One-step synthesis of mesoporous ZSM-11 composites through a dual-template method
Hong Li
Chen
,
Jian
Ding
and
Yi Meng
Wang
*
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, Shanghai 200062, PR China. E-mail: ymwang@chem.ecnu.edu.cn; Fax: +86 21 62232251; Tel: +86 21 62232251
First published on 11th October 2013
Abstract
Hierarchical porous ZSM-11 composites were hydrothermally synthesized through a one-step route using binary templates of cetyltrimethylammonium tosylate (CTATos) and tetrabutylammonium hydroxide (TBAOH). Influences of aluminum content and crystallization temperature on the morphology and structure were investigated. Powder X-ray diffraction (XRD) and N2 physisorption results show that the mesoporous ZSM-11 composites possess both a considerable mesoporous structure and zeolitic MEL-structure. Moreover, a large amount of aluminum in the gel composition slows the rate of zeolite crystallization, while a high SiO2/Al2O3 ratio is beneficial for the synthesis of mesoporous ZSM-11 without any phase separation. Mesoporous ZSM-11 aluminosilicates were compared to pure ordered mesoporous materials, zeolites and a mechanical mixture of meso and microphases for their catalytic activity in low-density polyethylene (LDPE) pyrolysis. The results suggest that all mesoporous ZSM-11 composites show higher catalytic performance for LDPE cracking.
1. Introduction
Zeolites show unique shape selectivity in various reactions due to molecular-sized microporosity, but suffer diffusion limitations due to the small size of the channels (less than ∼0.8 nm) and cavities (typically <1.5 nm).1,2 There has been increasing interest in expanding the pore sizes of zeotype materials from the microporous to mesoporous range in order to meet the demands in both industrial and fundamental studies with regards to the development of the oil industry and the treatment of large molecules.3 The development of ordered mesoporous materials has been used to shed light on reactions involving large molecules.4 However, the relatively low reactivity and hydrothermal stability of mesoporous aluminosilicates limit their potential applications as catalysts in petroleum refining and fine chemicals synthesis. Obviously, mesoporous zeolites, with the combined advantages of both mesoporous materials and crystalline microporous zeolites, are extremely desirable for catalysis and adsorption due to the possibility of overcoming the stability and diffusion limitations. Much effort has been devoted to combining the properties of meso and microporous materials. One effective route is to synthesize hierarchical zeolites,5,6 where mesopores are generated in zeolitic crystals via hard-casting template techniques,7–9 chemical leaching approaches (desilication and dealumination),10–12 the assembly of zeolite nanoparticles with inter-crystalline mesopores13–15 and a one-step hydrothermal treatment using specifically designed bifunctional cationic surfactants or binary templates of a mesotemplate and a microtemplate. However, the complexity of the template preparation has limited the industrial application of hard-casting techniques; and mesopores formed during leaching were predominantly cavities in the zeolite crystals rather than pores connecting the external surface with the interior of the crystal, which are so far unsuitable for significant enhancement of inter-crystalline diffusion.16 Besides, the inter-crystalline mesopores originated from spontaneous and random assembling of nano-sized primary particles possessing poor ordering. Ryoo and coworkers directly synthesized zeolite MFI nano-sheets with a thickness of only 2 nm and mesoporous MFI and LTA zeolites with a tunable mesostructure using specifically designed bifunctional cationic surfactants17 and amphiphilic organosilanes18 as a single template, respectively. However, both bifunctional cationic surfactants and amphiphilic organosilanes are complex and specifically designed, limiting the industrial application.Much work has been devoted to developing a dual-template method that can be used to obtain hierarchical micro- and mesoporous materials.19–21 Importantly, the surfactant or polymer used to generate the mesopores, and the small structure-directing agents (SDAs) leading to the micropores in the crystalline zeolite framework must work in a cooperative rather than a competitive manner in order to avoid the formation of physical mixtures of amorphous mesoporous materials and bulky zeolites, which are easily formed when dual templates are used. Mesoporous MFI and BEA-type composites were prepared by assembling the corresponding zeolite seeds with surfactant (CTAB) under hydrothermal conditions.22–25 Nevertheless, XRD patterns showed no diffraction peaks ascribed to crystalline zeolite, although an infrared (IR) band in the 550–600 cm−1 region confirmed the presence of five-membered ring subunits. Successful attempts aimed at synthesizing an intimate composite material composed of a highly ordered mesoporous material and a well-crystallized zeolite over microscale domains, which display a zeolitic diffraction pattern, have been undertaken.26–30 However, most of these materials were still mixtures of an ordered mesophase and zeolite crystals with inferior catalytic activity and stability. When traditional surfactants were directly mixed with the synthetic mixture of a zeolite containing an SDA, these two different templates worked in a competitive manner, which easily led to phase separation of amorphous mesoporous material and bulky zeolite. Gu et al. used tert-butyl alcohol and 1,3,5-trimethylbenzene as a co-solvent and additive, respectively, to enhance the stability of the surfactant (CTAB) micelles, resulting in hierarchical zeolite Y.31 Very recently, Shi and coworkers reported the direct hydrothermal synthesis of mesoporous ZSM-5 and TS-1 zeolites using CTAB and TPAOH as the meso and micro-porogens with the assistance of ethanol,32,33 where the presence of ethanol and ageing at low temperature hindered the overgrowth of zeolite crystals, slowed down the crystallization process34 and thus favored the self-assembly of zeolite sub-nanocrystals or nanocrystals by the cooperative templating of both micro- and mesopore directing agents. In short, the main point is to diminish a mismatch between the kinetics and thermodynamics in the fabrication of mesoporous zeolites. Obviously, a decrease in the crystal size of the zeolite is beneficial for the synthesis of uniform meso–microporous composites without phase separation of the mesoporous materials and bulky zeolite crystals. Compared with the MFI zeolite, the MEL zeolite, another member of pentasil family,35,36 tends to form nano-sized primary crystallites.37 It might be advantageous to keep the balance between mesoscopic and MEL-type zeolitic ordering in hierarchical porous materials.
In this report, hierarchical ZSM-11 composites are synthesized through a one-step hydrothermal treatment using dual templates of conventional surfactants CTA+ and tetrabutylammonium hydroxide (TBAOH) as the templates for the mesopores and micropores, respectively. Without the addition of an alcoholic solvent and other additives, the mesoporous ZSM-11 composites synthesized by the dual template method show no macroscopic phase separation. The influences of the aluminum content and crystallization temperature on the structure and morphology were investigated. Furthermore, the catalytic activity of the mesoporous ZSM-11 aluminosilicates in the pyrolysis of LDPE was compared to that of pure mesoporous materials, zeolites and a mechanical mixture of mesoporous materials and zeolites.
2. Experimental
2.1. Synthesis of mesoporous ZSM-11 materials
In a typical synthesis, cetyltrimethylammonium tosylate (CTATos, 4.55 g, 0.01 mol, Merck, 99%) was dissolved in water and stirred at room temperature for 30 min then at 60 °C for 1 h. Aluminium isopropoxide (Al(OiPr)3, CP, SCRC) was dissolved in tetramethylammonium hydroxide (TBAOH, 22.7 g, 0.035 mol, 40%, Alfa) aqueous solution followed by adding tetraethyl orthosilicate (TEOS, 20.8 g, 0.1 mol, SCRC, >97%). The aluminosilicate solution was completely hydrolyzed after stirring at room temperature for 30 min. Alcohol hydrolyzed from TEOS was removed by heating at 80 °C for 2 h. The obtained solution was added dropwise into CTATos solution. The collected gel composition was 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01/0.017Al2O3
0.01/0.017Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1CTATos
0.1CTATos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35TBAOH
0.35TBAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50H2O. The mixture was stirred continuously for 2 h at 60 °C and then divided into several aliquots and loaded into 20 ml Teflon-lined steel autoclaves. The samples were heated in the autoclaves under static conditions at selected temperatures and duration. The as-made samples were recovered by filtration, fully washed with water and dried at 100 °C overnight. Calcined samples were obtained after removal of the occluded templating molecules by heating the samples at 550 °C for 5 h in an air flow. The final obtained samples were labeled as MZSM-x-y-z, where x indicated the molar ratio of SiO2 to Al2O3, and y and z indicated the crystallization temperature and duration, respectively.
50H2O. The mixture was stirred continuously for 2 h at 60 °C and then divided into several aliquots and loaded into 20 ml Teflon-lined steel autoclaves. The samples were heated in the autoclaves under static conditions at selected temperatures and duration. The as-made samples were recovered by filtration, fully washed with water and dried at 100 °C overnight. Calcined samples were obtained after removal of the occluded templating molecules by heating the samples at 550 °C for 5 h in an air flow. The final obtained samples were labeled as MZSM-x-y-z, where x indicated the molar ratio of SiO2 to Al2O3, and y and z indicated the crystallization temperature and duration, respectively.
2.2. LDPE pyrolysis
LDPE (Alfa) pyrolysis was carried out in a PerkinElmer TGA analyzer. The polymer (0.15 g) and the catalyst (0.05 g), both in powder form, were carefully mixed in order to attain an intimate contact. The pyrolysis was performed in N2 (50 ml min−1), ramping the temperature from 25 to 700 °C with a heating rate of 10 °C min−1.2.3. Characterization
Powder X-ray diffraction patterns (XRD) were collected on a Bruker D8 Advance powder diffractometer using Cu Kα radiation (λ = 0.154 nm) over a 2θ range from 1° to 40°, and the accelerating voltage and the applied current were 35 kV and 30 mA, respectively. SEM was performed on a scanning electron microscope (type HITACHI S-4800) with an accelerating voltage of 3 kV. TEM of the as-synthesized samples was collected on a JEM-2010 operating at 200 kV. To prepare the samples for TEM, a dispersion of the sample in diluted ethanol was dropped onto the TEM sample bronze grid, and dried at room temperature for 1 h. FT-IR spectra were recorded on a Nicolet Fourier transform infrared spectrometer (NEXUS 670) using the KBr technique. Thermogravimetric analysis (TG) was performed using a PerkinElmer 457 TGA analyzer with a heating rate of 10 °C min−1 under an air flow. The specific BET surface area (SBET) and pore parameters of the samples were determined by nitrogen adsorption–desorption measurements at 77 K on a nitrogen adsorption apparatus (BELSORP-max). Before the measurements, the samples were outgassed at 300 °C in vacuum for 6 h. The pore size distributions were derived from the adsorption branches of the isotherms using the Barrett–Joyner–Halanda (BJH) method. The total pore volume (Vp) was estimated at a relative pressure of 0.99. 29Si solid-state MAS NMR and 27Al solid-state MAS NMR spectra were recorded on a VARIAN VNMRS-400WB spectrometer. The 29Si MAS NMR spectra were obtained with a frequency of 79.43 MHz, a spinning rate of 3.0 kHz, and a recycling delay of 60 s. The chemical shifts were referenced against Q8M8([(CH3)3SiO]8SiO12). The 27Al MAS NMR spectra were obtained with a frequency of 104.18 MHz, a spinning rate of 10.0 kHz, and a recycling delay of 4 s. The chemical shift was referenced against KAl(SO4)2·12H2O. The acidity was measured by an ammonia adsorption–desorption technique using the chemical adsorption instrument Quantachrome CHEMSORP-3000. The samples were outgassed at 823 K in a helium flow for 2 h, then cooled to 323 K and NH3 adsorbed until saturation. Desorption then occurred between 373 to 823 K, with the temperature increasing by 10 K min−1.3. Results and discussion
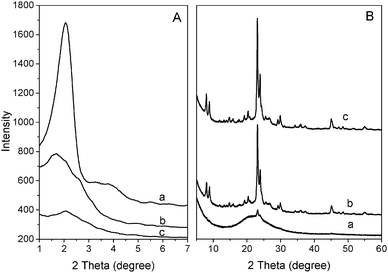
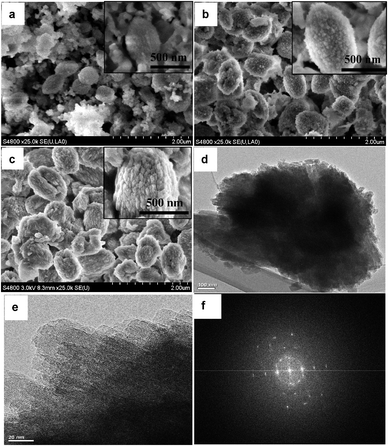
Fig. 1 displays the powder XRD patterns of the as-synthesized samples obtained at 160 °C over the period of 1–7 d. Sharp small-angle XRD peaks show that the MZSM-100-160-1 sample has an ordered hexagonal mesostructure similar to that of MCM-41 materials.4,38 Some small Bragg reflections, ascribed to the MEL-structure, appeared in the wide-angle region, indicating poor crystallization of the zeolitic structure. When the synthesis time was increased up to 3 d, the small-angle peak was broadened, indicating that the initial MCM-41-like mesostructure gradually transformed into a disordered mesostructure, while the intensity of the Bragg reflections in the wide-angle XRD region increased. Most of the intense peaks could be indexed to the crystalline structure of MEL zeolites, which have typical diffraction peaks in the region ranging from 2θ = 23–25°. The evolution of a meso and microstructure demonstrates that formation of zeolite ZSM-11 is accompanied by partial collapse of the MCM-41-like mesostructure during the crystallization process. Nevertheless, complete collapse of the mesostructure did not happen and a broad peak was observed in the small-angle XRD pattern, indicating that some disordered mesopores were retained after treatment at 160 °C for a duration as long as 7 d. It proves that the mesostructure directed by CTATos is hydrothermally stable to some extent.The SEM images in Fig. 2 are consistent with the XRD data discussed above. There are some irregularly-shaped gel lumps accompanied by some olive-like spheres of ∼600 nm in size randomly dispersed in the amorphous gel during the early stages of the hydrothermal reaction (1 d). With the crystallization time increased to 3 days, more irregularly-shaped gel transformed into olive-like spheres of ∼1 μm in size. After 7 d, almost all the amorphous gel had disappeared and well crystallized zeolite ZSM-11 had formed as olive-like agglomerates of nano-sized primary particles. Therefore, the combined results of XRD and SEM indicate that hierarchical MZSM-100-160-7 has a disordered mesostructure and highly crystallized zeolitic structure, without any obvious macroscopic phase separation. TEM images of MZSM-100-160-7 are shown in Fig. 2d and e. Separate particles of the MZSM-100-160-7 sample are presented in Fig. 2d. Fig. 2e shows that this composite consists of primary nanoparticles, which may result in the intercrystalline mesoporosity. Remarkable crystallization of MZSM-100-160-7 was proved by Fourier diffractograms, as shown in Fig. 2f, which also suggests that those nanoparticles may take orientation to some extent, because the SAED pattern is similar to that of a single crystal.
The effects of the SiO2/Al2O3 ratio on the morphology and structure were further investigated. Fig. 3 shows the XRD patterns of the samples with the SiO2/Al2O3 molar ratio varying from 100 to 60 when synthesized at 160 °C for 7 d and 12 d. Diffraction peaks due to the mesophase at around 2° and due to crystalline materials in the 2 theta region of 23–25° are observed for both MZSM-60-160-7 and MZSM-60-160-12. Compared to MZSM-100-160-7 with a high SiO2/Al2O3 ratio, MZSM-60-160-7 shows a poorer MEL-structure but better mesoscopic ordering. It has been reported that, during the crystallization of ZSM-5,39,40 an aluminum-rich gel was firstly formed at the bottom of the reactor, while tetrapropyl-ammonium (TPA+) ions in the presence of alkali ions interacted preferentially with the silicate species and the zeolite nucleates from the silica-rich solution. Similarly, with more aluminium introduced, less silicate species are available to interact with the TBA+ ions, slowing the nucleation and growth rates of zeolite. As a result, the reduced zeolitic structure and better mesoscopic ordering of MZSM-60-160-7 may be attributed to the low crystallization rate of the zeolite in an aluminum-rich synthetic system and the subsequent large retention of mesostructure.
 | ||
| Fig. 3 Small-angle (A) and wide-angle (B) XRD patterns of MZSM-11 synthesized at 160 °C for (a) 7 d and (b) 12 d with a SiO2/Al2O3 molar ratio of 60. | ||
SEM images of MZSM-60-160-7 and MZSM-60-160-12 are shown in Fig. 4a and b. Different from MZSM-100-160-7, the MZSM-60-160-7 composite shows a non-uniform morphology with some zeolitic nanoparticles embedded in layer-like materials (indicated by the white circles), further indicating the incomplete crystallization of the zeolite due to more Al being introduced. With the crystallization time prolonged, MZSM-60-160-12 exhibited a core–shell structure with zeolite nanoparticles for the core and a loose layer for the shell. Fig. 4c and d depict representative TEM images of MZSM-60-160-12. Although some amorphous mesoporous layers are deposited on the zeolite crystals, as pointed out by the black arrows, a few lattice fringes indicating a zeolitic structure along the long axis of the olive-like microspheres were clearly observed parallel to the white arrow. This shows that the layer-like shell is not totally amorphous, which is consistent with the distinct diffraction peaks of the MEL-structure in the wide-angle XRD pattern of Fig. 3B. All SEM and TEM analyses demonstrate that these composites are not a simple mixture of mesoporous materials and zeolite and have both parts intimately interconnected, possibly leading to full utilization of both mesoporosity and microporosity in certain applications.
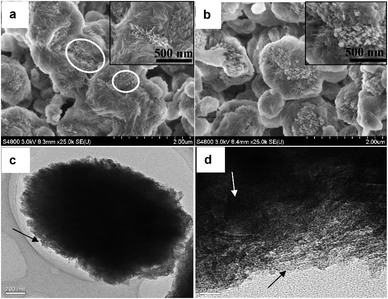 | ||
| Fig. 4 SEM images of MZSM-11 synthesized at 160 °C for (a) 7 d and (b) 12 d with a SiO2/Al2O3 molar ratio of 60. (c) and (d) are TEM images of MZSM-60-160-12. | ||
To investigate the influences of temperature on structure and morphology, hierarchical MZSM-11 samples were prepared at 150 °C and 175 °C when the molar ratio of SiO2/Al2O3 was fixed at 100. The XRD patterns in Fig. 5 suggest that a well crystallized zeolitic structure was obtained after hydrothermal synthesis for 7 d at 150 °C or 175 °C. In addition, a more highly ordered hexagonal mesostructure is observed at 150 °C, while a high temperature of 175 °C does not result in the complete collapse of the mesostructure, as shown in Fig. 5A. SEM images in Fig. 6 reveal the morphology of the obtained mesoporous ZSM-11 aggregates. Interestingly, all the samples synthesized with SiO2/AlO3 = 100 at 150–175 °C show relatively uniform olive-like crystals composed of nano-sized primary crystals. The size of the aggregates is about 1.0 μm without an obvious mesophase being observed throughout the entirety of the samples, which is quite different from MZSM-60-160-7 and MZSM-60-160-12. There are two possibilities for the formation of an ordered mesoporous structure detected by XRD. On the one hand, organized mesopores can form through the periodic arrangement of uniform nanosized particles.41 On the other hand, the presence of mesotemplate CTATos in the zeolite crystals might direct the disordered mesostructure during the hydrothermal treatment.
 | ||
| Fig. 5 Small-angle (A) and wide-angle (B) XRD patterns of MZSM-11 synthesized at (a) 150 °C and (b) 175 °C for 7 d with a SiO2/Al2O3 molar ratio of 100. | ||
 | ||
| Fig. 6 SEM images of MZSM-11 synthesized at (a) 150 °C and (b) 175 °C for 7 d with a SiO2/Al2O3 molar ratio of 100. | ||
To prove the important role of the surfactant CTATos retained in the pore channels in directing ordered mesopores during the hydrothermal crystallization process, the existence and evolution of mesotemplate CTA+ in the crystals were investigated by FT-IR spectra (Fig. 7) and thermogravimetric (TG) analysis (Fig. 8).
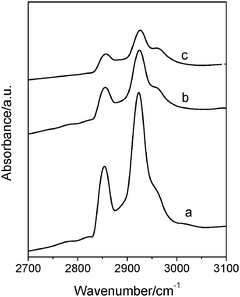 | ||
| Fig. 7 FT-IR spectra of as-prepared MZSM-11 with SiO2/AlO3 = 100 synthesized at (a) 150 °C, (b) 160 °C and (c) 175 °C for 7 d. | ||
 | ||
| Fig. 8 TG-DTG curves of as-prepared MZSM-11 with SiO2/AlO3 = 100 synthesized at (a) 150 °C, (b) 160 °C and (c) 175 °C for 7 d. | ||
The FT-IR spectra of as-prepared MZSM-11 with SiO2/AlO3 = 100 synthesized at different temperatures are shown in Fig. 7. The lowest frequency mode (460 cm−1) is used for intensity calibration. All samples show absorption bands at 2958 cm−1 and 2870 cm−1, which are assigned to the symmetric and asymmetric CH2 stretching modes, νs and νas, in long C16 hydrocarbon chains of CTA+ cations,42 demonstrating the absence of complete decomposition of CTA+ during the hydrothermal treatment. The existence of CTA+ could support the initially formed mesopore channels in the early stages of the crystallization, thus avoiding the severe collapse of the mesostructure during the hydrothermal process. The intensities of these two bands decrease with an increase in crystallization temperature, thereby indicating that some CTA+ ions are gradually excluded from the composites. It leads to a partial disappearance of mesoscopic ordering, which is in agreement with the results of the small-angle XRD shown in Fig. 5. In addition, TG analysis further proved the existence and evolution of mesotemplates during the crystallization process. As shown in Fig. 8, all three samples have an obvious weight loss in the 100–400 °C range, which is assigned to the decomposition of organic molecules including surfactant CTATos. The amount of surfactant trapped in the composite decreased greatly from 27.8 to 14.8 wt% with the increase of crystallization temperature, indicating that higher temperature leads to increased decomposition of surfactant. Furthermore, the decreased amount of physisorbed water below 100 °C with increasing temperature proves the high hydrophobicity of the composite due to increased condensation of silica during the crystallization at high temperature. Since no obvious separated mesophase was observed throughout the entire sample, the existence of surfactant trapped in the hierarchical MZSM-11 suggests that the disordered mesostructure detected by XRD is at least partially attributed to the surfactant retained in the zeolite aggregates.
It can be concluded that relatively uniform MZSM-11 composites can be obtained through a one-step hydrothermal method using binary templates CTATos and TBAOH as mesotemplate and microtemplate, respectively. Based on the influence of aluminum on the crystallization rate, a low SiO2/Al2O3 molar ratio (SiO2/Al2O3 = 60) leads to the formation of MZSM-11 composites with a core–shell structure, where nanosized zeolite aggregates lie in the core and layer materials form the shell. Moreover, mesoporous ZSM-11 aggregates without any obvious phase separation are prepared in the temperature range of 150–175 °C with a lower content of aluminum (SiO2/Al2O3 = 100).
The combination of meso- and microporosity was proven by nitrogen physisorption. N2 adsorption–desorption isotherms and pore size distribution (PSD) curves are shown in Fig. 9 and the textural properties are summarized in Table 1. The adsorbed volume of N2 for all the calcined samples at low partial pressure (P/P0 < 0.20) demonstrates the presence of micropores. Moreover, all MZSM-11 samples show clear hysteresis loops at relative pressure P/P0 of ∼0.45–0.85 caused by the capillary condensation in mesoporous channels as shown in Fig. 9A, indicating the hierarchical porous structure of MZSM-11 composites. Samples obtained during the early stages of crystallization (1 d and 3 d) show a narrow pore size distribution centered at 3.2 nm (Fig. 9B(a and b)), which is in agreement with the results reported in our previous work,43 where the pore size of MCM-41 synthesized using CTATos is in the range of 2.9–3.7 nm. MZSM-60-160-7 possesses highly ordered mesopores centered at 3.7 nm, while MZSM-60-160-12 shows a broad pore size distribution around 10 nm, demonstrating the collapse of ordered mesopores templated by CTATos with an increase in crystallization time due to the poor hydrothermal stability of ordered mesopores. Furthermore, the small micropore volume (0.09 cm3 g−1) of both MZSM-60-160-7 and MZSM-60-160-12 shown in Table 1 further proves the effect of aluminum on retarding the crystallization procedure, which is in agreement with the results detected by XRD and SEM.
| Samples | S BET /m2 g−1 | S ext /m2 g−1 | V total/cm3 g−1 | V micro /cm3 g−1 | V meso /cm3 g−1 | d p /nm |
|---|---|---|---|---|---|---|
| a Calculated by the BET method in the P/P0 range of 0.01–0.1. b Calculated using the t-plot method. c Calculated using Vpore − Vmicro. d Calculated from the adsorption branch using Barrett–Joyner–Halenda (BJH) method. | ||||||
| MZSM-100-160-1 | 556 | 540 | 0.71 | 0.06 | 0.65 | 3.2 |
| MZSM-100-160-3 | 495 | 307 | 0.41 | 0.09 | 0.32 | 3.6 |
| MZSM-100-150-7 | 567 | 385 | 0.56 | 0.09 | 0.47 | 3.2 |
| MZSM-100-160-7 | 520 | 301 | 0.46 | 0.11 | 0.35 | 9.1 |
| MZSM-100-175-7 | 498 | 245 | 0.40 | 0.12 | 0.28 | 13.9 |
| MZSM-60-160-7 | 455 | 203 | 0.35 | 0.09 | 0.26 | 3.7 |
| MZSM-60-160-12 | 458 | 255 | 0.44 | 0.09 | 0.35 | 10.5 |
Interestingly, because the MZSM-11 composites with SiO2/AlO3 = 100 synthesized at 150–175 °C show no obvious phase separation, broad pore distributions ranging from 3 nm to 14 nm in Fig. 9C imply the presence of some inter-crystalline mesopores. As shown in Table 1, the MZSM-11 crystallized at 150 °C possesses a significantly high BET surface area (567 m2 g−1) and large porosity (0.56 cm3 g−1). In general, a decrease in temperature leads to an increase in both the external surface area and mesopore volume accompanied by a decrease in the zeolitic micropore volume. It can be attributed to the fact that low temperature favors nucleation but reduces the growth rate of the zeolite nanodomains, since the activation energy needed for crystal growth is generally higher.44,45 Furthermore, an increase in temperature leads to an enlargement of the mesopore size, as shown in Fig. 9C. The relatively larger intercrystalline pore may be related to the consumption of mesopores during the crystallization process and the formation of bigger voids between the larger primary particles at high temperature, as shown in Fig. 6. The inter-crystalline mesopores are beneficial in that they connect the external surface with the interior zeolitic structure, which can lead to high catalytic performance. This is very different from mesoporous materials mechanically mixed with zeolites.
The local environment status of silicon and aluminum was investigated by magic angle spinning NMR techniques. As shown in Fig. 10A, strong resonance signals at −113 ppm accompanied by small peaks at −102 ppm are observed, which belong to Q4 [Si (4Si)] and Q3 [both Si (3Si, 1OH) and Si (3Si, 1Al)], respectively.46–48 With the crystallization time and the crystallization temperature both increasing, the relative intensity ratio of Q4/Q3 as inserted in Fig. 10A clearly increases, indicating better crystallization of zeolites. 27Al MAS NMR spectra were recorded to analyze the environment status of Al, as shown in Fig. 10B. The resonance bands at 54 and 0 ppm correspond to tetrahedrally (framework) and octahedrally (extra-framework) coordinated aluminum, respectively.49 The ratios of framework to extra-framework Al were calculated from the area of deconvoluted resonance peaks, as listed in Fig. 10B. The proportion of both framework Si and Al increased with increasing crystallization temperature and duration. This is attributed to improved condensation/crystallization. However, MZSM-60-160-7 and MZSM-60-160-12 both showed a relatively low content of framework Al because the large amount of aluminum in the gel composition slowed the rate of zeolite crystallization, leading to the formation of more extra-framework Al species.
Fig. 11 and Table 2 show NH3-TPD profiles and characteristics of MZSM-11. The amount of total acid sites increases with an increase in crystallization time at 160 °C, following the sequence: a < b < d. With a longer crystallization time of 7 d, samples of c, d and e synthesized at different temperatures show almost the same acidity. All catalysts show two NH3 desorption peaks. The peak centered at ∼200 °C is due to desorption of weakly bound NH3, followed by desorption at around 400 °C due to strongly bound NH3, which corresponds to weak and strong acid sites in zeolites, respectively. Moreover, most of the MZSM-11 composites (samples c and d) possess comparable total acid sites to that of conventional ZSM-11 (0.53 mmol g−1). As for the samples of f and g, more bulk Al species in the zeolites do not lead to more acid sites, which can possibly be ascribed to the relatively poor crystallization of these two samples.
 | ||
| Fig. 11 NH3-TPD profiles of the calcined MZSM-11: (a) MZSM-100-160-1, (b) MZSM-100-160-3, (c) MZSM-100-150-7, (d) MZSM-100-160-7, (e) MZSM-100-175-7, (f) MZSM-60-160-7 and (g) MZSM-60-160-12. | ||
| Sample | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| Total acid sites/mmol g−1 | 0.35 | 0.42 | 0.51 | 0.52 | 0.54 | 0.53 | 0.53 |
| Weak acid sites/mmol g−1 | 0.09 | 0.07 | 0.12 | 0.08 | 0.12 | 0.11 | 0.12 |
| Medium acid sites/mmol g−1 | 0.26 | 0.35 | 0.39 | 0.44 | 0.42 | 0.42 | 0.41 |
The ultimate goal of many zeolite fabrications is to achieve improved catalytic performance. The pyrolysis of low-density polyethylene (LDPE) was investigated, as it is a suitable probe reaction for diffusion limited reactions.50,51 This reaction is dominated by the external surface of the catalyst due to the bulky nature of the branched polyethylene chain (diameter 0.494 nm). In the case of ferrierite and ZSM-5, the generation of mesopores and structural defects coupled to a preservation of intrinsic zeolite properties has been proven to aid in the catalytic activity, hence lowering the degradation temperature of LDPE.12,52 Pure mesoporous Al-MCM-41 was synthesized in the gel composition of 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01Al2O3
0.01Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09CTATos
0.09CTATos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35TMAOH
0.35TMAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50H2O and zeolitic ZSM-11 was obtained in the synthetic composition of 1SiO2
50H2O and zeolitic ZSM-11 was obtained in the synthetic composition of 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01Al2O3
0.01Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35TBAOH
0.35TBAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60H2O as references. In addition, Al-MCM-41 and ZSM-11 were mechanically mixed with a mass ratio of 1 (denoted as Al-MCM-41@ZSM-11) to prove the catalytic advantages of hierarchical MZSM-11. Fig. 12 shows the correlation between the pyrolysis of LDPE and the catalysts with different porous structures. The introduction of the catalyst Al-MCM-41 distinctly shifts the degradation profile of LDPE by ca. 100 °C. The advanced catalytic properties of micropore-containing catalysts are more obvious than those of Al-MCM-41 due to the strong acidity of crystalline zeolites. The temperature at which 10% LDPE is converted (denoted as T10) was taken as a benchmark to compare the different catalysts. Samples g, h and i show the best catalytic properties, and the MZSM-100-160-7 catalyst is seen to be the most active with a T10 of 262 °C, 40 °C lower than the pure ZSM-11 and 174 °C lower than for the uncatalyzed pyrolysis of LDPE. It is attributed to their optimal combination of acidity and hierarchical porous structure, as suggested by the results of NMR, NH3-TPD and N2 adsorption, possessing acidic activity and shape selectivity of micropores and the free diffusion properties of mesopores. The improved catalytic activity illustrates that the intercrystalline mesopores connecting the external surface with the interior of the crystal play an important role in shortening the diffusion path length and increasing both accessibility and transport. It leads to a lower degradation temperature, indicating high performance in the pyrolysis of LDPE. The performances of hierarchical zeolites of SiO2/AlO3 = 100 without an obvious physical mixture (samples g, h and i) are followed by samples with a larger amount of aluminum (SiO2/AlO3 = 60) (samples j and k). All the MZSM-11 composites showed higher catalytic activity with much lower T10 than that of the mechanical mixture of Al-MCM-41 and ZSM-11 (sample d). Besides the influence of acidity, it can be concluded that the intimate contact of both meso- and microphases is unlike that in the mechanical mixture, which favors the reaction of large molecules.
60H2O as references. In addition, Al-MCM-41 and ZSM-11 were mechanically mixed with a mass ratio of 1 (denoted as Al-MCM-41@ZSM-11) to prove the catalytic advantages of hierarchical MZSM-11. Fig. 12 shows the correlation between the pyrolysis of LDPE and the catalysts with different porous structures. The introduction of the catalyst Al-MCM-41 distinctly shifts the degradation profile of LDPE by ca. 100 °C. The advanced catalytic properties of micropore-containing catalysts are more obvious than those of Al-MCM-41 due to the strong acidity of crystalline zeolites. The temperature at which 10% LDPE is converted (denoted as T10) was taken as a benchmark to compare the different catalysts. Samples g, h and i show the best catalytic properties, and the MZSM-100-160-7 catalyst is seen to be the most active with a T10 of 262 °C, 40 °C lower than the pure ZSM-11 and 174 °C lower than for the uncatalyzed pyrolysis of LDPE. It is attributed to their optimal combination of acidity and hierarchical porous structure, as suggested by the results of NMR, NH3-TPD and N2 adsorption, possessing acidic activity and shape selectivity of micropores and the free diffusion properties of mesopores. The improved catalytic activity illustrates that the intercrystalline mesopores connecting the external surface with the interior of the crystal play an important role in shortening the diffusion path length and increasing both accessibility and transport. It leads to a lower degradation temperature, indicating high performance in the pyrolysis of LDPE. The performances of hierarchical zeolites of SiO2/AlO3 = 100 without an obvious physical mixture (samples g, h and i) are followed by samples with a larger amount of aluminum (SiO2/AlO3 = 60) (samples j and k). All the MZSM-11 composites showed higher catalytic activity with much lower T10 than that of the mechanical mixture of Al-MCM-41 and ZSM-11 (sample d). Besides the influence of acidity, it can be concluded that the intimate contact of both meso- and microphases is unlike that in the mechanical mixture, which favors the reaction of large molecules.
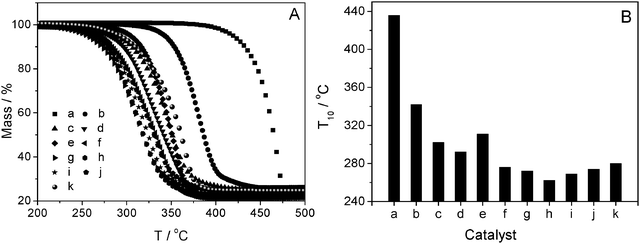 | ||
| Fig. 12 (A) Low-density polyethylene (LDPE) pyrolysis tests, and (B) correlation between the catalytic activity (T10, temperature at 10% conversion) from the conversion profiles in Fig. 10A and catalysts with different porous structure. LDPE with (a) no catalyst, (b) Al-MCM-41, (c) ZSM-11, (d) Al-MCM-41@ZSM-11, (e) MZSM-100-160-1, (f) MZSM-100-160-3, (g) MZSM-100-150-7, (h) MZSM-100-160-7, (i) MZSM-100-175-7, (j) MZSM-60-160-7 and (k) MZSM-600-160-12. | ||
4. Conclusions
Hierarchical porous materials combine the advantages of both zeolitic acidity and the diffusion advantages of mesoporous materials. Furthermore, intimate contact of both the meso- and microphases is very important for making full use of the porosities of both mesoporous materials and zeolites in a catalytic process. In this present work, hierarchical MZSM-11 composites were synthesized through a one-step method using binary templates of CTATos and TBAOH as the mesotemplate and microtemplate, respectively. Relatively uniform olive-like crystals composed of nano-sized primary crystals without any obvious phase separation were synthesized in a wide range of temperatures when the molar ratio of SiO2/Al2O3 was 100. In the case of SiO2/Al2O3 = 60, more aluminum slowed the nucleation and growth rates of the zeolite, leading to incompletely crystallized core–shell spheres of mesophase and zeolite. All MZSM-11 aggregates possessed considerable amounts of both meso and micropores and distinctly decreased the degradation temperature of LDPE in the pyrolysis of LDPE compared to pure mesoporous materials, zeolites and a mechanical mixture of meso- and microphases.Acknowledgements
This work is supported by the National Key Technology R&D Program (No. 2012BAE05B02) and National Science Foundation of China (20890122). Prof. Wang thanks the Fundamental Research Funds for the Central Universities and the Program for New Century Excellent Talents in University (NCET-11-0145), Ministry of Education of China.Notes and references
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS PubMed.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1 CrossRef CAS PubMed.
- X. S. Zhao, G. Q. M. Lu and G. J. Millar, Ind. Eng. Chem. Res., 1996, 35, 2075 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- Y. S. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896 CrossRef CAS PubMed.
- J. Cejka and S. Mintova, Catal. Rev. Sci. Eng., 2007, 49, 457 CAS.
- Y. M. Fang and H. Q. Hu, J. Am. Chem. Soc., 2006, 128, 10636 CrossRef CAS PubMed.
- J. Perez-Ramirez, C. H. Christensen, K. Egeblad and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- X. J. Meng, F. Nawaz and F. S. Xiao, Nano Today, 2009, 4, 292 CrossRef CAS PubMed.
- J. C. Groen, T. Bach, U. Ziese, A. M. Paulaime-van Donk, K. P. de Jong, J. A. Moulijn and J. Pérez-Ramírez, J. Am. Chem. Soc., 2005, 127, 10792 CrossRef CAS PubMed.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Perez-Ramirez, Chem.–Eur. J., 2005, 11, 4983 CrossRef CAS PubMed.
- D. Verboekend, J. C. Groen and J. Pérez-Ramírez, Adv. Funct. Mater., 2010, 20, 1441 CrossRef CAS.
- Y. M. Fang, H. Q. Hu and G. H. Chen, Chem. Mater., 2008, 20, 1670 CrossRef CAS.
- L. Chen, S. Y. Zhu, H. M. Wang and Y. M. Wang, Solid State Sci., 2011, 13, 2024 CrossRef PubMed.
- T. Xue, L. Chen, Y. M. Wang and M. Y. He, Microporous Mesoporous Mater., 2012, 156, 97 CrossRef CAS PubMed.
- S. van Donk, A. H. Janssen, J. H. Bitter and K. P. de Jong, Catal. Rev. Sci. Eng., 2003, 45, 297 CAS.
- (a) M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246 CrossRef CAS PubMed; (b) K. Na, M. Choi, W. Park, Y. Sakamoto, O. Terasaki and R. Ryoo, J. Am. Chem. Soc., 2010, 132, 4169 CrossRef CAS PubMed.
- M. Choi, R. Srivastava and R. Ryoo, Chem. Commun., 2006, 4380 RSC.
- A. Karlsson, M. Stöcker and R. Schmidt, Microporous Mesoporous Mater., 1999, 27, 181 CrossRef CAS.
- N. Petkov, M. Hölzl, T. H. Metzger, S. Mintova and T. Bein, J. Phys. Chem. B, 2005, 109, 4485 CrossRef CAS PubMed.
- W. P. Guo, L. M. Huang, P. Deng, Z. Y. Xue and Q. Z. Li, Microporous Mesoporous Mater., 2001, 44–45, 427 CrossRef CAS.
- S. P. Naik, A. S. T. Chiang, R. W. Thompson, F. C. Huang and H. M. Kao, Microporous Mesoporous Mater., 2003, 60, 213 CrossRef CAS.
- Y. Liu, W. Z. Zhang and T. J. Pinnavaia, Angew. Chem., Int. Ed., 2001, 40, 1255 CrossRef CAS.
- K. S. Triantafyllidis, E. F. Iliopoulou, E. V. Antonakou, A. A. Lappas, H. Wang and T. J. Pinnavaia, Microporous Mesoporous Mater., 2007, 99, 132 CrossRef CAS PubMed.
- X. B. Wang, X. F. Zhang, Y. Wang, H. O. Liu, J. S. Qiu, J. Q. Wang, W. Han and K. L. Yeung, Chem. Mater., 2011, 23, 4469 CrossRef CAS.
- J. Perez-Pariente, I. Diaz and J. Agundez, C. R. Chim., 2005, 8, 569 CrossRef CAS PubMed.
- A. K. Kinage, A. K. Prashar, G. Berlier and R. K. Chaturvedi, Mater. Charact., 2011, 62, 1166 CrossRef CAS PubMed.
- L. Frunz, R. Prins and G. D. Pirngruber, Microporous Mesoporous Mater., 2006, 88, 152 CrossRef CAS PubMed.
- L. M. Huang, W. P. Guo, P. Deng, Z. Y. Xue and Q. Z. Li, J. Phys. Chem. B, 2000, 104, 2817 CrossRef CAS.
- S. A. Bagshaw, N. I. Baxter, D. R. M. Brew, C. F. Hosie, N. Yuntong, S. Jaenicke and C. G. Khuan, J. Mater. Chem., 2006, 16, 2235 RSC.
- F. N. Gu, F. Wei, J. Y. Yang, N. Lin, W. G. Lin, Y. Wang and J. H. Zhu, Chem. Mater., 2010, 22, 2442 CrossRef CAS.
- Y. Zhu, Z. L. Hua, J. Zhou, L. J. Wang, J. J. Zhao, Y. Gong, W. Wu, M. L. Ruan and J. L. Shi, Chem.–Eur. J., 2011, 17, 14618 CrossRef CAS PubMed.
- Y. Zhu, Z. L. Hua, X. X. Zhou, Y. D. Song, Y. Gong, J. Zhou, J. J. Zhao and J. L. Shi, RSC Adv., 2013, 3, 4193 RSC.
- W. Song, R. E. Justice, C. A. Jones, V. H. Grassian and S. C. Larsen, Langmuir, 2004, 20, 8301 CrossRef CAS PubMed.
- G. T. Kokotqilo, S. L. Lawton, D. H. Olson and W. M. Meier, Nature, 1978, 272, 437 CrossRef.
- G. T. Kokotqilo, P. Chu and S. L. Lawton, Nature, 1978, 275, 119 CrossRef.
- J. P. Dong, J. Zou and Y. C. Long, Microporous Mesoporous Mater., 2003, 57, 9 CrossRef CAS.
- R. Ryoo and J. M. Kim, J. Chem. Soc., Chem. Commun., 1995, 711 RSC.
- R. von Ballmoos and W. M. Meier, Nature, 1981, 289, 782 CrossRef CAS.
- E. G. Derouane, J. P. Gilson, Z. Gabelica, C. Mousty-Desbuquoit and J. Verbist, J. Catal., 1981, 71, 447 CrossRef CAS.
- (a) T. Yokoi, Y. Sakamoto, O. Terasaki, Y. Kubota, T. Okubo and T. Tatsumi, J. Am. Chem. Soc., 2006, 128, 13664 CrossRef CAS PubMed; (b) W. Q. Jiao, M. B. Yue, Y. M. Wang and M. Y. He, Microporous Mesoporous Mater., 2012, 147, 167 CrossRef PubMed.
- K. Zhang, B. Albela, M. Y. He, Y. M. Wang and L. Bonneviot, Phys. Chem. Chem. Phys., 2009, 11, 2912 RSC.
- K. Zhang, H. L. Chen, B. Albela, J. G. Jiang, Y. M. Wang, M. Y. He and L. Bonneviot, Eur. J. Inorg. Chem., 2011, 59 CrossRef CAS.
- N. N. Feoktistova, S. P. Zhdanov, W. Lutz and M. Büllow, Zeolites, 1989, 9, 136 CrossRef CAS.
- S. Mintova, V. Valtchev and I. Kanev, Zeolites, 1993, 13, 102 CrossRef CAS.
- G. Boxhoorn, A. G. T. G. Kortbeek, G. R. Hays and N. C. M. Alma, Zeolites, 1984, 4, 15 CrossRef CAS.
- J. M. Chezeau, L. Delmotte, J. L. Guth and Z. Gabelica, Zeolites, 1991, 11, 598 CrossRef CAS.
- J. B. Nagy, Z. Gabelica and E. G. Derouane, Chem. Lett., 1982, 1105 CrossRef CAS.
- P. Marturano, L. Drozdov', A. Kogelbauer and R. Prins, J. Catal., 2000, 192, 236 CrossRef CAS.
- M. S. Renzini, U. Sedran and L. B. Pierella, J. Anal. Appl. Pyrolysis, 2009, 86, 215 CrossRef CAS PubMed.
- L. C. Lerici, M. S. Renzini, U. Sedran and L. B. Pierella, Energy Fuels, 2013, 27, 2202 CrossRef CAS.
- A. Bonilla, D. Baudouin and J. Pérez-Ramírez, J. Catal., 2009, 265, 170 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |