CuInS2 quantum dots@silica near-infrared fluorescent nanoprobe for cell imaging
Zihan
Lin
a,
Xiaofang
Fei
b,
Qiang
Ma
a,
Xue
Gao
a and
Xingguang
Su
*a
aDepartment of Analytical Chemistry, College of Chemistry, Jilin University, Changchun 130012, PR China. E-mail: suxg@jlu.edu.cn; Tel: +86-431-85168352
bCollege of Life Sciences, Jilin University, Changchun 130012, PR China
First published on 11th September 2013
Abstract
In this paper, near-infrared (NIR) fluorescent CuInS2 QDs@SiO2 nanobeads were prepared and used as fluorescent nanoprobes for prostate cancer cells imaging. The core–shell CuInS2 QDs@SiO2 nanobeads with controllable particle sizes were synthesized via a reverse microemulsion method. Further surface modifications were performed for grafting amino groups on the surface of the NIR CuInS2 QDs@SiO2 nanobeads. For prostate cancer cell imaging, anti-PSCA antibody was conjugated to the NIR CuInS2 QDs@SiO2 nanobeads to prepare the anti-PSCA–QDs@SiO2 nanoprobe. The specific binding of the antibody conjugated CuInS2 QDs@SiO2 nanobeads to the surface of human prostate cancer cells (PC-3M) was confirmed by fluorescence microscopy. MTT assay and fluorescence microscopy images showed that the anti-PSCA-conjugated NIR CuInS2 QDs@SiO2 nanoprobe was a non-toxic nanoprobe and had high-specificity in cell imaging. The CuInS2 QDs@SiO2 nanoprobe as an efficient NIR imaging nanoprobe could be used for target imaging, biological assays and early diagnosis of cancer.
1. Introduction
Quantum dots (QDs), also named semiconductor nanocrystals, show great potential in biomolecule labeling, medical identification and cell imaging.1–4 QDs offer many advantages over traditional organic dyes such as high photoluminescence efficiency, size-dependent emission wavelengths, narrow and symmetric emission spectra, broad absorption spectra, and photostability.5–7 The use of near-infrared emission quantum dots (NIR QDs) in biomedical imaging has attracted much attention during the past few years. The near-infrared (NIR) region, between 650–900 nm, is very useful for biological imaging and detection. NIR QDs not only retain the advantages of the conventional visible emitting QDs, but also counteract the interference which was attributed to the scattering, absorbance and auto-fluorescence of the tissues in the visible region because of their longer emission wavelength at the NIR spectrum window.8–11 NIR QDs are compatible with many infrared sensing and imaging technologies,12 including in vitro and in vivo bioimaging and biolabeling,13 deep tissue imaging,14 and diagnostics.15 Much effort has been devoted to the development of various of NIR QDs,16 such as CdSeTe/CdS,17 CdHgTe/CdS,17 CdTe1−xSex/CdS,18 Ag2S,19 InAs/InP/ZnSe,20 CdTe/CdS,21 CuInSe/ZnS22 and PbS.23 Nonetheless, NIR QDs are still facing some unsolved problems such as cytotoxicity of heavy metal ions in the NIR QDs and organic groups of the surface of NIR QDs, the ultra-sensitivity of their fluorescence to the surface states, toxicity in the process of organic synthesis.24 Most NIR QDs are synthesized via an organometallic precursor route in an organic solvent that is hazardous to the environment and to the health of people.25 Thus, the development of less toxic and more environmentally friendly ternary NIR QDs materials has attracted considerable attention.26–28 The water-soluble CuInS2 QDs used in this study is a new kind of biocompatible non-toxic NIR material.29,30Prostate cancer (CaP) has emerged as the most commonly diagnosed malignancy and the second leading cause of cancer-related death in men in the Western countries.31 Early diagnosis of prostate cancer is problematic due to the lack of a biomarker that has high diagnostic sensitivity and specificity. With the widespread use of screening tests (for example, prostate-specific antigen testing), patients are diagnosed with less-advanced disease, and the prostate-cancer-specific mortality has declined over the past few years.32 The prostate-specific membrane antigen PSCA is an integral membrane glycoprotein expressed on the surface of prostate carcinoma, with limited expression in extraprostatic normal tissues.33 PSCA expression correlates with tumor stage, grade and androgen independence and may have prognostic utility. Because expression on the surface of prostate cancer cells increases with tumor progression, PSCA may be a useful molecular target in early prostate cancer. Molecules able to bind tightly and specifically to the surface of malignant cells would greatly benefit cancer diagnosis and treatment. Therefore, anti-PSCA antibodies have the ability to specifically recognize tumor cell markers.
Silica is an ideal inert material for surface coating. Silica material is a non-toxic substance and can be easily modified with functionalized groups which can conjugate with biomolecules.4,34 Because of the resistance of silica to both aqueous and non-aqueous solvents, leakage can be avoided.35 The basic principle of the experimental system is shown in Scheme 1. In the present study, we embedded aqueous NIR CuInS2 QDs in silica nanospheres by a reverse microemulsion method. The prepared CuInS2 QDs@SiO2 nanobeads were linked with anti-PSCA antibody via covalent binding. Subsequently, the anti-PSCA conjugated NIR CuInS2 QDs@SiO2 nanoprobe was used for PC-3M cells imaging. The specific targeting ability of the anti-PSCA conjugated NIR CuInS2 QDs@SiO2 nanoprobe to prostate cancer cells was confirmed by fluorescence microscopy.
 | ||
| Scheme 1 Schematic illustration of NIR CuInS2 QDs@SiO2 nanobeads used as fluorescent nanoprobes for prostate cancer cells imaging. | ||
2. Experimental section
2.1 Materials
All chemicals used were of analytical reagent grade without further purification. Copper(II) chloride dehydrate (CuCl2·2H2O), sodium hydroxide (NaOH), sulfourea (CS(NH2)2), sodium dihydrogen phosphate (NaH2PO4) and disodium hydrogen phosphate (Na2HPO4) were purchased from Beijing Chemical Works. Mercaptopropionic acid (MPA), indium chloride tetrahydrate (InCl3·4H2O), cyclohexane, Triton X-100, n-hexanol, tetraethoxysilane (TEOS), 3-aminopropyltrimethoxysilane (APS) and 3-(trihydroxysilyl)propylmethylphosphonate (THPMP) were purchased from Sigma-Aldrich Corporation. 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) was purchased from Aldrich Chemical Co. Ltd. The rabbit anti-PSCA was purchased from Beijing Biosynthesis Biotechnology Co. Ltd. IMDM was obtained from Invitrogen Corporation. Fetal bovine serum (FBS) was purchased from Hyclone. 3-(4,5-Dimethylthiazol-2-yl)-2 and 5-diphenyl tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Amresco Inc. All the solutions for cells incubation were prepared with triply distilled water.2.2 Preparation of CuInS2 QDs
NIR CuInS2 QDs were prepared in aqueous solution via a hydrothermal synthesis method based on our previous report.36 In a typical experiment, CuCl2·2H2O (0.15 mmol) and InCl3·4H2O (0.15 mmol) were dissolved in distilled water (10.5 ml), then MPA (1.8 mmol) was injected into the solution. The pH value of the mixture solution was adjusted to 11.3 by adding 2 mol L−1 NaOH solution with stirring. After stirring for 10 min, CS(NH2)2 (0.30 mmol) was dissolved in the solution. The Cu-to-In-to-S and Cu-to-MPA precursor ratios were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, respectively. All the above mentioned experimental procedures were performed at room temperature, and then the solution was transferred into a Teflon-lined stainless steel autoclave with a volume of 15 mL. The autoclave was maintained at 150 °C for 21 h and then cooled down to room temperature. Ethanol was added to the stock solution to obtain the CuInS2 QDs precipitate, and the process was repeated three times. The remaining impurities were removed by cycled washing. The purified CuInS2 QDs solution was dissolved in PBS buffer (0.01 mol L−1, pH 7.4), and stored in the dark. The final concentration of CuInS2 QDs was calculated to be 1.5 mmol L−1 according to the addition of In3+ concentration in the synthesis process.36 In our work, the optimal reactant molar ratio of MPA/Cu was chosen as 12
12, respectively. All the above mentioned experimental procedures were performed at room temperature, and then the solution was transferred into a Teflon-lined stainless steel autoclave with a volume of 15 mL. The autoclave was maintained at 150 °C for 21 h and then cooled down to room temperature. Ethanol was added to the stock solution to obtain the CuInS2 QDs precipitate, and the process was repeated three times. The remaining impurities were removed by cycled washing. The purified CuInS2 QDs solution was dissolved in PBS buffer (0.01 mol L−1, pH 7.4), and stored in the dark. The final concentration of CuInS2 QDs was calculated to be 1.5 mmol L−1 according to the addition of In3+ concentration in the synthesis process.36 In our work, the optimal reactant molar ratio of MPA/Cu was chosen as 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the PL quantum yield of the ternary CuInS2 QDs synthesized was 3.3%, which was higher than that synthesized in organic solvent (QY = 3%).37
1 and the PL quantum yield of the ternary CuInS2 QDs synthesized was 3.3%, which was higher than that synthesized in organic solvent (QY = 3%).37
2.3 Synthesis of NIR CuInS2 QDs@SiO2 nanobeads
Fluorescent core–shell NIR CuInS2 QDs@SiO2 nanobeads were prepared via a reverse microemulsion method. In this method, cyclohexane was used as a continuous phase, and Triton X-100 and n-hexanol were used as surfactant and cosurfactant respectively. In detail, 500 μL of aqueous solution of the as-prepared CuInS2 QDs and 40 μL of ammonia aqueous solution (25 wt%) were introduced into a liquid system containing 15 mL of cyclohexane, 3.6 mL of n-hexanol, and 3.6 mL of Triton X-100. Subsequently, various amounts of TEOS were introduced under vigorous magnetic stirring. The reaction system was then sealed and kept under stirring in the dark at room temperature for 24 h. The microemulsion was broken by adding 20 mL of acetone to the reaction system. And the resultant precipitate was NIR CuInS2 QDs@SiO2 nanobeads which were washed three times in sequence with isopropyl alcohol, ethanol and water. During each washing procedure, the composite nanobeads dispersion was first subjected to fast speed centrifugation (9400g, 10 min), followed by decanting of the supernatant and redispersion of the precipitate in the next solvent with the aid of supersonication. Ultimately, aqueous dispersions of the NIR CuInS2 silica nanobeads were obtained for further experiments. In this work, the PL quantum yield of the NIR CuInS2 QDs@SiO2 nanobeads synthesized under the reaction conditions was 1.5%.2.4 Preparation of NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobe
Proper surface functionalization of the NIR CuInS2 QDs@SiO2 nanobeads is important for further application as a fluorescent label. To graft amine groups on the surface of NIR CuInS2 QDs@SiO2 nanobeads, we added 20 μL 3-aminopropyltrimethyloxysilane (APS) and 80 μL 3-(trihydroxysilyl)propylmethylphosphonate (THPMP) into the reaction system 24 h after the hydrolysis and condensation of TEOS. The reaction system was then kept stirring for one more day. The resultant amine-functionalized NIR CuInS2 QDs@SiO2 nanobeads were obtained by the same purification procedures as those for NIR CuInS2 QDs@SiO2 nanobeads.The anti-PSCA–NIR CuInS2 QDs@SiO2 nanoprobe was prepared as follow: 0.1 mL of 0.02 mg mL−1 anti-PSCA solution was added to 0.1 mL of 5 mM EDC solution in PBS buffer (pH 7.4). After 0.5 h, 1 mL of NIR CuInS2 QDs@SiO2 nanoprobe (8.6 mg mL−1) was added under gentle stirring. And the solution was incubated for 2 h for antibody conjugation at room temperature. Unconjugated antibodies and unreacted chemicals were removed by centrifugation. After that, the precipitate dissolved in 1 mL of PBS buffer was mixed with 0.1 mL BSA solution (1 mg mL−1) and reacted for 0.5 h with gentle agitation. There were many nonspecific binding sites on the NIR anti-PSCA CuInS2 QDs@SiO2 nanoprobe, which can lead to non-specific recognition. BSA was used commonly to block the nonspecific binding sites. The excess proteins and byproducts were removed by centrifugation again.
2.5 MTT assay
PC-3M prostate cancer cells were provided from the college of medicine, Jilin University, China. PC-3M cells were cultivated in IMDM medium containing 10% FBS at 37 °C in a humidified environment of 5% CO2.PC-3M cells were seeded into 96-well plates at a density of 4 × 104 cells per well and incubated at 37 °C. After 24 h to allow cell attachment, the cells were washed with PBS and incubated with 0, 10, 25, 50, 75, 100, 150 μg mL−1 NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobe and NIR CuInS2 QDs@SiO2 nanobeads respectively for 24 h at 37 °C with 5% CO2. Before 20 μL MTT (5 mg ml−1 MTT in PBS) was added, it was necessary to replace the medium by serum-free IMDM. After 4 h, when the MTT fully integrated with cells, 150 μL DMSO was added to dissolve the dark blue formazan crystals. The plates were oscillated for 10 min making sure all the formazan had dissolved in DMSO. Finally, we measured the absorbance at 490 nm using a microplate reader.
2.6 Cell imaging
PC-3M cells were seeded into a 6-well plate (Corning Incorporated, Corning, NY, US) at a density of 3 × 104 cells per well and incubated at 37 °C. Human hepatoma cells HepG2 were seeded into a 6-well plate at a density of 3 × 104 cells per well and incubated at 37 °C. When the cultured cells reached about 70% confluences, we washed with PBS three times. After thoroughly rinsing with PBS, the cells were incubated with serum-free medium containing the NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobe at 37 °C for 0.5 h. Then cells were washed three times with PBS for 5 min each. Finally cells were fixed with 4% paraformaldehyde for 15 min and washed with PBS at least three times. Fluorescent photos of the cells were taken by inverted fluorescence microscope equipped with a Nuance system.2.7 Characterization
Fluorescence measurements were performed on a Shimadzu RF-5301 PC spectrofluorophotometer (Shimadzu Co., Kyoto, Japan), measuring the wavelength range 220–750 nm, and a 1 cm path-length quartz cuvette was used in experiments. UV-vis absorption spectra were obtained using a GBC Cintra 10 e UV-vis spectrometer. Transmission electron microscopy (TEM) experiments were performed on a Philips Tecnai F20 TEM operating at 200 kV. TEM samples were prepared by dropping the CuInS2 QDs–SiO2 composite nanobeads dispersed in absolute ethanol solution onto carbon-coated copper grids, while the excess solvent evaporated. A dynamic light scattering technique (Nano ZS model, ZEN 3600, Malvern Instruments) was used to determine the zeta potential of amine-functionalized NIR CuInS2 QDs@SiO2 nanobeads. All pH measurements were taken with a PHS-3C pH meter (Tuopu Co., Hangzhou, China). In the MTT assay, absorbance was measured in a TRITURUS microplate reader (Bio-Rad 680, USA). An inverted fluorescence microscope (Olympus FV1000 IX71) equipped with a multispectral imaging system (Nuance, CRI, Woburn, MA, USA) was used to observe the morphological changes of cells. Fluorescence excitation model is U-MWIY2, with wide band interference yellow excitation, exciter filter BP545-580, dichroic beamsplitter DM600, barrier filter BA610-IF.3. Results and discussion
Characterization of NIR CuInS2 QDs@SiO2 nanobeads
The UV-vis and fluorescence emission spectra of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads are shown in Fig. 1. It can be seen that the emission band of the CuInS2 QDs was narrow and symmetrical with the emission peak around 655 nm, excitation peak around 620 nm and the UV-vis absorption peak was around 570 nm.36,37 The PL emission peak of NIR CuInS2 QDs@SiO2 nanobeads was red shifted from 655 nm to 670 nm. Therefore, NIR CuInS2 QDs@SiO2 nanobeads are more suitable for biological imaging and detection.The formation of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads are directly visualized by transmission electron microscopy (TEM). Fig. 2A shows the TEM micrograph of CuInS2 QDs. It can be seen that the size distribution of CuInS2 QDs was reasonably uniform. The particle size of CuInS2 QDs was approximate 2 nm. As shown in Fig. 2B, the NIR CuInS2 QDs@SiO2 nanobeads was homogeneous with a size of about 47 nm, and the silica composite nanobeads were kept apart from each other showing monodispersity. The zeta potentials of CuInS2 QDs, CuInS2 QDs@SiO2 nanobeads and amine-functionalized CuInS2 QDs@SiO2 nanobeads are also shown in Fig. 3. Solutions with zeta potential above +20 mV and below −20 mV are considered stable.38 It is significant to note that the zeta potentials of CuInS2 QDs, CuInS2 QDs@SiO2 nanobeads and amine-functionalized CuInS2 QDs@SiO2 nanobeads are −28.64 mV, −22.53 mV and +9.54 mV, respectively. The zeta potentials indicate that they are dispersed uniformly and stable in water. The change of zeta potentials is due to different surface charges, there are many carboxyl groups on CuInS2 QDs and amino-groups on amine-functionalized CuInS2 QDs@SiO2 nanobeads.36 It indicates the successful amino-functionalized modification on the surface of CuInS2 QDs@SiO2 nanobeads.
Stability of NIR CuInS2 QDs@SiO2 nanobeads
As an ideal NIR bio-probe, it needs to have good stability in the cell culture buffer. Therefore, the influences of ionic strength and pH on the PL properties of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads were investigated in this study. Fig. 4 and 5 show the effect of both ionic strength and pH on the PL intensity of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads. It can be seen that the PL intensity of CuInS2 QDs increased with the increase of NaCl concentration, and remained nearly constant after NaCl concentration reached 10 mmol L−1. Meanwhile, the PL intensity of NIR CuInS2 QDs@SiO2 nanobeads kept almost invariable with the increase of NaCl concentration, indicating that CuInS2 QDs with silica shell protection can reduce the impact of ionic strength. As shown in Fig. 5, with the increase of the pH value, the PL intensity of both of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads also increased. Compared with CuInS2 QDs, the PL intensity of NIR CuInS2 QDs@SiO2 nanobeads showed better stability to pH change, especially under acidic conditions. All the above results demonstrated that NIR CuInS2 QDs@SiO2 nanobeads are more stable than the naked CuInS2 QDs. Therefore, CuInS2 QDs@SiO2 nanobeads are very suitable to be applied to in vitro tests as NIR biological probes.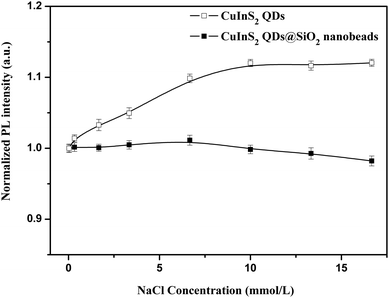 | ||
| Fig. 4 The influence of ionic strength on the PL emission of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads. | ||
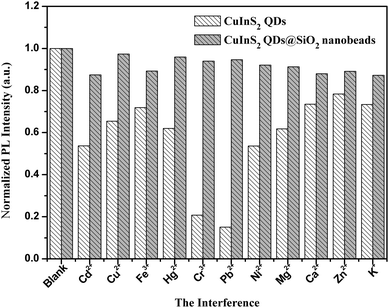
Fig. 6 shows the influence of a series of metal ions on the PL intensity of CuInS2 QDs and NIR CuInS2 QDs@SiO2 nanobeads. It can be seen that various inorganic metal ions have more or less impact on the PL intensity of CuInS2 QDs. The impact of Cr3+ and Pb2+ on the PL intensity of CuInS2 QDs is particularly obvious, for which the fluorescence quenching of CuInS2 QDs was more than 80%. The influence of metal ions Cd2+, Cu2+, Hg2+, Ni2+ and Mg2+ on the fluorescence quenching of CuInS2 QDs was more than 40%, and the fluorescence quenching of CuInS2 QDs by Fe3+, Ca2+, Zn2+ and K+ was more than 20%. Under the same conditions, the impact of Cu2+, Hg2+, Cr3+, Pb2+, Ni2+ and Mg2+ on the fluorescence intensity of NIR CuInS2 QDs@SiO2 nanobeads was less than 10%. The impact of Cd2+, Fe3+, Ca2+, Zn2+ and K+ on the fluorescence intensity of NIR CuInS2 QDs@SiO2 nanobeads was less than 15%. So when the CuInS2 QDs were embedded in silica nanobeads, the stability of the PL emission of the CuInS2 QDs could be enhanced. Further, the PL intensity of NIR CuInS2 QDs@SiO2 nanobeads after being placed at room temperature and dark storage for two months is also very stable. The above-mentioned results indicate that the PL emission of CuInS2 QDs can be well protected by the silica network structure.
The photostability and chemical stability of the anti-PSCA–QDs@SiO2 nanoprobe is critical for its practical applications, we utilized a 150 W xenon lamp for continuous intensive excitation at 590 nm for 4 h, and got the photobleaching curves of the anti-PSCA–QDs@SiO2 nanoprobe in the biological buffer media. From Fig. 7, we clearly observed that the fluorescence emission intensity of the QDs was almost constant during a 4 h irradiation process. In addition, the anti-PSCA–QDs@SiO2 nanoprobe in the biological buffer media also has a high chemical stability. The excellent photostability, chemical stability and no toxic composition allow the anti-PSCA–QDs@SiO2 nanoprobe to be used as a new class of fluorescent label in biomedical imaging and assays.
In vitro study
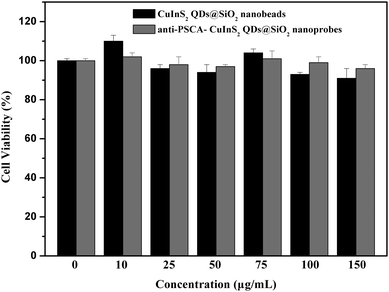
In order to verify the potential application of the NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes in bioimaging, it is necessary to evaluate its in vitro cytotoxicity. We evaluated the cytotoxicity of NIR CuInS2 QDs@SiO2 nanobeads and anti-PSCA conjugated NIR CuInS2 QDs@SiO2 nanoprobes by MTT assay on PC-3M cells. The optical absorbance of formazan (produced by the cleavage of MTT by dehydrogenases in living cells) at a wavelength of 490 nm is directly proportional to the number of live cells in the MTT assay. Fig. 8 shows that the NIR CuInS2 QDs@SiO2 nanobeads and anti-PSCA conjugated NIR CuInS2 QDs@SiO2 nanoprobes showed no significant cytotoxic effect on PC-3M cells in the concentration range of 10–150 μg mL−1. After 24 h of incubation, more than 90% of the PC-3M cells survived, even at a high concentration (150 μg mL−1) of NIR CuInS2 QDs@SiO2 nanobeads and anti-PSCA conjugated NIR CuInS2 QDs@SiO2 nanoprobes. The cell viability test shows the non-toxicity of the anti-PSCA conjugated CuInS2 QDs@SiO2 nanoprobes, and suggests that the PSCA conjugated NIR CuInS2 QDs@SiO2 nanoprobes can be used for a long term imaging of prostatic neoplasms in vivo.In the in vitro study, we used NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes to label human prostate cancer cells (PC-3M). Prostate stem cell antigen (PSCA), a glycosylphosphatidylinositol (GPI)-anchored cell surface glycoprotein, is highly expressed in both local and metastatic prostate cancer. The targeting ability of NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes to the tumor cells was examined by using fluorescence microscopy. After incubation of NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes with the PC-3M cells, the NIR signals from the NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes can be observed to be distributed throughout the plasmalemma region of the cell (Fig. 9a and 9b), indicating that NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes could specifically bind to the PSCA receptors at the cell surface. To test the selectivity of NIR CuInS2 QDs@SiO2 nanoprobes, anti-PSCA–NIR CuInS2 QDs@SiO2 nanoprobes were added to the PC-3M cell line and human hepatoma HepG2 cell line in vitro. Compared with the PC-3M cell line, when HepG2 cancer cells were incubated with NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes for 0.5 h, weak fluorescence signals of NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes were detected (as shown in Fig. 10). It demonstrated the high selectivity of CuInS2 QDs@SiO2 nanoprobes to PSCA-positive cancer cells. Therefore, as an active tumor targeting nanomaterial with antibody, CuInS2 QDs@SiO2 nanobeads can hopefully increase the specificity of tumor identification. The results showed that the NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes can serve as a potential biocompatible targeted nanoprobe to specifically diagnose human prostate cancer cells.
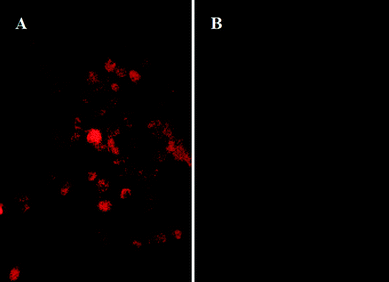 | ||
| Fig. 10 Fluorescent images of PC-3M cells (A) and HepG2 cells (B) counterstained with 0.043 mg mL−1 NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes at incubation time of 0.5 h. | ||
4. Conclusions
In this paper, NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes have been successfully prepared by an inverse microemulsion method. By modifying the surface of NIR CuInS2@SiO2 nanobeads with amino groups, functionalized and monodisperse silica composite nanoparticles can be obtained. Comparing with naked CuInS2 QDs, the resultant NIR CuInS2@SiO2 nanobeads are relatively stable. By using the NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes, we have successfully performed in vitro imaging of human prostate cancer cells PC-3M. The cellular experiment indicates that the prepared NIR anti-PSCA–CuInS2 QDs@SiO2 nanoprobes are particularly suitable for long-term and high-specificity cell imaging.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21075050, 21005029).References
- N. R. Jana, C. Earhart and J. Y. Ying, Chem. Mater., 2007, 19, 5074–5082 CrossRef CAS.
- M. A. Emril, Y. Zheng, H.-h. Yu and J. Y. Ying, Anal. Chem., 2007, 79, 9452–9458 CrossRef PubMed.
- C. Wang, Q. Ma, W. Dou, S. Kanwal, G. Wang, P. Yuan and X. Su, Talanta, 2009, 77, 1358–1364 CrossRef CAS PubMed.
- Q. Ma, W. Yu and X. Su, Talanta, 2010, 82, 51–55 CrossRef CAS PubMed.
- A. P. Alivisatos, J. Phys. Chem., 1996, 100, 13226–13239 CrossRef CAS.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CAS.
- J. A. Smyder and T. D. Krauss, Mater. Today, 2011, 14, 382–387 CrossRef.
- S. B. Rizvi, S. Ghaderi, M. Keshtgar and A. M. Seifalian, Nano Rev., 2010, 1, 5161–5175 Search PubMed.
- F. Zhang, X.-W. He, W.-Y. Li and Y.-K. Zhang, J. Mater. Chem., 2012, 22, 22250–22257 RSC.
- Q. Ma and X. G. Su, Analyst, 2010, 135, 1867–1877 RSC.
- X. He, K. Wang and Z. Cheng, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 349–366 CrossRef CAS PubMed.
- E. H. Sargent, Adv. Mater., 2005, 17, 515–522 CrossRef CAS.
- C.-A. J. Lin, T. Liedl, R. A. Sperling, M. T. Fernandez-Arguelles, J. M. Costa-Fernandez, R. Pereiro, A. Sanz-Medel, W. H. Chang and W. J. Parak, J. Mater. Chem., 2007, 17, 1343–1346 RSC.
- R. Aswathy, Y. Yoshida, T. Maekawa and D. Kumar, Anal. Bioanal. Chem., 2010, 397, 1417–1435 CrossRef CAS PubMed.
- H. M. E. Azzazy, M. M. H. Mansour and S. C. Kazmierczak, Clin. Biochem., 2007, 40, 917–927 CrossRef CAS PubMed.
- X. He, J. Gao, S. S. Gambhir and Z. Cheng, Trends Mol. Med., 2010, 16, 574–583 CrossRef CAS PubMed.
- H. Y. Chen, S. S. Cui, Z. Z. Tu, J. Z. Ji, J. Zhang and Y. Q. Gu, Photochem. Photobiol., 2011, 87, 72–81 CrossRef CAS PubMed.
- R. Hu, K.-T. Yong, I. Roy, H. Ding, W.-C. Law, H. Cai, X. Zhang, L. A. Vathy, E. J. Bergey and P. N. Prasad, Nanotechnology, 2010, 21, 145105–145113 CrossRef PubMed.
- Y. Zhang, G. Hong, Y. Zhang, G. Chen, F. Li, H. Dai and Q. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS PubMed.
- J. Gao, K. Chen, R. Xie, J. Xie, Y. Yan, Z. Cheng, X. Peng and X. Chen, Bioconjugate Chem., 2010, 21, 604–609 CrossRef CAS PubMed.
- L.-N. Chen, J. Wang, W.-T. Li and H.-Y. Han, Chem. Commun., 2012, 48, 4971–4973 RSC.
- J. Park, C. Dvoracek, K. H. Lee, J. F. Galloway, H. C. Bhang, M. G. Pomper and P. C. Searson, Small, 2011, 7, 3148–3152 CrossRef CAS PubMed.
- D. Wang, J. Qian, F. Cai, S. He, S. Han and Y. Mu, Nanotechnology, 2012, 23, 245701–245709 CrossRef CAS PubMed.
- Y. Yang, L. Jing, X. Yu, D. Yan and M. Gao, Chem. Mater., 2007, 19, 4123–4128 CrossRef CAS.
- G. H. T. Au, W. Y. Shih, S.-J. Tseng and W.-H. Shih, Nanotechnology, 2012, 23, 275601–275609 CrossRef PubMed.
- D. Deng, Y. Chen, J. Tian, Z. Qian, S. Achilefu and Y. Gu, Chem. Mater., 2012, 24, 3029–3037 CrossRef CAS.
- C.-H. Quek and K. W. Leong, Nanomaterials, 2012, 2, 92–112 CrossRef CAS.
- H. Zhong, Z. Wang, E. Bovero, Z. Lu, F. C. J. M. V. Veggel and G. D. Scholes, J. Phys. Chem. C, 2011, 115, 12396–12402 CAS.
- D. C. Pan, L. J. An, Z. M. Sun, W. Hou, Y. Yang, Z. Z. Yang and Y. F. Lu, J. Am. Chem. Soc., 2008, 130, 5620–5621 CAS.
- R. G. Xie, M. Rutherford and X. G. Peng, J. Am. Chem. Soc., 2009, 131, 5691–5697 CrossRef CAS PubMed.
- Z. Zhao, W. Ma, G. Zeng, D. Qi, L. Ou and Y. Liang, Urol. Oncol.: Semin. Orig. Invest., 2013, 31, 343–351 CrossRef CAS PubMed.
- E. D. Pressly, R. A. Pierce, L. A. Connal, C. J. Hawker and Y. Liu, Bioconjugate Chem., 2013, 24, 196–204 CrossRef CAS PubMed.
- R. E. Reiter, Z. Gu, T. Watabe, G. Thomas, K. Szigetti, E. Davis, M. Wahl, S. Nisitani, J. Yamashiro, M. M. L. Beau, M. Loda and O. N. Witte, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 1735–1740 CrossRef CAS.
- J.-C. Hsu, C.-C. Huang, K.-L. Ou, N. Lu, F.-D. Mai, J.-K. Chen and J.-Y. Chang, J. Mater. Chem., 2011, 21, 19257–19266 RSC.
- S. Santra, P. Zhang, K. Wang, R. Tapec and W. Tan, Anal. Chem., 2001, 73, 4988–4993 CrossRef CAS.
- S. Y. Liu, H. Zhang, Y. Qiao and X. G. Su, RSC Adv., 2012, 2, 819–825 RSC.
- R. Xie, M. Rutherford and X. Peng, J. Am. Chem. Soc., 2009, 131(15), 5691–5697 CrossRef CAS PubMed.
- T. C. Prathna, N. Chandrasekaran and A. Mukherjee, Colloids Surf., A, 2011, 390, 216–224 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |