Denitration of hydrazinium nitroformate to form hydrazinium dinitromethanide†‡
Young-Hyuk
Joo
*a and
Byoung Sun
Min
b
aDepartment of Energetic Materials and Pyrotechnics, Hanwha Corporation Defence R&D Center, Daejeon, 305-156, Korea. E-mail: joo2011@hanwha.com; Fax: +82 42 829 2623; Tel: +82 42 829 2749
bAdvanced Propulsion Technology Center, The 4th R&D Institute, Agency for Defense Development, Daejeon, 305-600, Korea
First published on 22nd October 2013
Abstract
Thermally unstable hydrazinium dinitromethanide was synthesized by denitration of hydrazinium nitroformate with hydrazine hydrate and fully characterized using IR, Raman and multinuclear (1H, 13C, 15N) NMR spectroscopy, and single crystal X-ray diffraction as well as thermal, impact and friction stability measurements.
Polynitro compounds are an interesting class of energetic materials. Compounds containing nitro groups have been investigated both theoretically and experimentally because the nitro group can offer improved oxygen balance and higher density.1 Some of the most prominent members of these energetic materials are TNT, RDX, HMX, and CL-20. A description of recent advances is given while addressing issues that have emerged during the attempts to replace ammonium perchlorate (AP) in solid propellants. Finding replacements has unfortunately proven to be a difficult challenge, primarily due to the small number of available and suitable oxidizing agents. Three main candidates, ammonium dinitramide (ADN), hydrazinium nitroformate (HNF) and 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20), are being considered to replace AP as an oxidizer.2
HNF is one oxidizer that is being seriously studied as a replacement for AP in order to increase the performance of the presently used AP/Hydroxy-terminated polybutadiene (HTPB)-based solid composite propellants. However, despite significant advances in recent years, there are several issues that argue against the use of HNF. There are unresolved problems concerning thermal stability, and impact and friction sensitivity.3,4 HNF has a high specific impulse, does not produce chlorine rich combustion products, and has no white smoke trail. Recently, many energetic salts containing the nitroformate anion have been synthesized by metathesis or acid base reactions and subsequently characterized.5 However, only HNF has been scaled up for further testing and investigation as a high performing, halogen free oxidizer for advanced propellant formulations.4,6
Nitroform (NF) is the key starting material for the production of HNF. Hantzsch first reported the synthesis of NF by nitration of acetic anhydride to tetranitromethane, followed by its conversion to NF through reaction with potassium hydroxide and sulfuric acid.7 Many other methods for the synthesis of nitroform were developed involving nitration of acetylene, acetone, or isopropyl alcohol;5c,6g,8 the only effective route for large-scale production is the reaction of isopropyl alcohol and nitric acid.8a
Dinitromethanide salts, including substituted imidazolium cations, exhibit good liquid characteristics with desirable long liquid ranges of more than 200 °C.9 Recently, our investigation of polynitro compounds has offered a different approach to explosives development.10 Now in this work we report the synthesis and characterization of hydrazinium dinitromethanide, obtained by denitration of HNF with hydrazine hydrate.
The experimental details of the reaction of potassium nitroformate (1) with sulfuric acid and hydrazine hydrate to form hydrazinium nitroformate (HNF) (2) have already been reported.6f However, further reaction of the latter to form hydrazinium dinitromethanide (HDM) (3), which was thermally unstable, has not been reported. We now wish to issue a warning regarding the potential hazards associated with the preparation and handling of 3.
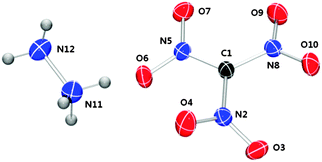
Potassium nitroform (1) was synthesized from tetranitromethane and potassium hydroxide according to a literature procedure in good yield5b,7 (Scheme 1). The new energetic salt hydrazinium dinitromethanide (3) was obtained by reacting nitroform with a slight excess of hydrazine hydrate or by reacting HNF (2) and hydrazine hydrate (see Experimental). The structure of 3 is supported by IR, Raman, 1H, 13C, and 15N NMR spectroscopic data (see ESI‡). Structural confirmations of 2 and 3 by single crystal X-ray diffraction analysis are given in Fig. 1 for 2, and for 3 in Fig. 2.
A systematic study of the crystal structure was of interest to us in order to obtain reliable values for parameters such as density, cell volume and the number of formula units for the unit cell. The influence of the many factors that affect the structure of the nitroformate anion is complex and has been the subject of several publications.5a,b,11 However HNF (2) has been characterized by single crystal X-ray diffraction analysis previously.5b,12 It is also reported here as a comparison with the crystal structure of HDM (3) (Fig. 1). The nitroformate anion in HNF (2) exists in the characteristic C–N3 plane with the typical conformation of the nitro groups. Two of the nitro groups are almost coplanar with the C–N3 plane, whereas the third one is twisted out of plane at a certain angle.
The light brown crystals of hydrazinium dinitromethanide (3) were grown by slow evaporation of an aqueous solution. Compound 3 crystallizes in the monoclinic space group P21/n with four molecules in the unit cell, and has a density of 1.681 g cm−3 (at 296 K). A selection of structural parameters is given in Table 1.
| a R 1 = ∑||Fo| – |Fc||/∑|Fo|. b wR2 = {∑[w(Fo2 – Fc2)2]/∑[w(Fo2)2]}1/2. c CCDC numbers 955657 and 955658. | ||
|---|---|---|
| Chemical formula | CH5N5O6 | CH6N4O4 |
| Formula mass | 183.10 | 138.10 |
| Crystal system | Monoclinic | Monoclinic |
| a/Å | 8.0012(5) | 3.701(3) |
| b/Å | 11.8726(7) | 13.875(11) |
| c/Å | 14.0979(9) | 10.753(9) |
| β/° | 104.441(10) | 98.833(9) |
| Unit cell volume/Å3 | 1296.92(14) | 545.6(8) |
| Temperature/K | 296(2) | 296(2) |
| Space group | P2(1)/n | P2(1)/n |
| No. of formula units per unit cell, Z | 8 | 4 |
| Absorption coefficient, μ mm−1 | 0.190 | 0.164 |
| Density calcd/g cm−3 | 1.875 | 1.681 |
| No. of reflections measured | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 211 211 |
9535 |
| No. of independent reflections | 3207 | 1360 |
| R int | 0.0626 | 0.1425 |
| Final R1 values (I > 2σ(I))a | 0.0390 | 0.0415 |
| Final wR2 values (I > 2σ(I))b | 0.0938 | 0.0843 |
| Final R1 values (all data) | 0.0525 | 0.1428 |
| Final wR2 values (all data) | 0.1000 | 0.1064 |
| Goodness of fit on F2 | 0.989 | 0.856 |
| CCDC numberc | 955657 | 955658 |
The dinitromethanide anion of 3 is nearly planar and four similar N–O bond lengths of nitro groups are observed [N1–O4 = 1,266(2), N1–O5 = 1.254(2), N3–O6 = 1.264(2), N3–O7 = 1.256(3)]. There are many hydrogen bonds which stabilize this structure and assist with charge delocalization. The extended structure of 3 (Fig. 2b) shows some of these strong hydrogen bonds and indicates the layer structure formed by alternating cationic hydrazinium and the dinitro-methanide anion. As shown in Fig. 2b, there are intramolecular hydrogen bonds in 3 to link the molecules into a three dimensional network structure. The hydrogen bond distances and angles are summarized in ESI.‡ These extensive hydrogen bonds also make an important contribution to the stability of the energetic salts.13
13C NMR and 15N NMR spectra of HNF (2) and HDM (3) were measured in deuterated dimethyl sulfoxide solution. The chemical shift data of 2 and 3 are shown in Fig. 3. In the 13C NMR spectra, the resonance for the carbon anion (150.8 ppm) of 2 with three nitro groups is observed at a relatively lower field than the carbon anion of 3 (122.4 ppm) with two nitro groups because of the strong electronegativity effect (Fig. 3 top). For 15N NMR spectra of 2 and 3, the values of the chemical shifts of hydrazinum moieties, NH2, and +NH3 groups can be assigned to the resonances at the highest field based on comparison with the literature14 (Fig. 3 bottom). The nitrogen signals of NO2 from both compounds 2 and 3 have very similar chemical shifts at −29.3 ppm and −20.7 ppm, respectively.
 | ||
| Fig. 3 13C NMR spectra of 2 and 3 (top). 15N NMR spectra of 2 and 3: chemical shifts are given with respect to CH3NO2 as the external standard (delay of 5 s between the pulses) (bottom). | ||
Thermal behavior studies of energetic materials are very important for the evaluation of the thermodynamic properties and stabilities of the materials. The thermal stability of 3 was measured using differential scanning calorimetry (DSC) and thermogravimetric (TG) analyses at a heating rate of 10 °C min−1. The DSC or TG measurements were made on a sample of ca. 0.5 mg. Typical DSC and TG curves are presented in the ESI.‡ Based on the thermograms, the sample decomposed at 86 °C without melting. HDM (3) shows a lower decomposition temperature than 2.3 The thermal decomposition process of 3 was also investigated at different heating rates using DSC. The DSC curves of 3 obtained at heating rates of 2, 5, 10, 15, 20 °C min−1 are shown in the ESI.‡ The decomposition temperatures of HDM cover a range of roughly 15 °C when heated at a rate of 2 °C min−1 compared to a rate of 20 °C min−1.
Calculations were carried out by using the Gaussian 09W suite of programs15 at the B3LYP/6-31+G**//MP2/6-311++G** level. All of the optimized structures were characterized to be true local energy minima on the potential-energy surface without having imaginary frequencies. Atomization energies were calculated by the G2 method.16
Based on Born–Haber energy cycles (see ESI‡), the heats of formation of salts can be simplified as eqn (1):
| ΔHof (salt, 298 K) = ΔHof (cation, 298 K) + ΔHof (anion, 298 K) − ΔHL | (1) |
The lattice potential energies (UPOT) and lattice enthalpies (ΔHL) were calculated according to the equations provided by Jenkins and are summarized in ref. 17 in which ΔHL is the lattice energy of the salt. With the values of the heats of formation and density of the energetic salts, the detonation pressures (P) and detonation velocities (D) were calculated using the program EXPLO5 V5.05.18 In Table 2 it is seen that 2 and 3 are exothermic. Both of the energetic salts exhibit negative heats of formation at −56 kJ mol−1 and −27.5 kJ mol−1, respectively. The X-ray densities of salts 2 and 3 are 1.875 g cm−3 and 1.681 g cm−3, respectively; the latter is slightly lower than that of RDX (1.816 g cm−3). The detonation pressure of 3 is calculated to be P = 33.55 GPa. This value is slightly lower than that of 2 (35.40 GPa) or RDX (35.2 GPa). The detonation velocity of 3 is also calculated to be D = 8935 m s−1, which is similar to 2 (8858 m s−1) or RDX (8977 m s−1). Impact and friction sensitivity measurements were made using a standard BAM drop hammer and a BAM friction tester, respectively.19 In Table 2, the value of impact sensitivities from the relatively similar sensitive 3 (8 J) compared to RDX (7.4 J) is listed. The friction sensitivity is found to be 96 N for 3.
| Compd | T m [°C] | T dec [°C] | Densityb [g cm−3] | ΔHoL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [kJ mol−1] c [kJ mol−1] |
ΔHocationc [kJ mol−1] | ΔHoanionc [kJ mol−1] | ΔHof![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [kJ mol−1] c [kJ mol−1] |
ΔHof![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [kJ g−1] c [kJ g−1] |
P [GPa] | D [m s−1] | ISf [J] | FSg [N] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Melting and thermal decomposition temperature under nitrogen gas (DSC, 10 °C min−1). b X-ray density (25 °C). c Heat of formation (calculated via Gaussian 09). d Calculated detonation pressure (EXPLO5 V5.05). e Calculated detonation velocity (EXPLO5 V5.05). f Impact sensitivity (BAM drop hammer). g Friction sensitivity (BAM friction tester). h Ref. 1. | ||||||||||||
| 2 | 129 | 131 | 1.875 | 539 | 770 | −287 | −56.0 | −0.306 | 35.40 | 8858 | 4 | 28 |
| 3 | — | 86 | 1.681 | 565 | 770 | −233 | −27.5 | −0.199 | 33.55 | 8935 | 8 | 96 |
| RDXh | 206 | 230 | 1.816 | — | — | — | 92.6 | 0.42 | 35.2 | 8977 | 7.4 | 120 |
In conclusion, hydrazinium dinitromethanide (HDM) (3) was synthesized by reacting hydrazinium nitroformate (HNF) (2) with excess hydrazine hydrate, and was characterized by using IR, Raman and multinuclear NMR (1H, 13C, 15N) spectroscopy as well as single crystal X-ray diffraction analysis. Compound 3 is slightly less sensitive to impact and friction than 2, but similar to RDX. Decomposing at 86 °C, compound 3 is less thermally stable than 2.
Experimental
Safety precautions20
While we have experienced no difficulties with the impact instability of the HNF (2) and HDM (3), they must be synthesized only in ∼3.00 g amounts. Manipulations must be carried out in a hood behind a safety shield. Eye protection and leather gloves must be worn. Caution should be exercised at all times during the synthesis, characterization, and handling of any of these materials, and mechanical actions involving scratching or scraping must be avoided. Samples of 3 have undergone spontaneous slow energetic decomposition in our laboratories; some within days of preparation, others after storage at ambient temperature for 1 month. Do not store in a closed vial.Hydrazinium dinitromethanide (3)
Method A
At 0 °C, 11.3 g (113 mmol) of 98% sulfuric acid was dropped slowly to the suspension of 21.3 g (113 mmol) of potassium nitroformate (1) in 100 mL of methylene chloride. The reaction mixture was stirred at 10–15 °C for 1 hour. The inorganic salt was filtered off and washed two times with 50 mL of methylene chloride. The filtrate was stirred at ambient temperature and 98% hydrazine hydrate was added by dropping very slowly until pH = 9. The reaction mixture was stirred for 1 hour. The methylene chloride phase was separated by decanting and the residue was dissolved in methanol. After removing the solvent under a stream of air, a 2.33 g portion (16.9 mmol, 15%) of light yellow crystalline 3 was obtained.Method B
To a solution of 5.00 g (27.3 mmol) of HNF (2) in 100 mL of methanol was added 2.79 g (54.6 mmol) of 98% hydrazine hydrate. After stirring at ambient temperature for 1 h, the solvent was removed under a stream of air. A 1.55 g portion (11.2 mmol, 41%) of light yellow crystalline 3 was obtained.
3: light yellow crystal; Tdec. 86 °C. IR ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) /cm−1 3322, 3280, 3135, 3073, 2637, 1609, 1581, 1542, 1427, 1398, 1371, 1305, 1224, 1098, 1079, 975, 782, 738, 708. Raman (532 nm, 50 mW)
/cm−1 3322, 3280, 3135, 3073, 2637, 1609, 1581, 1542, 1427, 1398, 1371, 1305, 1224, 1098, 1079, 975, 782, 738, 708. Raman (532 nm, 50 mW) ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) /cm−1 3282, 3140, 1495, 1356, 1001, 786, 479, 270. δH (300 MHz, DMSO[D6]) 6.90 (5H, br. s, NH2–NH3+), 8.20 (1H, s). δC (75.5 MHz, DMSO[D6]) 122.4. δN (60.8 MHz, DMSO[D6]) −330.6, −20.7.
/cm−1 3282, 3140, 1495, 1356, 1001, 786, 479, 270. δH (300 MHz, DMSO[D6]) 6.90 (5H, br. s, NH2–NH3+), 8.20 (1H, s). δC (75.5 MHz, DMSO[D6]) 122.4. δN (60.8 MHz, DMSO[D6]) −330.6, −20.7.
Acknowledgements
The authors gratefully acknowledge the support of the Agency for Defense Development (Korea).Notes and references
- H. H. Krause, in Energetic Materials, ed. U. Teipel, VCH, Weinheim, 2005, ch. 1 Search PubMed.
- J. Giles, Nature, 2004, 427, 580 CrossRef CAS PubMed.
- W. P. C. de Klerk, A. E. D. M. van der Heijden and W. H. M. Veltmans, J. Therm. Anal. Calorim., 2001, 64, 973 CrossRef CAS.
- P. S. Dendage, D. B. Sarwade, A. B. Mandale and S. N. Asthana, J. Energ. Mater., 2003, 21, 167 CrossRef CAS.
- (a) Y. Huang, H. Gao, B. Twamley and J. M. Shreeve, Eur. J. Inorg. Chem., 2007, 2025 CrossRef CAS; (b) M. Göbel and T. M. Klapötke, Z. Anorg. Allg. Chem., 2007, 633, 1006 CrossRef; (c) H. S. Jadhav, M. B. Talawar, D. D. Dhavale, S. N. Asthana and V. N. Krishnamurthy, Indian J. Chem. Technol., 2005, 12, 187 CAS.
- (a) A. I. Atwood, T. L. Boggs, P. O. Curran, T. P. Parr, D. M. Hanson-Parr, C. F. Price and J. Wiknich, J. Propul. Power, 1999, 15, 740 CrossRef CAS; (b) A. I. Atwood, T. L. Boggs, P. O. Curran, T. P. Parr, D. M. Hanson-Parrand, C. F. Price and J. Wiknich, J. Propul. Power, 1999, 15, 748 CrossRef CAS PubMed; (c) H. F. R. Schöyer, W. H. M. Welland-Veltmans, J. Louwers, P. A. O. G. Korting, A. E. D. M. van der Heijden, H. L. J. Keizers and R. P. van den Berg, J. Propul. Power, 2002, 18, 131 CrossRef; (d) H. F. R. Schöyer, W. H. M. Welland-Veltmans, J. Louwers, P. A. O. G. Korting, A. E. D. M. van der Heijden, H. L. J. Keizers and R. P. van den Berg, J. Propul. Power, 2002, 18, 138 CrossRef PubMed; (e) A. J. Cesaroni, M. J. Dennett and J. Louwers, US Pat., 7022196, 2006 Search PubMed; (f) P. S. Dendage, D. B. Sarwade, S. N. Asthana and H. Singh, J. Energ. Mater., 2001, 19, 41 CrossRef CAS; (g) J. Louwers, G. M. H. J. L. Gadiot, M. Q. Brewster, S. F. Son, T. Parr and D. Hanson-Parr, J. Propul. Power, 1999, 15, 772 CrossRef CAS.
- (a) A. Hantzsch and A. Rinckenberger, Ber. Dtsch. Chem. Ges., 1899, 32, 628 CrossRef CAS; (b) P. Liang, Org. Synth., 1941, 21, 105 CAS; (c) H. Shechter and H. L. Cates Jr., J. Org. Chem., 1961, 26, 51 CrossRef CAS.
- (a) M. B. Frankel, F. C. Gunderloy Jr. and D. O. Woolery II, US Pat., 4122124, 1978 Search PubMed; (b) A. Wetterhom, Tetrahedron, 1963, 19(Suppl. 1), 155 CrossRef; (c) G. A. Wetterholm and E. Lennart, US Pat., 2658084, 1953 Search PubMed.
- L. He, G.-H. Tao, D. A. Parrish and J. M. Shreeve, Inorg. Chem., 2011, 50, 679 CrossRef CAS PubMed.
- (a) M.-J. Sul, W. B. Jeong and Y.-C. Park, Korea Institute of Military Science and Technology 2012 Annual Conference, Kyungju, 2012, 1405; (b) M.-J. Sul, Y.-H. Joo, W. B. Jeong and Y.-C. Park, The Korean Society of Propulsion Engineers Spring Conference, Busan, 2013, 575.
- M. Göbel, T. M. Klapötke and P. Mayer, Z. Anorg. Allg. Chem., 2006, 632, 1043 CrossRef.
- B. Dickens, Chem. Commun., 1967, 246 RSC.
- C. Knoll, D. Muller, G. Giester, J. Ofner, B. Lendl, P. Weinberger and G. Steinhauser, New J. Chem., 2013 10.1039/C3NJ00492A.
- (a) T. M. Klapötke, P. Mayer and J. Stierstorfer, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2393 CrossRef; (b) Y.-H. Joo and J. M. Shreeve, Chem.–Eur. J., 2009, 15, 3198 CrossRef CAS PubMed; (c) Y.-H. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2010, 49, 7320 CrossRef CAS PubMed.
- M. J. Frisch, et al.Gaussian 09 (Revision C.01), Gaussian Inc., Wallingford, CT, USA, 2010 Search PubMed.
- R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989 Search PubMed.
- H. D. B. Jenkins, D. Tudela and L. Glasser, Inorg. Chem., 2002, 41, 2364 CrossRef CAS PubMed.
- M. Sućeska, EXPLO5 V5.05 program, Zagreb, Croatia, 2011 Search PubMed.
- A portion of 30 mg of HNF (2) or HDM (3) was subjected to a drop-hammer test using a 1 or 5 kg weight dropped. A range of impact and friction sensitivities according to the UN Recommendations (Impact: insensitive >40 J, less sensitive ≥35 J, sensitive ≥4 J, very sensitive ≤3 J; friction: insensitive >360 N, less sensitive: 360 N, sensitive: 80–360 N, very sensitive: <80 N, extremely sensitive: ≤10 N).
- T. M. Klapötke, B. Krumm, F. X. Steemann and G. Steinhauser, Saf. Sci., 2010, 48, 28 CrossRef PubMed.
Footnotes |
| † In Memory of Professor Malcolm M. Renfrew. |
| ‡ Electronic supplementary information (ESI) available: DSC and TGA data, IR, Raman, 1H, 13C and NMR spectra. CCDC numbers 955657 (2) and 955658 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3nj01203d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |