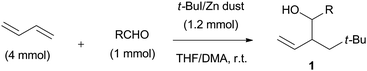
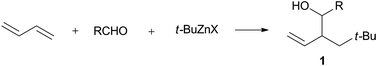C–C bond formation via 1,2-addition of a tert-butylzinc reagent and carbonyls across conjugated dienes†
Yuki
Ohira
,
Maya
Hayashi
,
Takamichi
Mori
,
Gen
Onodera
and
Masanari
Kimura
*
Graduate School of Engineering, Nagasaki University, 1-14 Bunkyo machi, Nagasaki 852-8521, Japan. E-mail: masanari@nagasaki-u.ac.jp; Fax: +81 95 819 2677; Tel: +81 95 819 2677
First published on 19th November 2013
Abstract
A mixture of t-butylzinc halide and an aldehyde reacts with conjugated dienes to provide 2-neopentyl homoallyl alcohols in high yields by 1,2-addition. Without the aldehyde, under carbon dioxide atmospheric pressure, the three components of t-butylzinc halide, butadiene, and carbon dioxide combine in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to give 2-neopentyl-3-butenoic acid in excellent yield.
1 ratio to give 2-neopentyl-3-butenoic acid in excellent yield.
Introduction
Multi-component coupling reactions involving conjugated dienes are among the most efficient and useful synthetic strategies for natural products and complex molecules.1 We have developed a Ni catalyst that accelerates the three-component coupling reaction of aldehydes, conjugated dienes, and dimethylzinc in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
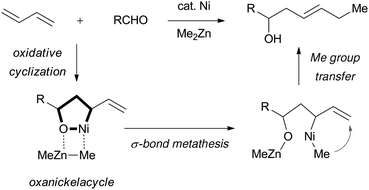
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to provide homoallyl alcohols (Scheme 1).2 In this case, oxidative cyclization of 1,3-butadiene and an aldehyde proceeds smoothly to form an oxanickelacycle intermediate followed by σ-bond metathesis with dimethylzinc leading to the allylmethylnickel species. Methyl group transfer from the Ni metal center to the allylic terminus then leads to the homoallyl alcohol via 1,4-addition.
1 ratio to provide homoallyl alcohols (Scheme 1).2 In this case, oxidative cyclization of 1,3-butadiene and an aldehyde proceeds smoothly to form an oxanickelacycle intermediate followed by σ-bond metathesis with dimethylzinc leading to the allylmethylnickel species. Methyl group transfer from the Ni metal center to the allylic terminus then leads to the homoallyl alcohol via 1,4-addition.
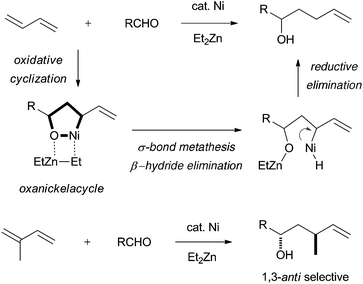
When diethylzinc was employed in place of dimethylzinc, the reaction features changed, and the homoallylation of the aldehyde with the conjugated diene proceeded via the key oxanickelacycle intermediate (Scheme 2).3 Ethyl group transfer from diethylzinc to the oxanickelacycle provided the allylethylnickel which underwent β-hydride elimination to give rise to the allylnickel hydride species. Reductive elimination of Ni(0) metal from the allylnickel hydride species formed the homoallylation product, a bis-homoallyl alcohol, predominantly with retention of configuration. When isoprene was used as the diene, the reductive coupling reaction proceeded with high regio- and stereoselectivities to provide 1,3-anti bis-homoallyl alcohols exclusively. Thus, diethylzinc serves as a reducing agent as well as a promoter of stereocontrolled homoallylation reactions.
Based on the results of Schemes 1 and 2, we studied the reaction further using various kinds of organozinc reagents. Herein, we report that t-butylzinc halides react with a mixture of conjugated dienes and aldehydes to provide 2-neopentyl homoallyl alcohols 1 (Scheme 3). It is notable that the coupling reaction proceeds in the absence of Ni catalyst, and the t-butyl group and aldehyde add to the C–C double bond of 1,3-butadiene via 1,2-addition to give branched type homoallyl alcohols in contrast to the regioselectivities of the Ni-catalyzed reaction systems. A similar coupling reaction proceeds under the atmospheric pressure of carbon dioxide to give the 2-neopentyl-3-butenoic acids exclusively with high regio- and stereoselectivities.
Results and discussions
Reaction with t-BuZnBr
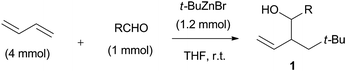
The three-component coupling reaction was conducted in the presence of a wide variety of aldehydes, 1,3-butadiene, and commercially available t-BuZnBr THF solution. t-BuZnBr was introduced into a mixture of 1,3-butadiene and aldehyde, and the reaction mixture was stirred at room temperature under a nitrogen atmosphere. The results of the coupling reactions with various carbonyl compounds are shown in Table 1.| Entry | Electrophile | Time (h) | Yield (%) [anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn] syn] |
|---|---|---|---|
| a The reaction was undertaken in the presence of butadiene (4 mmol), aldehyde (1 mmol), and t-BuZnBr (1.2 mmol) at room temperature in THF (5 mL) under nitrogen atmosphere. | |||
| 1 | PhCHO | 6 |
1a (98) [10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 2 | (p-OMe)PhCHO | 24 |
1b (91) [6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 3 | (p-Cl)PhCHO | 24 |
1c (93) [9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 4 | c-C6H11CHO | 24 |
1d (91) [12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 5 | n-C5H11CHO | 24 |
1e (42) [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 6 | Acetone | 24 | 1f (70) |
Irrespective of the kinds of aromatic and aliphatic aldehydes, t-BuZnBr reacted with 1,3-butadiene at the C1 position and the aldehydes reacted at the C2 position to provide 2-neopentyl homoallyl alcohols 1 in excellent yields (entries 1–4). The reaction proceeded with high regio- and stereoselectivities and anti isomers were formed predominantly. Although n-hexanal could participate in the coupling reaction, non-selective formation of syn and anti diastereoisomers was observed (entry 5).
Besides aromatic and aliphatic aldehydes, ketones also took part in the coupling reaction. When acetone was used as the carbonyl electrophile, the desired homoallyl alcohol was obtained in reasonable yield (entry 6, Table 1). As there was no change in reactivities and selectivities regardless of the presence or absence of an Ni catalyst,4 the coupling reactions did not involve nickelacycles, but an alternative multi-component coupling mechanism might be active.
Reaction with t-BuZnI prepared from t-BuI and Zn dust
The t-BuZnI reagent, which was prepared from Zn dust and t-BuI in situ, was useful for a similar coupling reaction providing homoallyl alcohols 1 efficiently (Table 2). t-BuI, butadiene, and various kinds of aldehydes were introduced to the Zn dust suspension, and the reaction mixture was stirred at room temperature. As a result of many investigations in various kinds of solvents, such as DMSO (dimethyl sulfoxide), DMF (N,N-dimethylformamide), DMA (N,N-dimethylacetamide), nitromethane, toluene, and dichloromethane, it was found that a combination of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v THF and DMA was the most effective solvent for producing good yields and stereoselectivities, compared to the reaction in THF alone (entries 1 and 2, Table 2).5
1 v/v THF and DMA was the most effective solvent for producing good yields and stereoselectivities, compared to the reaction in THF alone (entries 1 and 2, Table 2).5
| Entry | Electrophile | Yield (%) [anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn] syn] |
|---|---|---|
| a The reaction was undertaken in the presence of Zn dust (1.2 mmol) in THF–DMA solution (3 mL/1 mL) by employment of butadiene (4 mmol), aldehyde (1 mmol), and t-BuI (1.2 mmol) at room temperature for 24 hours under nitrogen atmosphere. b THF (5 mL) was used as solvent. | ||
| 1 | PhCHO |
1a (77) [7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 2 | PhCHO |
1a (75) [4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]b 1]b |
| 3 | (p-OMe)PhCHO |
1b (87) [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 4 | (p-Cl)PhCHO |
1c (84) [5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 5 | c-C6H11CHO |
1d (84) [10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 6 | n-C5H11CHO |
1e (57) [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 7 | Acetone | 1f (10) |
In comparison with the result of t-BuZnBr in Table 1, the reactions using t-BuI and zinc dust showed similar reactivities; however, the stereoselectivities were slightly lower than that with t-BuZnBr (entries 1–5, Table 2). Although the isolated yield of the reaction with n-hexanal was improved, the stereoselectivity did not change (entry 6, Table 2). Acetone provided the desired product in modest yield, although by-products, such as direct coupling products derived from alkylzinc reagent and carbonyls were not produced at all (entry 7, Table 2).
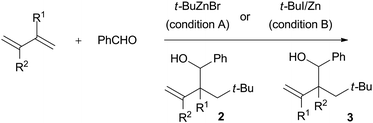
Reaction with various dienes
These coupling reactions utilizing t-BuZnBr and t-BuZnI prepared from Zn dust and t-BuI were capable of the efficient and straightforward stereodefined construction of homoallyl alcohols. The results using various substituted dienes are shown in Table 3.| Entry | Diene | Isolated yield (%) [anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn] syn] |
|
|---|---|---|---|
| Condition A | Condition B | ||
| a Condition A: diene (4 mmol), benzaldehyde (1 mmol), and t-BuZnBr (1.2 mmol) in THF (5 mL) at r.t. for 24 h; condition B: Zn dust (1.2 mmol) in THF–DMA (3 mL/1 mL), diene (4 mmol), benzaldehyde (1 mmol), t-BuI (1.2 mmol) at r.t. for 24 h. | |||
| 1 | Isoprene (R1 = Me, R2 = H) |
2a (63) [10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
2a (55) [6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
3a (20) [9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
3a (12) [8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
||
| 2 | 2,3-Dimethyl-1,3-butadiene (R1, R2 = Me) |
2b (62) [3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
2b (79) [2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 3 | Myrcene (R1 = C6H11, R2 = H) |
2c (60) [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
2c (58) [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
3c (36) [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
3c (18) [single] | ||
| 4 | Cyclohexadiene | No reaction | No reaction |
| 5 | Methyl sorbate | No reaction | No reaction |
The reactions with a wide variety of conjugated dienes were conducted with both commercially available t-BuZnBr (condition A) and t-BuZnI reagent prepared from Zn dust and t-BuI (condition B). In the case of isoprene, the t-Bu group added to the diene at the C1 position and benzaldehyde reacted at the C2 position to construct the sterically congested quaternary carbon center giving rise to the homoallyl alcohol 2a along with the regioisomer 3a as a minor product (entry 1, Table 3). Irrespective of the conditions, anti stereoselectivities were predominantly observed in 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios.
1 ratios.
2,3-Dimethyl-1,3-butadiene provided the desired product in modest to good yields, but with lower anti stereoselectivities than that of 1,3-butadiene and isoprene (entry 2, Table 3). In benzene solvent, the hetero Diels–Alder reaction adduct from 2,3-dimethyl-1,3-butadiene and benzaldehyde was obtained exclusively, instead of the homoallyl alcohol (Scheme 4). While the combination of benzaldehyde and conjugated dienes is generally reluctant to undergo the hetero Diels–Alder reaction,6 3,6-dihydro-2H-pyran is afforded smoothly under benzene. Myrcene could participate in the coupling reaction as well as isoprene, but the diastereoselectivities were by no means satisfactory (entry 3, Table 3). No reaction took place at all with cyclohexadiene and electron deficient dienes such as methyl sorbate (entries 4 and 5, Table 3).
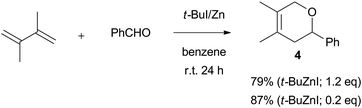 | ||
| Scheme 4 Hetero Diels–Alder reaction of PhCHO and 2,3-dimethyl-1,3-butadiene promoted by Zn dust and t-BuI. | ||
Structure determination
The structure of product 1a was determined unequivocally by conversion to the six-membered ring acetonide by ozonolysis, reduction with NaBH4, and acetonization with acetone dimethylacetal. NOE enhancement of the boldface protons by irradiation at Ha and the coupling constant between the vicinal diaxial Ha and Hb protons of the acetonide 5 are illustrated in Scheme 5. These results confirmed the relative configuration of 5 as the anti form. Thus, the stereochemistry of the major homoallyl alcohol product was unambiguously determined as the anti form by means of chemical derivatization and spectral analysis.7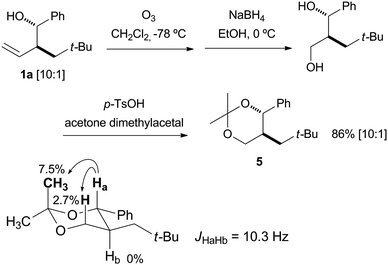 | ||
| Scheme 5 Structure determination and % NOE enhancement upon irradiation of Ha and the coupling constant. | ||
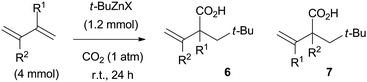
Coupling reaction under CO2
A series of these coupling reactions in the absence of aldehydes was conducted under a carbon dioxide atmosphere (1 atm). In the presence of t-BuZnBr or t-BuZnI, 1,3-butadiene underwent the three-component coupling reaction to provide 2-neopentyl-3-butenoic acid 6a as a single isomer by addition of the t-Bu group and carbon dioxide in a 1,2-addition manner (entry 1, Table 4). When using 2,3-dimethyl-1,3-butadiene, t-BuZnBr reacted with the diene at the C1 position and carbon dioxide reacted at the C2 position to provide the desired product 6b in excellent yield with high regioselectivity, whereas t-BuZnI provided the same product 6b in modest yields (entry 2, Table 4). Isoprene and myrcene also underwent 1,2-addition to afford more congested carboxylic acids 6c and 6d along with the less substituted regioisomers of 7c and 7d as minor products (entries 3 and 4, Table 4). It is noteworthy that t-BuZn remains intact under carbon dioxide atmospheric pressure and does not undergo direct coupling with carbon dioxide to give the corresponding carboxylic acids in comparison to the reaction with Grignard reagents.8 Instead, the three-component coupling reaction of conjugated dienes, t-Bu groups, and carbon dioxide predominates over the direct coupling reaction of t-Bu and carbon dioxide.| Entry | Diene | Isolated yield (%) | |
|---|---|---|---|
| Condition A | Condition B | ||
| a Condition A: diene (4 mmol), and t-BuZnBr (1.2 mmol) in THF (2 mL) at r.t. under CO2 (1 atm); condition B: diene (4 mmol), Zn dust (1.2 mmol), t-BuI (1.2 mmol) in THF (2 mL) at r.t. under CO2 (1 atm). | |||
| 1 | Butadiene (R1, R2 = H) | 6a (86) | 6a (61) |
| 2 | 2,3-Dimethyl-1,3-butadiene (R1, R2 = Me) | 6b (86) | 6b (41) |
| 3 | Isoprene (R1 = Me, R2 = H) | 6c (60), 7c (28) | 6c (12), 7c (5) |
| 4 | Myrcene (R1 = C6H11, R2 = H) | 6d (54), 7d (23) | 6d (0), 7d (11) |
Plausible reaction mechanism
A plausible reaction mechanism for the three-component coupling reaction is shown in Scheme 6. The C–Zn bonds of t-BuZnX are readily cleaved by homolysis to form t-Bu and ZnX radicals, which then add to the 1,3-butadiene in 1,4-addition fashion forming allylzinc species I and II in equilibrium with each other. The carbonyl species add to the γ-position of the allylzinc species to form the six-membered transition states III and IV. The more stable allylzinc species I with an aldehyde would undergo the coupling reaction via six-membered transition state III predominating over IV to avoid steric repulsion between the neopentyl group and the substituents on the aldehyde, and result in the formation of homoallyl alcohols with anti stereoselectivity.9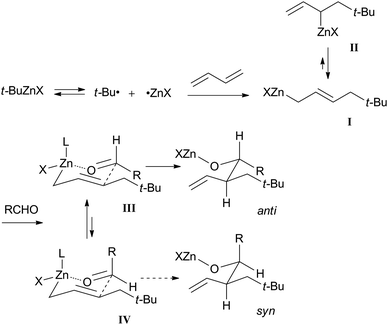 | ||
| Scheme 6 A plausible reaction mechanism for three-component coupling reaction of t-Bu group, butadiene, and aldehyde. | ||
Conclusions
In summary, the addition of t-butylzinc halide to the reaction mixture of butadiene and an aldehyde provides 2-neopentyl homoallyl alcohols in high yields by 1,2-addition of the aldehyde and the t-butyl group to butadiene. Furthermore, t-BuZnI prepared from Zn dust and t-BuI was also useful for the coupling reaction to provide homoallyl alcohols with high regio- and stereoselectivities. Without the aldehyde, under carbon dioxide atmospheric pressure, the three components of t-butylzinc halide, butadiene, and carbon dioxide combine to give 2-neopentyl-3-butenoic acids in excellent yields.Experimental
General procedures
Distillation were carried out in a Kugelrohr apparatus (SIBATA glass tube oven GTO-350RG). Boiling points are meant to refer to the oven temperature (±1 °C). Microanalyses were performed by the Instrumental Analysis Center of Nagasaki University. Analysis agreed with the calculated values within ±0.4%. High resolution mass spectra (HRMS) were measured with JEOL JMSDX303. Infrared spectra were recorded with a JASCO A-100 or SHIMAZU FTIR-8700 infrared spectrophotometer. 1H and 13C magnetic resonance spectra were measured on JEOL-GX400 instrument with tetramethylsilane as an internal standard. Chemical shift values were given in ppm downfield from the internal standard.Tetrahydrofuran was dried and distilled from benzophenone-sodium immediately prior to use under nitrogen atmosphere. DMA were distilled over calcium hydride. Benzaldehyde, p-anisaldehyde, cyclohexanecarbaldehyde, n-hexanal, isoprene, 2,3-dimethyl-1,3-butadiene, myrcene, cyclohexadiene, methyl sorbate were distilled via Kugelrohr apparatus under reduced pressure prior to use. t-BuZnBr (0.5 M THF, Aldrich), t-BuI (Aldrich), Zinc dust (Aldrich), Ni(cod)2 (KANTO Kagaku) were used without further purification. 1,3-Butadiene (Tokyo Kasei Kogyo Co., Ltd) was purchased, and was liquefied by cooling at −78 °C (dry ice/isopropanol) prior to use under argon atmosphere. 1,3-Butadiene could be measured by syringe kept cool in the freezer as well beforehand, and then was introduced into the reaction mixture at room temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 7
syn = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3423 (s), 2866 (s), 1495 (s), 1001 (s), 910 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) d 0.75 (s, 9H), 1.18 (dd, J = 2.8, 13.9 Hz, 1H), 1.24 (dd, J = 8.8, 13.9 Hz, 1H), 2.34 (d, J = 2.1 Hz, 1H), 2.45 (br dq, J = 2.8, 8.8 Hz, 1H), 4.28 (br dd, J = 2.1, 8.1 Hz, 1H), 5.20 (br dd, J = 1.8, 17.2 Hz, 1H), 5.23 (br dd, J = 1.8, 10.3 Hz, 1H), 5.72 (ddd, J = 9.5, 10.3, 17.2 Hz, 1H), 7.13–7.53 (m, 5H); 13C NMR (100 MHz, CDCl3, anti-isomer) d 30.0, 31.0, 44.1, 49.4, 77.4, 118.2, 127.4, 127.6, 128.2, 141.9, 142.3; 1H NMR (400 MHz, CDCl3, syn-isomer) d 0.81 (s, 9H), 1.16 (dd, J = 9.5, 13.9 Hz, 1H), 1.47 (dd, J = 2.2, 13.9 Hz, 1H), 2.14 (br d, J = 5.3 Hz, 1H), 2.54 (br dq, J = 2.2, 9.5 Hz, 1H), 4.58 (br t, J = 5.3 Hz, 1H), 5.08 (br d, J = 10.8, 1H), 5.09 (br dd, J = 16.9 Hz, 1H), 5.62 (ddd, J = 9.0, 10.8, 16.9 Hz, 1H), 7.13–7.53 (m, 5H); 13C NMR (100 MHz, CDCl3, syn-isomer) d 30.1, 31.0, 42.9, 47.8, 77.4, 116.6, 126.8, 127.2, 127.9, 141.4, 142.4; high-resolution MS, calcd for C15H22O: 218.1671. Found m/z (relative intensity) 218.1692 (M+, 100), 201 (88), 147 (34), 146 (32).
1 ratio).
IR (neat) 3423 (s), 2866 (s), 1495 (s), 1001 (s), 910 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) d 0.75 (s, 9H), 1.18 (dd, J = 2.8, 13.9 Hz, 1H), 1.24 (dd, J = 8.8, 13.9 Hz, 1H), 2.34 (d, J = 2.1 Hz, 1H), 2.45 (br dq, J = 2.8, 8.8 Hz, 1H), 4.28 (br dd, J = 2.1, 8.1 Hz, 1H), 5.20 (br dd, J = 1.8, 17.2 Hz, 1H), 5.23 (br dd, J = 1.8, 10.3 Hz, 1H), 5.72 (ddd, J = 9.5, 10.3, 17.2 Hz, 1H), 7.13–7.53 (m, 5H); 13C NMR (100 MHz, CDCl3, anti-isomer) d 30.0, 31.0, 44.1, 49.4, 77.4, 118.2, 127.4, 127.6, 128.2, 141.9, 142.3; 1H NMR (400 MHz, CDCl3, syn-isomer) d 0.81 (s, 9H), 1.16 (dd, J = 9.5, 13.9 Hz, 1H), 1.47 (dd, J = 2.2, 13.9 Hz, 1H), 2.14 (br d, J = 5.3 Hz, 1H), 2.54 (br dq, J = 2.2, 9.5 Hz, 1H), 4.58 (br t, J = 5.3 Hz, 1H), 5.08 (br d, J = 10.8, 1H), 5.09 (br dd, J = 16.9 Hz, 1H), 5.62 (ddd, J = 9.0, 10.8, 16.9 Hz, 1H), 7.13–7.53 (m, 5H); 13C NMR (100 MHz, CDCl3, syn-isomer) d 30.1, 31.0, 42.9, 47.8, 77.4, 116.6, 126.8, 127.2, 127.9, 141.4, 142.4; high-resolution MS, calcd for C15H22O: 218.1671. Found m/z (relative intensity) 218.1692 (M+, 100), 201 (88), 147 (34), 146 (32).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 6
syn = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3452 (m), 2866 (m), 1612 (m), 1514 (s), 1248 (s), 1038 (s), 833 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.76 (s, 9H), 1.17 (dd, J = 3.0, 14.0 Hz, 1H), 1.23 (dd, J = 8.6, 14.0 Hz, 1H), 2.27 (d, J = 1.7 Hz, 1H), 2.42 (ddm, J = 3.0, 8.6 Hz, 1H), 3.80 (s, 3H), 4.22 (dd, J = 1.7, 8.1 Hz, 1H), 5.20 (dd, J = 1.8, 17.1 Hz, 1H), 5.23 (dd, J = 1.8, 10.2 Hz, 1H), 5.72 (ddd, J = 9.3, 10.2, 17.1 Hz, 1H), 6.86 (d, J = 8.5 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 30.0, 31.0, 44.1, 49.5, 55.2, 76.4, 113.4, 118.0, 128.3, 134.2, 142.1, 158.9; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.83 (s, 9H), 2.51–2.53 (m, 1H), 3.80 (s, 3H), 4.53 (t, J = 5.1 Hz, 1H), 5.05 (dd, J = 1.9, 9.1 Hz, 1H), 5.07 (dd, J = 1.9, 16.8 Hz, 1H), 5.62 (ddd, J = 9.1, 10.5, 16.8 Hz, 1H); 13C NMR (400 MHz, CDCl3) δ 30.1, 43.2, 47.8, 113.2, 116.5, 127.8, 134.4, 141.2; high-resolution MS, calcd for C15H21ClO: 248.1776, found m/z (relative intensity): 248.1767 (M+, 75), 215 (100).
1 ratio).
IR (neat) 3452 (m), 2866 (m), 1612 (m), 1514 (s), 1248 (s), 1038 (s), 833 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.76 (s, 9H), 1.17 (dd, J = 3.0, 14.0 Hz, 1H), 1.23 (dd, J = 8.6, 14.0 Hz, 1H), 2.27 (d, J = 1.7 Hz, 1H), 2.42 (ddm, J = 3.0, 8.6 Hz, 1H), 3.80 (s, 3H), 4.22 (dd, J = 1.7, 8.1 Hz, 1H), 5.20 (dd, J = 1.8, 17.1 Hz, 1H), 5.23 (dd, J = 1.8, 10.2 Hz, 1H), 5.72 (ddd, J = 9.3, 10.2, 17.1 Hz, 1H), 6.86 (d, J = 8.5 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 30.0, 31.0, 44.1, 49.5, 55.2, 76.4, 113.4, 118.0, 128.3, 134.2, 142.1, 158.9; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.83 (s, 9H), 2.51–2.53 (m, 1H), 3.80 (s, 3H), 4.53 (t, J = 5.1 Hz, 1H), 5.05 (dd, J = 1.9, 9.1 Hz, 1H), 5.07 (dd, J = 1.9, 16.8 Hz, 1H), 5.62 (ddd, J = 9.1, 10.5, 16.8 Hz, 1H); 13C NMR (400 MHz, CDCl3) δ 30.1, 43.2, 47.8, 113.2, 116.5, 127.8, 134.4, 141.2; high-resolution MS, calcd for C15H21ClO: 248.1776, found m/z (relative intensity): 248.1767 (M+, 75), 215 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 9
syn = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3433 (m), 2909 (s), 1638 (w), 1090 (s), 831 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.76 (s, 9H), 1.16 (dd, J = 2.4, 13.9 Hz, 1H), 1.25 (dd, J = 9.0, 13.9 Hz, 1H), 2.32 (br s, 1H), 2.39 (dddd, J = 2.4, 7.8, 9.0, 9.3 Hz, 1H), 4.57 (d, J = 7.8 Hz, 1H), 5.17 (dd, J = 1.3, 17.2 Hz, 1H), 5.18 (dd, J = 1.3, 10.2 Hz, 1H), 5.70 (ddd, J = 9.3, 10.2, 17.2 Hz, 1H), 7.25 (d, J = 8.7 Hz, 2H), 7.30 (d, J = 8.7 Hz, 2H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 30.0, 31.0, 44.0, 49.4, 76.2, 118.5, 128.1, 128.6, 133.1, 140.6, 141.3; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.81 (s, 9H), 2.50–2.52 (m, 1H), 4.27 (t, J = 4.6 Hz, 1H), 5.08 (dm, J = 17.2 Hz, 1H), 5.10 (dm, J = 10.4 Hz, 1H), 5.60 (ddd, J = 9.3, 10.4, 17.2 Hz, 1H); 13C NMR (400 MHz, CDCl3, syn-isomer) δ 30.1, 42.8, 47.7, 116.9, 140.7, 140.8; high-resolution MS, calcd for C15H21ClO: 252.1281, found m/z (relative intensity): 252.1295 (M+, 68), 219 (100).
1 ratio).
IR (neat) 3433 (m), 2909 (s), 1638 (w), 1090 (s), 831 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.76 (s, 9H), 1.16 (dd, J = 2.4, 13.9 Hz, 1H), 1.25 (dd, J = 9.0, 13.9 Hz, 1H), 2.32 (br s, 1H), 2.39 (dddd, J = 2.4, 7.8, 9.0, 9.3 Hz, 1H), 4.57 (d, J = 7.8 Hz, 1H), 5.17 (dd, J = 1.3, 17.2 Hz, 1H), 5.18 (dd, J = 1.3, 10.2 Hz, 1H), 5.70 (ddd, J = 9.3, 10.2, 17.2 Hz, 1H), 7.25 (d, J = 8.7 Hz, 2H), 7.30 (d, J = 8.7 Hz, 2H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 30.0, 31.0, 44.0, 49.4, 76.2, 118.5, 128.1, 128.6, 133.1, 140.6, 141.3; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.81 (s, 9H), 2.50–2.52 (m, 1H), 4.27 (t, J = 4.6 Hz, 1H), 5.08 (dm, J = 17.2 Hz, 1H), 5.10 (dm, J = 10.4 Hz, 1H), 5.60 (ddd, J = 9.3, 10.4, 17.2 Hz, 1H); 13C NMR (400 MHz, CDCl3, syn-isomer) δ 30.1, 42.8, 47.7, 116.9, 140.7, 140.8; high-resolution MS, calcd for C15H21ClO: 252.1281, found m/z (relative intensity): 252.1295 (M+, 68), 219 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 9
syn = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3368 (br m), 3071 (s), 2853 (s), 1636 (w) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.90 (s, 9H), 0.96–1.30 (m, 5H), 1.32–1.42 (m, 3H), 1.64–1.76 (m, 4H), 1.81–1.86 (m, 1H), 2.37 (dq, J = 5.2, 9.2 Hz, 1H), 3.07 (t, J = 5.2 Hz, 1H), 5.08 (ddd, J = 0.7, 1.9, 17.3 Hz, 1H), 5.14 (dd, J = 1.9, 10.4 Hz, 1H), 5.73 (ddd, J = 9.2, 10.4, 17.3 Hz, 1H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 26.1, 26.3, 26.5, 27.8, 30.0, 30.1, 31.3, 40.3, 42.9, 45.5, 79.1, 116.7, 140.8; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.86 (s, 9H), 3.13 (m, 1H), 5.02 (dm, J = 1.0 Hz, 1H), 5.07 (dm, J = 1.0 Hz, 1H), 5.75 (dd, J = 8.5, 17.6 Hz, 1H); 13C NMR (400 MHz, CDCl3, syn-isomer) δ 27.2, 29.2, 30.3, 30.4, 39.9, 42.7, 43.8, 80.2, 115.0, 143.1; high-resolution MS, calcd for C15H28O: 224.2140, found m/z (relative intensity): 224.2079 (M+, 24), 223 (100).
1 ratio).
IR (neat) 3368 (br m), 3071 (s), 2853 (s), 1636 (w) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.90 (s, 9H), 0.96–1.30 (m, 5H), 1.32–1.42 (m, 3H), 1.64–1.76 (m, 4H), 1.81–1.86 (m, 1H), 2.37 (dq, J = 5.2, 9.2 Hz, 1H), 3.07 (t, J = 5.2 Hz, 1H), 5.08 (ddd, J = 0.7, 1.9, 17.3 Hz, 1H), 5.14 (dd, J = 1.9, 10.4 Hz, 1H), 5.73 (ddd, J = 9.2, 10.4, 17.3 Hz, 1H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 26.1, 26.3, 26.5, 27.8, 30.0, 30.1, 31.3, 40.3, 42.9, 45.5, 79.1, 116.7, 140.8; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.86 (s, 9H), 3.13 (m, 1H), 5.02 (dm, J = 1.0 Hz, 1H), 5.07 (dm, J = 1.0 Hz, 1H), 5.75 (dd, J = 8.5, 17.6 Hz, 1H); 13C NMR (400 MHz, CDCl3, syn-isomer) δ 27.2, 29.2, 30.3, 30.4, 39.9, 42.7, 43.8, 80.2, 115.0, 143.1; high-resolution MS, calcd for C15H28O: 224.2140, found m/z (relative intensity): 224.2079 (M+, 24), 223 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 1
syn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3362 (w), 2934 (s), 2862 (s), 1638 (w) cm−1; (one isomer): 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.87 (t, J = 5.6 Hz, 3H), 0.89 (s, 9H), 1.21–1.39 (ddm, J = 4.6, 7.8 Hz, 10H), 2.28 (dq, J = 4.1, 8.3 Hz, 1H), 3.33–3.40 (m, 1H), 5.08 (dd, J = 1.7, 18.5 Hz, 1H), 5.13 (dd, J = 1.7, 10.0, Hz, 1H), 5.69 (ddd, J = 8.3, 10.0, 18.5 Hz, 1H); 13C NMR (400 MHz, CDCl3, one isomer) δ 14.0, 22.6, 26.0, 30.1, 31.1, 33.2, 44.1, 75.7, 116.4, 141.5; (minor isomer): 1H NMR (400 MHz, CDCl3, other isomer) δ 0.90 (s, 9H), 0.94 (t, J = 7.2 Hz, 3H), 2.12–2.18 (dddm, J = 0.7, 4.6, 8.8 Hz, 1H), 5.09 (dd, J = 0.7, 15.9 Hz, 1H), 5.09 (d, J = 11.5 Hz, 1H), 5.68 (ddd, J = 8.8, 11.5, 15.9 Hz, 1H); 13C NMR (400 MHz, CDCl3, other isomer) δ 25.6, 30.2, 31.2, 31.9, 34.4, 44.9, 46.8, 74.9, 116.9, 141.2; high-resolution MS, calcd for C12H24O: 212.214, found m/z (relative intensity): 212.2099 (M+, 46), 197 (100).
1 ratio).
IR (neat) 3362 (w), 2934 (s), 2862 (s), 1638 (w) cm−1; (one isomer): 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.87 (t, J = 5.6 Hz, 3H), 0.89 (s, 9H), 1.21–1.39 (ddm, J = 4.6, 7.8 Hz, 10H), 2.28 (dq, J = 4.1, 8.3 Hz, 1H), 3.33–3.40 (m, 1H), 5.08 (dd, J = 1.7, 18.5 Hz, 1H), 5.13 (dd, J = 1.7, 10.0, Hz, 1H), 5.69 (ddd, J = 8.3, 10.0, 18.5 Hz, 1H); 13C NMR (400 MHz, CDCl3, one isomer) δ 14.0, 22.6, 26.0, 30.1, 31.1, 33.2, 44.1, 75.7, 116.4, 141.5; (minor isomer): 1H NMR (400 MHz, CDCl3, other isomer) δ 0.90 (s, 9H), 0.94 (t, J = 7.2 Hz, 3H), 2.12–2.18 (dddm, J = 0.7, 4.6, 8.8 Hz, 1H), 5.09 (dd, J = 0.7, 15.9 Hz, 1H), 5.09 (d, J = 11.5 Hz, 1H), 5.68 (ddd, J = 8.8, 11.5, 15.9 Hz, 1H); 13C NMR (400 MHz, CDCl3, other isomer) δ 25.6, 30.2, 31.2, 31.9, 34.4, 44.9, 46.8, 74.9, 116.9, 141.2; high-resolution MS, calcd for C12H24O: 212.214, found m/z (relative intensity): 212.2099 (M+, 46), 197 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 10
syn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3462 (br m), 3030 (m), 2953 (s), 1634 (w), 1454 (s), 1022 (s), 910 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.90 (s, 9H), 1.10 (s, 3H), 1.24 (d, J = 13.8 Hz, 1H), 1.55 (d, J = 13.8 Hz, 1H), 2.14 (d, J = 1.7 Hz, 1H), 4.24 (d, J = 1.7 Hz, 1H), 5.12 (dd, J = 1.2, 17.6 Hz, 1H), 5.26 (dd, J = 1.2, 10.9 Hz, 1H), 5.98 (dd, J = 10.9, 17.6 Hz, 1H), 7.20–7.34 (m, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 17.2, 29.7, 31.9, 46.9, 50.7, 80.7, 114.8, 126.2, 127.2, 128.3, 139.9, 145.9; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.76 (s, 9H), 1.23 (s, 3H), 1.23 (d, J = 4.3 Hz, 1H), 1.41 (d, J = 4.3 Hz, 1H), 2.04 (d, J = 6.0 Hz, 1H), 4.27 (d, J = 6.0 Hz, 1H), 5.01 (dd, J = 1.3, 17.6 Hz, 1H), 5.15 (dd, J = 1.3, 11.8 Hz, 1H), 5.97 (dd, J = 11.8, 17.6 Hz, 1H); 13C NMR (400 MHz, CDCl3, syn-isomer) δ 19.9, 30.7, 32.2, 46.1, 50.1, 82.3, 113.9, 126.9, 127.3, 127.9, 141.3, 144.7; high-resolution MS, calcd for C16H24O: 232.1827, found m/z (relative intensity): 232.1823 (M+, 2), 199 (100).
1 ratio).
IR (neat) 3462 (br m), 3030 (m), 2953 (s), 1634 (w), 1454 (s), 1022 (s), 910 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.90 (s, 9H), 1.10 (s, 3H), 1.24 (d, J = 13.8 Hz, 1H), 1.55 (d, J = 13.8 Hz, 1H), 2.14 (d, J = 1.7 Hz, 1H), 4.24 (d, J = 1.7 Hz, 1H), 5.12 (dd, J = 1.2, 17.6 Hz, 1H), 5.26 (dd, J = 1.2, 10.9 Hz, 1H), 5.98 (dd, J = 10.9, 17.6 Hz, 1H), 7.20–7.34 (m, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 17.2, 29.7, 31.9, 46.9, 50.7, 80.7, 114.8, 126.2, 127.2, 128.3, 139.9, 145.9; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.76 (s, 9H), 1.23 (s, 3H), 1.23 (d, J = 4.3 Hz, 1H), 1.41 (d, J = 4.3 Hz, 1H), 2.04 (d, J = 6.0 Hz, 1H), 4.27 (d, J = 6.0 Hz, 1H), 5.01 (dd, J = 1.3, 17.6 Hz, 1H), 5.15 (dd, J = 1.3, 11.8 Hz, 1H), 5.97 (dd, J = 11.8, 17.6 Hz, 1H); 13C NMR (400 MHz, CDCl3, syn-isomer) δ 19.9, 30.7, 32.2, 46.1, 50.1, 82.3, 113.9, 126.9, 127.3, 127.9, 141.3, 144.7; high-resolution MS, calcd for C16H24O: 232.1827, found m/z (relative intensity): 232.1823 (M+, 2), 199 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 9
syn = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3470 (m), 3030 (w), 2866 (m), 1641 (w), 1196 (m), 1022 (m), 889 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.71 (s, 9H), 0.97 (dd, J = 2.0, 14.1 Hz, 1H), 1.39 (dd, J = 9.6, 14.1 Hz, 1H), 1.78 (dd, J = 0.7, 1.5 Hz, 3H), 2.35 (br s, 1H), 2.52 (dt, J = 2.0, 9.6 Hz, 1H), 4.27 (d, J = 9.6 Hz, 1H), 5.04 (dd, J = 0.7, 1.8 Hz, 1H), 5.07 (dd, J = 1.5, 1.8, Hz, 1H), 7.33 (d, J = 4.4 Hz, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 18.9, 29.5, 31.0, 41.2, 52.6, 75.5, 116.1, 127.4, 127.5, 128.0, 142.5, 146.9; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.90 (s, 9H), 0.97 (dm, J = 10.0 Hz, 1H), 1.39 (dm, J = 10.0 Hz, 1H), 1.69 (dd, J = 0.7, 1.5 Hz, 3H), 4.99 (d, J = 0.7 Hz, 1H), 5.03 (d, J = 1.5 Hz, 1H); high-resolution MS, calcd for C16H24O: 232.1827, found m/z (relative intensity): 232.1823 (M+, 13), 199 (100).
1 ratio).
IR (neat) 3470 (m), 3030 (w), 2866 (m), 1641 (w), 1196 (m), 1022 (m), 889 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.71 (s, 9H), 0.97 (dd, J = 2.0, 14.1 Hz, 1H), 1.39 (dd, J = 9.6, 14.1 Hz, 1H), 1.78 (dd, J = 0.7, 1.5 Hz, 3H), 2.35 (br s, 1H), 2.52 (dt, J = 2.0, 9.6 Hz, 1H), 4.27 (d, J = 9.6 Hz, 1H), 5.04 (dd, J = 0.7, 1.8 Hz, 1H), 5.07 (dd, J = 1.5, 1.8, Hz, 1H), 7.33 (d, J = 4.4 Hz, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 18.9, 29.5, 31.0, 41.2, 52.6, 75.5, 116.1, 127.4, 127.5, 128.0, 142.5, 146.9; 1H NMR (400 MHz, CDCl3, syn-isomer) δ 0.90 (s, 9H), 0.97 (dm, J = 10.0 Hz, 1H), 1.39 (dm, J = 10.0 Hz, 1H), 1.69 (dd, J = 0.7, 1.5 Hz, 3H), 4.99 (d, J = 0.7 Hz, 1H), 5.03 (d, J = 1.5 Hz, 1H); high-resolution MS, calcd for C16H24O: 232.1827, found m/z (relative intensity): 232.1823 (M+, 13), 199 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 3
syn = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3462 (w), 2953 (s), 2872 (s), 1630 (w), 1452 (m), 1364 (m), 1242 (m), 1190 (m), 1043 (m), 1022 (m), 894 (m), 702 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.87 (s, 9H), 1.05 (d, J = 14.5 Hz, 1H), 1.10 (s, 3H), 1.72 (d, J = 14.5 Hz, 1H), 1.95 (br s, 3H), 4.45 (br s, 1H), 5.10 (br s, 1H), 5.18 (br s, 1H), 7.24–7.35 (m, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 18.5, 21.9, 26.5, 32.2, 48.5, 49.0, 80.4, 114.0, 127.2, 128.1, 141.0, 148.9; (1,2-syn isomer): 13C NMR (400 MHz, CDCl3, syn-isomer) δ 17.9, 20.3, 26.4, 31.6, 45.8, 47.4, 78.2, 115.9, 127.4, 128.9, 140.3, 149.7; high-resolution MS, calcd for C17H26O: 246.1984, found m/z (relative intensity): 246.1992 (M+, 1), 245 (4), 244 (6), 229 (100).
1 ratio).
IR (neat) 3462 (w), 2953 (s), 2872 (s), 1630 (w), 1452 (m), 1364 (m), 1242 (m), 1190 (m), 1043 (m), 1022 (m), 894 (m), 702 (s) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.87 (s, 9H), 1.05 (d, J = 14.5 Hz, 1H), 1.10 (s, 3H), 1.72 (d, J = 14.5 Hz, 1H), 1.95 (br s, 3H), 4.45 (br s, 1H), 5.10 (br s, 1H), 5.18 (br s, 1H), 7.24–7.35 (m, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 18.5, 21.9, 26.5, 32.2, 48.5, 49.0, 80.4, 114.0, 127.2, 128.1, 141.0, 148.9; (1,2-syn isomer): 13C NMR (400 MHz, CDCl3, syn-isomer) δ 17.9, 20.3, 26.4, 31.6, 45.8, 47.4, 78.2, 115.9, 127.4, 128.9, 140.3, 149.7; high-resolution MS, calcd for C17H26O: 246.1984, found m/z (relative intensity): 246.1992 (M+, 1), 245 (4), 244 (6), 229 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 1
syn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3470 (m), 3030 (m), 2934 (s), 1634 (w), 1196 (m), 899 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 1.02 (s, 9H), 1.42 (ddd, J = 2.4, 8.4, 19.8, Hz, 1H), 1.43 (dd, J = 8.4, 19.8, Hz, 1H), 1.57 (s, 3H), 1.64 (d, J = 1.2 Hz, 3H), 1.75 (s, 2H), 1.89 (d, J = 4.1 Hz, 1H), 2.04 (ddm, J = 7.1, 8.4, Hz, 2H), 4.64 (d, J = 4.1 Hz, 1H), 4.96 (dt, J = 1.2, 7.1 Hz, 1H), 4.96 (dd, J = 18.1, 1.3 Hz, 1H), 5.20 (dd, J = 11.2, 1.3 Hz, 1H), 5.94 (dd, J = 18.1, 11.2 Hz, 1H), 7.25–7.31 (dm, J = 6.1 Hz, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 23.0, 25.7, 32.1, 32.1, 33.0, 44.1, 48.6, 79.1, 114.6, 124.6, 127.3, 128.2, 141.2, 143.3; high-resolution MS, calcd for C21H32O: 300.2453, found m/z (relative intensity): 300.2461 (M+, 11), 282 (100).
1 ratio).
IR (neat) 3470 (m), 3030 (m), 2934 (s), 1634 (w), 1196 (m), 899 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 1.02 (s, 9H), 1.42 (ddd, J = 2.4, 8.4, 19.8, Hz, 1H), 1.43 (dd, J = 8.4, 19.8, Hz, 1H), 1.57 (s, 3H), 1.64 (d, J = 1.2 Hz, 3H), 1.75 (s, 2H), 1.89 (d, J = 4.1 Hz, 1H), 2.04 (ddm, J = 7.1, 8.4, Hz, 2H), 4.64 (d, J = 4.1 Hz, 1H), 4.96 (dt, J = 1.2, 7.1 Hz, 1H), 4.96 (dd, J = 18.1, 1.3 Hz, 1H), 5.20 (dd, J = 11.2, 1.3 Hz, 1H), 5.94 (dd, J = 18.1, 11.2 Hz, 1H), 7.25–7.31 (dm, J = 6.1 Hz, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 23.0, 25.7, 32.1, 32.1, 33.0, 44.1, 48.6, 79.1, 114.6, 124.6, 127.3, 128.2, 141.2, 143.3; high-resolution MS, calcd for C21H32O: 300.2453, found m/z (relative intensity): 300.2461 (M+, 11), 282 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) syn = 1
syn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 ratio).
IR (neat) 3470 (br m), 3030 (m), 2866 (m), 1634 (w), 1196 (m), 899 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.71 (s, 9H), 1.03 (dd, J = 2.0, 14.1 Hz, 1H), 1.44 (dd, J = 9.8, 14.1 Hz, 1H), 1.63 (s, 3H), 1.70 (d, J = 1.2 Hz, 3H), 2.04 (dt, J = 6.8, 8.2 Hz, 2H), 2.16–2.24 (m, 2H), 2.34 (d, J = 2.4 Hz, 1H), 2.51 (ddd, J = 2.0, 9.0, 9.8 Hz, 1H), 4.32 (dd, J = 2.4, 9.0 Hz, 1H), 5.11 (d, J = 1.7 Hz, 1H), 5.12 (d, J = 1.2 Hz, 1H), 5.14 (tq, J = 1.2, 6.8 Hz, 1H), 7.31 (d, J = 4.4 Hz, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 17.7, 25.6, 26.1, 29.6, 31.0, 32.3, 41.9, 52.9, 75.9, 113.9, 123.9, 127.3, 127.5, 128.0, 132.0, 142.6, 150.7; high-resolution MS, calcd for C21H32O: 300.2453, found m/z (relative intensity): 300.2461 (M+, 11), 282 (100).
1 ratio).
IR (neat) 3470 (br m), 3030 (m), 2866 (m), 1634 (w), 1196 (m), 899 (m) cm−1; 1H NMR (400 MHz, CDCl3, anti-isomer) δ 0.71 (s, 9H), 1.03 (dd, J = 2.0, 14.1 Hz, 1H), 1.44 (dd, J = 9.8, 14.1 Hz, 1H), 1.63 (s, 3H), 1.70 (d, J = 1.2 Hz, 3H), 2.04 (dt, J = 6.8, 8.2 Hz, 2H), 2.16–2.24 (m, 2H), 2.34 (d, J = 2.4 Hz, 1H), 2.51 (ddd, J = 2.0, 9.0, 9.8 Hz, 1H), 4.32 (dd, J = 2.4, 9.0 Hz, 1H), 5.11 (d, J = 1.7 Hz, 1H), 5.12 (d, J = 1.2 Hz, 1H), 5.14 (tq, J = 1.2, 6.8 Hz, 1H), 7.31 (d, J = 4.4 Hz, 5H); 13C NMR (400 MHz, CDCl3, anti-isomer) δ 17.7, 25.6, 26.1, 29.6, 31.0, 32.3, 41.9, 52.9, 75.9, 113.9, 123.9, 127.3, 127.5, 128.0, 132.0, 142.6, 150.7; high-resolution MS, calcd for C21H32O: 300.2453, found m/z (relative intensity): 300.2461 (M+, 11), 282 (100).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1, major isomer was assigned).
mp = 79.5–80.1 °C. IR (KBr) 2995 (s), 2955 (s), 1059 (s), 1026 (s) cm−1; 1H NMR (100 MHz, CDCl3) δ 0.58 (s, 9H), 0.78 (dd, J = 7.5, 14.5 Hz, 1H), 0.99 (dd, J = 1.5, 14.5 Hz, 1H), 1.48 (s, 3H), 1.56 (s, 3H), 1.86–1.95 (m, 1H, coalescing to ddm, J = 1.5, 5.3, 7.5 Hz by irradiation at 4.41), 3.70 (t, J = 11.6 Hz, 1H), 4.00 (dd, J = 5.3, 11.6 Hz, 1H), 4.41 (d, J = 10.3 Hz, 1H), 7.20–7.43 (m, 5H), 13C NMR (100 MHz, CDCl3) δ 19.1, 29.5, 29.9, 30.4, 37.8, 40.6, 66.8, 78.0, 98.5, 128.1, 128.2, 128.3, 140.3; high-resolution MS, calcd for C17H26O2: 262.1933. Found m/z (relative intensity) 262.1934 (M+, 25), 247 (79), 165 (94), 163 (100).
1, major isomer was assigned).
mp = 79.5–80.1 °C. IR (KBr) 2995 (s), 2955 (s), 1059 (s), 1026 (s) cm−1; 1H NMR (100 MHz, CDCl3) δ 0.58 (s, 9H), 0.78 (dd, J = 7.5, 14.5 Hz, 1H), 0.99 (dd, J = 1.5, 14.5 Hz, 1H), 1.48 (s, 3H), 1.56 (s, 3H), 1.86–1.95 (m, 1H, coalescing to ddm, J = 1.5, 5.3, 7.5 Hz by irradiation at 4.41), 3.70 (t, J = 11.6 Hz, 1H), 4.00 (dd, J = 5.3, 11.6 Hz, 1H), 4.41 (d, J = 10.3 Hz, 1H), 7.20–7.43 (m, 5H), 13C NMR (100 MHz, CDCl3) δ 19.1, 29.5, 29.9, 30.4, 37.8, 40.6, 66.8, 78.0, 98.5, 128.1, 128.2, 128.3, 140.3; high-resolution MS, calcd for C17H26O2: 262.1933. Found m/z (relative intensity) 262.1934 (M+, 25), 247 (79), 165 (94), 163 (100).
Typical procedure for the three-component coupling reaction of diene, carbon dioxide, and t-BuZnI reagent prepared from t-BuI and Zn dust (entry 1, Table 4, condition B): into a carbon dioxide-purged flask containing zinc dust (78 mg, 1.2 mmol) were introduced successively THF (2 mL), t-BuI (220 mg, 1.2 mmol), 1,3-butadiene (0.4 mL, 4 mmol) via syringes. The reaction mixture was stirred at room temperature for 24 h under carbon dioxide atmospheric pressure, during which the reaction was monitored by TLC. After dilution with ethyl acetate (30 mL), the mixture was washed successively with 2 N-HCl, and brine, and then dried (MgSO4) and concentrated in vacuo. The residual oil was subjected to column chromatography over silica gel (hexane/ethyl acetate = 16/1, v/v) to give an analytically pure sample of 6a (114 mg, 61%).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT).Notes and references
- (a) Modern Organic Chemistry, ed. Y. Tamaru, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) J. Montgomery, Angew. Chem., Int. Ed., 2004, 43, 3890 CrossRef CAS PubMed; (c) J. Tsuji, Transition Metal Regents and Catalysts, Wiley & Sons, Chichester, 2000 Search PubMed; (d) G. Wilke, Angew. Chem., Int. Ed., 2003, 42, 5000 CrossRef CAS PubMed; (e) Y. Sato, M. Takimoto and M. Mori, J. Am. Chem. Soc., 2000, 122, 1624 CrossRef CAS and references therein; (f) P. W. Jolly and G. Wilke, The Organic Chemistry of Nickel, Academic, New York, vol. 1, 1974 Search PubMed.
- (a) T. Mori, T. Nakamura, G. Onodera and M. Kimura, Synthesis, 2012, 2333 CAS; (b) K. Kojima, M. Kimura and Y. Tamaru, Chem. Commun., 2005, 4717 RSC; (c) M. Kimura, K. Kojima, T. Inoue and Y. Tamaru, Synthesis, 2004, 3089 CAS; (d) A. Ezoe, M. Kimura, T. Inoue, M. Mori and Y. Tamaru, Angew. Chem., Int. Ed., 2002, 41, 2784 CrossRef CAS; (e) K. Shibata, M. Kimura, M. Shimizu and Y. Tamaru, Org. Lett., 2001, 3, 2181 CrossRef CAS PubMed; (f) K. Shibata, M. Kimura, K. Kojima, S. Tanaka and Y. Tamaru, J. Organomet. Chem., 2001, 624, 348 CrossRef CAS; (g) M. Kimura, K. Shibata, Y. Koudahashi and Y. Tamaru, Tetrahedron Lett., 2000, 41, 6789 CrossRef CAS; (h) M. Kimura, S. Matsuo, K. Shibata and Y. Tamaru, Angew. Chem., Int. Ed., 1999, 38, 3386 CrossRef CAS.
- (a) M. Kimura, Org. Synth., 2013, 90, 105 Search PubMed; (b) M. Kimura, A. Ezoe, M. Mori, K. Iwata and Y. Tamaru, J. Am. Chem. Soc., 2006, 128, 8559 CrossRef CAS PubMed; (c) Y. Sato, N. Saito and M. Mori, J. Org. Chem., 2002, 67, 9310 CrossRef CAS PubMed; (d) M. Kimura, H. Fujimatsu, A. Ezoe, K. Shibata, M. Shimizu, S. Matsumoto and Y. Tamaru, Angew. Chem., Int. Ed., 1999, 38, 397 CrossRef CAS; (e) M. Kimura, A. Ezoe, K. Shibata and Y. Tamaru, J. Am. Chem. Soc., 1998, 120, 4033 CrossRef CAS; (f) Y. Sato, N. Saito and M. Mori, Tetrahedron, 1998, 54, 1153 CrossRef CAS; (g) M. Takimoto, Y. Hiraga, Y. Sato and M. Mori, Tetrahedron Lett., 1998, 39, 4543 CrossRef CAS; (h) Y. Sato, M. Takimoto, K. Hayashi, T. Katsuhara, K. Takagi and M. Mori, J. Am. Chem. Soc., 1994, 116, 9771 CrossRef CAS.
- Transition metal catalyzed carbomagnesiation of diene with sec- and tert-alkyl Grignard reagents; (a) H. Toda, J. Terao, H. Watanabe, H. Kuniyasu and N. Kambe, Chem. Commun., 2008, 1332 RSC; (b) N. B. Viktorov and L. M. Zubritskii, Russ. J. Gen. Chem., 2001, 71, 1773 CrossRef CAS.
- Effective preparation of organozinc reagents from zinc dust and iodoesters in the presence of DMA as co-solvent have been developed by Y. Tamaru, et al. (a) H. Ochiai, Y. Tamaru, K. Tsubaki and Z.-I. Yoshida, J. Org. Chem., 1987, 52, 4418 CrossRef CAS; (b) Y. Tamaru, H. Ochiai, T. Nakamura and Z.-I. Yoshida, Tetrahedron Lett., 1986, 27, 955 CrossRef CAS; (c) Y. Tamaru, H. Ochiai, T. Nakamura, K. Tsubaki and Z.-I. Yoshida, Tetrahedron Lett., 1985, 26, 5559 CrossRef CAS.
- (a) M. R. Dintzner, A. J. Little, M. Pacilli, D. J. Pileggi, Z. R. Osner and T. W. Lyons, Tetrahedron Lett., 2007, 48, 1577 CrossRef CAS PubMed; (b) S. Oi, K. Kashiwagi, E. Terada, K. Ohuchi and Y. Inoue, Tetrahedron Lett., 1996, 37, 6351 CrossRef CAS.
- See the Experimental section.
- (a) F. Barbot and P. Miginlac, J. Organomet. Chem., 1978, 145, 269 CrossRef CAS; (b) S. Akutagawa and S. Otsuka, J. Am. Chem. Soc., 1975, 97, 6870 CrossRef CAS.
- Cr-mediated three-component coupling reaction of alkyl halides, diene, and carbonyls via radical mechanism; K. Takai, N. Matsukawa, A. Takahashi and T. Fujii, Angew. Chem., Int. Ed., 1998, 37, 152 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nj00992k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |