizadeh and J. Mokhtari, Tetrahedron, 2013, 69, 6313 CrossRef CAS PubMed.
- Y. Gu, R. De Sousa, G. Frapper, C. Bachmann, J. Barraulta and F. Jerome, Green Chem., 2009, 11, 1968 RSC.
- (a) D. K. Walker, M. J. Ackland, G. C. James, G. J. Muirhead, D. J. Rance, P. Wastall and P. A. Wright, Xenobiotica, 1999, 29, 297 CrossRef CAS PubMed; (b) J. K. Yano, T. T. Denton, M. A. Cerny, X. Zhang, E. F. Johnson and J. R. Cashman, J. Med. Chem., 2006, 49, 6987 CrossRef CAS PubMed; (c) J. Elguero, P. Goya, N. Jagerovic and A. M. S. Silva, Targets Heterocycl. Syst., 2002, 6, 52 CAS.
- (a) Y. Li, H.-Q. Zhang, J. Liu, X.-P. Yang and Z.-J. Liu, J. Agric. Food Chem., 2006, 54, 3636 CrossRef CAS; (b) D. N. Gandhale, A. S. Patil, B. G. Awate and L. M. Naik, Pesticides, 1982, 16, 27 CAS.
- (a) M. J. Kim and S. B. Park, Tetrahedron Lett., 2008, 49, 5080 CrossRef PubMed; (b) B. K. R. Sagar Srivastava, A. Joharapurkar, S. Raval, J. Z. Patel, R. Soni, P. Raval, A. Gite, A. Goswami, N. Sadhwani, N. Gandhi, H. Patel, B. Mishra, M. Solanki, B. Pandey, M. R. Jain and P. R. Patel, J. Med. Chem., 2007, 50, 5951 CrossRef PubMed; (c) S. Prekupec, D. Makuc, J. Plavec, L. Suman, M. Kralj, K. Pavelic, J. Balzarini, E. D. Clercq, M. Mintas and S. Raic-Malic, J. Med. Chem., 2007, 50, 3037 CrossRef CAS PubMed.
- (a) A. Agarwal, K. Srivastava, S. K. Purib and P. M. S. Chauhana, Bioorg. Med. Chem., 2005, 13, 4645 CrossRef CAS PubMed; (b) S. K. Singh, S. Vobbalareddy, S. Shivaramakrishna, A. Krishnamraju, S. A. Rajjak, S. R. Casturi, V. Akhilab and Y. K. Raoa, Bioorg. Med. Chem. Lett., 2004, 14, 1683 CrossRef CAS PubMed; (c) K. L. Kees, J. J. Fitzgerald Jr, K. E. Steiner, J. F. Mattes, B. Mihan, T. Tosi, D. Mondoro and M. L. McCaleb, J. Med. Chem., 1996, 39, 3920 CrossRef CAS PubMed.
- (a) R. Sagar, M. J. Kim and S. B. Park, Tetrahedron Lett., 2008, 49, 5080 CrossRef CAS PubMed; (b) B. Chandrakantha, A. M. Isloor, S. K. Peethambar and P. Shetty, Pharma Chem., 2012, 4, 1723 CAS.
- (a) H. Dai, Y. Q. Li, D. DU, X. Qin, X. Zhang, H. B. Yu and J. X. Fang, J. Agric. Food Chem., 2008, 56, 10805 CrossRef CAS PubMed; (b) E. Miklos, F. Waczek, G. Ker, S. Lany and L. Orfi, Univ. “Politeh.” Bucharest, Sci. Bull., Ser. B, 2010, 72, 75 CAS; (c) T. T. Dang, T. T. Dang, C. Fischer, H. Görls and P. Langer, Tetrahedron, 2008, 64, 2207 CrossRef CAS PubMed; (d) S. K. Singh, P. G. Reddy, K. S. Rao, B. B. Lohray, P. Misra, S. A. Rajjak, Y. K. Rao and A. Venkateswarlu, Bioorg. Med. Chem. Lett., 2004, 14, 499 CrossRef CAS PubMed; (e) A. M. Zonouz, I. Eskandari and H. R. Khavasi, Tetrahedron Lett., 2012, 53, 5519 CrossRef CAS PubMed.
- (a) N. K. Terrett, A. S. Bell, D. Brown and P. Ellis, Bioorg. Med. Chem. Lett., 1996, 6, 1819 CrossRef; (b) F. Barth and M. Rinaldi-Carmona, Curr. Med. Chem., 1999, 6, 745 CAS.
- T. D. Penning, J. J Talley, S. R. Bertenshaw, J. S. Carter, P. W. Collins, S. Docter, M. J. Graneto, L. F. Lee, J. W. Malecha, J. M. Miyashiro, R. S. Rogers, D. J. Rogier, S. S. Yu, G. D. Anderson, E. G. Burton, J. N. Cogburn, S. A. Gregory, C. M. Koboldt, W. E. Perkins, K. Seibert, A. W. Veenhuizen, Y. Y. Zhang and P. C. Isakson, J. Med. Chem., 1997, 40, 1347 CrossRef CAS PubMed.
- (a) E. R. Froesch and M. Wolavogel, Mol. Pharmacol., 1967, 3, 429–442 CAS; (b) W. G. Ryan, D. Carithers, K. Moldave and M. Bell, Int. J. Appl. Res. Vet. Med., 2010, 8, 114 Search PubMed; (c) J. M. Uslaner, S. P. Batteur, R. B. Flick, N. O. Surles, J. S. H. Lam, C. H. McNaughton, M. A. Jacobson and P. H. Hutson, Neuropharmacology, 2009, 57, 531 CrossRef CAS PubMed; (d) S. P. Batteur, J. A. OBrien, S. Doran, S. J. Nguyen, R. B. Flick, J. M. Uslaner, H. Chen, E. N. Finger, T. M. Williams, M. A. Jacobson and P. H. Hutson, Neuropharmacology, 2012, 62, 1453 CrossRef PubMed.
- M. Srivastava, J. Singh, S. B. Singh, K. Tiwari, V. K. Pathak and J. Singh, Green Chem., 2012, 14, 901 RSC.
- A. Hasaninejad and S. Firoozi, Mol. Diversity, 2013, 17, 459 CrossRef CAS PubMed; M. Srivastava, P. Rai, J. Singh and J. Singh, RSC Adv., 2013, 3, 16994 RSC.
- J. Sun, E. Xia, Q. Wu and C. Yan, ACS Comb. Sci., 2011, 13, 421 CrossRef CAS PubMed.
- N. Nishiwaki, K. Kobiro, S. Hirao, J. Sawayama, K. Saigo, Y. Ise, Y. Okajima and M. Ariga, Org. Biomol. Chem., 2011, 9, 6750 CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |