Design, synthesis and biochemical investigation, by in vitro luciferase reporter system, of peptide nucleic acids as new inhibitors of miR-509-3p involved in the regulation of cystic fibrosis disease-gene expression†
Felice
Amato
ab,
Rossella
Tomaiuolo
ab,
Nicola
Borbone
c,
Ausilia
Elce
abd,
Jussara
Amato
c,
Stefano
D'Errico
c,
Giuseppe
De Rosa
c,
Laura
Mayol
c,
Gennaro
Piccialli
c,
Giorgia
Oliviero
*c and
Giuseppe
Castaldo
ab
aDipartimento di Medicina Molecolare e Biotecnologie Mediche, Via Pansini 5, 80131, Napoli, Italy
bCEINGE-Biotecnologie avanzate, Napoli, Italy
cDipartimento di Farmacia, Università degli Studi di Napoli Federico II, Via D. Montesano 49, 80131, Napoli, Italy. E-mail: golivier@unina.it
dUniversità Telematica Pegaso, Napoli, Italy
First published on 30th October 2013
Abstract
Recently, microRNAs (miRNAs) were identified as being able to inhibit the expression of the Cystic Fibrosis Transmembrane Regulator (CFTR) disease-gene of Cystic Fibrosis (CF) and CFTR-Related Disorders (CFTR-RD). This study shows the use of peptide nucleic acids (PNAs) as inhibitors of miR-509-3p, one of the CFTR regulating miRNAs, by reverting the expression of the luciferase gene containing the 3′UTR of CFTR gene.
Cystic Fibrosis (CF) is the most common lethal genetic disorder among Caucasians with one in every 3000 newborns affected. CF is due to mutations in the CFTR gene encoding the CFTR chloride channel expressed by most epithelial cells.1 The phenotypic expression of CF typically includes an altered sweat test, pancreatic insufficiency and pulmonary infections that gradually lead to respiratory insufficiency. However, less severe forms of CF, called CFTR-RD, are characterized by pancreatic sufficiency, normal or borderline sweat test and single-organ involvement.2 CF displays a wide clinical variability and outcome.3 However, the CFTR genotype (more than 1900 mutations described so far) correlates only with the pancreatic expression of the disease. On the contrary, meconium ileus, diabetes and lung and liver disease show a strong CFTR-independent genetic control. Modifier genes such as SERPINC, mannose binding lectin (MBL)2 and tissue growth factor (TGF)β play a role in modulating the lung and liver involvement in CF patients and disease outcome.4,5 A set of miRNAs inhibit the CFTR expression,6 and our group has shown that mutations in the 3′UTR of the CFTR gene may have a pathogenic effect enhancing the affinity for miR-509-3p.7 Ramachandran et al. confirmed that miR-509-3p inhibits CFTR expression and then may have a role in CF pathogenesis.8 In fact, miRNAs regulate (mainly inhibiting) the expression of specific genes at the post-transcriptional level, by forming miRNA/mRNA complexes.9,10 Although miRNAs are part of the physiological regulation of gene expression, their deregulation can cause diseases. For this reason, many investigators are considering miRNAs as potential therapeutic targets.11
Recently, Yin's group has conducted a study on the effect of peptide nucleic acid (PNA) analogues as inhibitors of miRNA-4661-3p.12 They have demonstrated the possibility of modulating the effect of miRNAs through the use of PNAs. PNAs are DNA mimics in which the native ribose–phosphate skeleton is replaced by 2-aminoethyl-glycine residues.13 These molecules are resistant to enzymatic hydrolysis and recognize with a high affinity complementary fragments of DNA or RNA by Watson–Crick hydrogen bond formation.14 Many studies have been performed on the binding capability of PNAs and on the topological way in which they can recognize nucleic acids in single strand, duplex or quadruplex arrangements to form heteroduplex, heterotriplex complexes15–18 or to act as quadruplex ligands.19,20 The higher affinity of PNAs for RNA, even when the latter is in a duplex conformation, indicates the PNAs as ideal candidates for the targeting of miRNAs.
Very promising results have been obtained by using PNAs conjugated with carrier or cationic peptides.12,21,22 In particular, poly-lysine tails are widely used to overcome the poor water solubility of PNAs and to increase their cellular uptake. Cationic lipids, largely employed as transfecting agents for the DNA fragments,23 have also been used for PNAs hybridized with complementary DNA fragments or for PNAs covalently bound to an oligonucleotide (PNA–DNA chimeras).24 Recent studies report on the feasibility of a miRNA regulation approach by using “wild type” PNAs and PNAs conjugated with peptides or hydrophilic groups.25,26
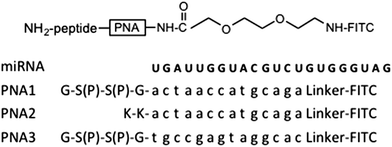
Herein, we propose the use of PNAs capable to antagonize the CFTR-inhibiting miRNAs as a new potential therapeutic approach for CF. In particular, we have synthesized two PNAs complementary to the 5′-end of miR-509-3p for a length of fourteen base pairs and containing two negative charges (PNA1) or positive charges (PNA2) with the aim of increasing their water solubility and facilitating their complexation with transfectant agents. In addition, we have performed preliminary studies on the ability of PNA1 and PNA2 to cross the cell membranes and to interact with the CFTR-regulating miR-509-3p. Thus, PNA1 and PNA2 carrying two serine–phosphate residues or two lysines, respectively, at their C-end and complementary to the whole seed region of miR-509-3p were synthesized. The negatively charged PNA3 with a scrambled base sequence was also synthesized and used as a negative control. The sequences and complete structures of the PNAs are shown in Table 1.
The PNA1–3 were synthesized using a standard Fmoc-solid-phase strategy on the Rink-amide resin. In the “on line” synthetic approach reported here, at first the peptide tract was assembled, followed by the construction of the PNA portion on which the N-terminal fluorescein isothiocyanate (FITC) was attached using an ethoxyethyl (AEEA) linker.
All the PNAs were assembled using 5.0 eq. excess of monomer building blocks in the presence of HATU (3.6 eq.)/DIPEA (5.0 eq.)/lutidine (6.0 eq.) in an NMP/DMF solution. The bis-phosphate PNA1 and PNA3 were obtained using Fmoc-Ser[PO(OBzl)OH]-OH as building blocks. After the assembly of the PNA tract, the terminal amino group was coupled with a 2-[2-(Fmoc-amino)ethoxy]ethoxyacetic acid linker on which, after the removal of the Fmoc group, FITC was attached. The final standard treatment with TFA/anisole/ethanedithiol allowed the detachment from the solid support and the complete deprotection of the synthesized oligomers. After HPLC purification the structures of PNA1–3 were confirmed by mass spectrometry (see ESI†).
The recognition phenomena between miR-509-3p and the PNA1 or PNA2 were studied by electrophoretic mobility shift assay (EMSA), UV and CD spectroscopies.
In the EMSA, PNA1 is able to migrate due to the presence of negative charges in the two phosphate groups present in the molecule (Fig. 1, red arrow). The formation of miR-509-3p/PNA1 heteroduplex is evidenced by the corresponding upwards-shifted band, both for the miRNA/PNA1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (lane 1) and 1
1.5 (lane 1) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (lane 2) ratios. In contrast to PNA1, PNA2 alone is not able to migrate due to the absence of negative charges. In this case, the formation of the miR-509-3p/PNA2 heteroduplex is evidenced by the corresponding downwards-shifted bands, as for PNA1, for both the 1
5 (lane 2) ratios. In contrast to PNA1, PNA2 alone is not able to migrate due to the absence of negative charges. In this case, the formation of the miR-509-3p/PNA2 heteroduplex is evidenced by the corresponding downwards-shifted bands, as for PNA1, for both the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 miRNA/PNA2 ratios (lane 5 and 6, respectively).
5 miRNA/PNA2 ratios (lane 5 and 6, respectively).
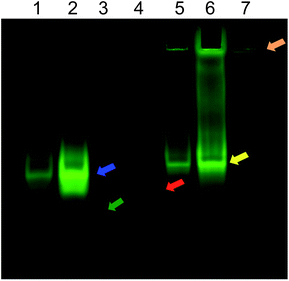 | ||
Fig. 1 EMSA of miR-509-3p alone (lane 3, green arrow; visualized by EtBr staining in Fig. S8 in ESI†) and in the presence of PNA1 (lane 1, miRNA/PNA1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5; lane 2, miRNA/PNA1 1 1.5; lane 2, miRNA/PNA1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; blue arrow) or PNA2 (lane 5, miRNA/PNA2 1 5; blue arrow) or PNA2 (lane 5, miRNA/PNA2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5; lane 6, miRNA/PNA2 1 1.5; lane 6, miRNA/PNA2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; yellow arrow). The red and orange arrows show the mobility of PNA1 alone (lane 4) and PNA2 alone (lane 7), respectively. 5; yellow arrow). The red and orange arrows show the mobility of PNA1 alone (lane 4) and PNA2 alone (lane 7), respectively. | ||
The UV spectra of miRNA/PNA complexes (K+ buffer, pH 7) formed by PNA1 (Fig. S1, ESI†) and PNA2 (Fig. S2, ESI†) displayed a lower value of absorbance than the arithmetic sum of each component alone, thus evidencing that stacking interactions between the miRNA strand and the complexed PNAs occurred. Furthermore, the sigmoidal profiles of the UV melting experiment for both complexes (Fig. S3 and S4, ESI†) were indicative of a duplex/single strand transition. The calculated apparent melting temperatures of the two miRNA/PNA heteroduplexes were 74 and 80 °C for PNA1 and PNA2, respectively. These data suggested that the anionic tail of PNA1 does not reduce significantly the stability of the miR-509-3p/PNA1 heteroduplex.
The CD spectra of miRNA/PNA complexes were recorded in PBS buffer at pH 7 after the annealing procedure. Both complexes showed the typical CD profile of antiparallel RNA/PNA heteroduplexes characterized by maxima at around 260 and 220 nm and minima at around 235 and 196 nm (Fig. S5, ESI†).14
At this point, we have examined the potential of these PNAs as miRNA inhibitors in a biological context. For this purpose we have tested the ability of PNA1 and PNA2 to revert the reduction of luciferase activity induced by transfection of miR-509-3p in A549 cells. To mimic a more physiological context in which miRNAs are already expressed in the cells and to avoid any pre-formation of the miRNA/PNA heteroduplex in the transfection mix reaction, the A549 cells were treated with PNA1 and PNA2 (100 μM) twelve hours after the transfection of the pLuc-CFTR-3′UTR plasmid (a reporter luciferase construct sensitive to the miR-509-3p mimic action for the presence of the 3′UTR of the CFTR gene) and miR-509-3p mimic. As shown in the ESI (Fig. S6,† panel C) the PNA1 and PNA2 were not able to inhibit the activity of miR-509-3p. A plausible explanation for this result could be found in the unfavorable cellular localization of PNAs. Indeed, images of fluorescence microscopy (Fig. S6, panel A and B in ESI†) showed a punctuate fluorescence into the cells that is not compatible with the free diffusion of PNAs into the cytoplasm.
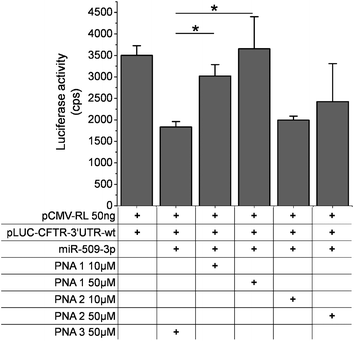
In order to improve the PNAs cellular uptake, we used a lipid transfection reagent (see text and Fig. S7 in ESI†). Thus, we transfected the PNA1–3 in A549 cells. In the presence of a scrambled PNA, the miR-509-3p reduced the luciferase activity by up to 40–50%. The transfection of PNA1 rescued the luciferase activity in a dose-dependent manner (Fig. 2), whereas the transfection of PNA2 appeared to inhibit miR-509-3p, but not in a statistically significant manner. This latter result might be due to the less effective transfection of PNA2 into cells because of the presence of the positive charges. As expected, the PNA3 was not able to rescue the luciferase activity.
Conclusions
In conclusion, spectroscopic and EMSA data confirmed the ability of the negatively charged PNA1 and positively charged PNA2 to bind their complementary miRNA target by forming stable miRNA/PNA heteroduplexes. In addition, the in vitro studies on A549 cell lines indicated that the use of cationic transfection agents greatly enhanced the cellular uptake and the biological activity of the negatively charged PNA1. Furthermore, it has been pointed out that a serine phosphate tail could be a suitable means to improve the PNA cellular uptake. Such data confirm the use of PNAs as a suitable approach to interfere with miRNA inhibition of gene expression pointing to a possible role of our novel PNAs in the modulation of the disease-gene of CF.Notes and references
- I. McIntosh and G. R. Cutting, FASEB J., 1992, 6, 2775 CAS.
- F. Amato, C. Bellia, G. Cardillo, G. Castaldo, M. Ciaccio, A. Elce, F. Lembo and R. Tomaiuolo, J. Mol. Diagn., 2011, 14, 81 CrossRef PubMed.
- G. R. Cutting, Annu. Rev. Genomics Hum. Genet., 2005, 6, 237 CrossRef CAS PubMed.
- F. Salvatore, O. Scudiero and G. Castaldo, Am. J. Med. Genet., 2002, 111, 88 CrossRef PubMed.
- R. Tomaiuolo, D. Degiorgio, D. A. Coviello, A. Baccarelli, A. Elce, V. Raia, V. Motta, M. Seia, G. Castaldo and C. Colombo, Dig. Liver Dis., 2009, 41, 817 CrossRef CAS PubMed.
- A. E. Gillen, N. Gosalia, S. H. Leir and A. Harris, Biochem. J., 2011, 438, 25 CrossRef CAS PubMed.
- F. Amato, M. Seia, S. Giordano, A. Elce, F. Zarrilli, G. Castaldo and R. Tomaiuolo, PLoS One, 2013, 8, e60448 CAS.
- S. Ramachandran, P. H. Karp, S. R. Osterhaus, P. Jiang, C. Wohlford-Lenane, K. A. Lennox, A. M. Jacobi, K. Praekh, S. D. Rose, M. A. Behlke, Y. Xing, M. J. Welsh and P. B. McCray Jr, Am. J. Respir. Cell Mol. Biol., 2013, 49, 544 CrossRef CAS PubMed.
- S. K. Singh, M. Pal Bhadra, H. J. Girschick and U. Bhadra, FEBS J., 2008, 275, 4929 CrossRef CAS PubMed.
- G. Castaldo, F. Lembo and R. Tomaiuolo, Clin. Chem. Lab. Med., 2010, 48, 973 CrossRef CAS PubMed.
- A. G. Bader, D. Brown and M. Winkler, Cancer Res., 2010, 70, 7027 CrossRef CAS PubMed.
- P. N. Brown and H. Yin, Chem. Commun., 2012, 49, 4415 RSC.
- P. E. Nielsen, M. Egholm, R. H. Berg and O. Buchardt, Science, 1991, 254, 1497 CAS.
- M. Pooga, T. Land, T. Bartfai and U. Langel, Biomol. Eng., 2001, 17, 183 CrossRef CAS.
- X. Zhang, T. Ishihara and D. R. Corey, Nucleic Acids Res., 2000, 28, 3332 CrossRef CAS PubMed.
- S. A. Kushon, J. P. Jordan, J. L. Seifert, H. Nielsen, P. E. Nielsen and B. A. Armitage, J. Am. Chem. Soc., 2001, 123, 10805 CrossRef CAS PubMed.
- R. Besch, C. Giovannangeli, T. Schuh, C. Kammerbauer and K. Degitz, J. Mol. Biol., 2004, 341, 979 CrossRef CAS PubMed.
- P. E. Nielsen, Chem. Biodiversity, 2010, 7, 786 CAS.
- J. Amato, G. Oliviero, E. De Pauw and V. Gabelica, Biopolymers, 2009, 91, 244 CrossRef CAS PubMed.
- J. Amato, B. Pagano, N. Borbone, G. Oliviero, V. Gabelica, E. D. Pauw, S. D'Errico, V. Piccialli, M. Varra, C. Giancola, G. Piccialli and L. Mayol, Bioconjugate Chem., 2011, 22, 654 CrossRef CAS PubMed.
- M. M. Fabani, C. Abreu-Goodger, D. Williams, P. A. Lyons, A. G. Torres, K. G. Smith, A. J. Enright, M. J. Gait and E. Vigorito, Nucleic Acids Res., 2010, 38, 4466 CrossRef CAS PubMed.
- A. G. Torres, M. M. Fabani, E. Vigorito, D. Williams, N. Al-Obaidi, F. Wojciechowski, R. H. Hudson, O. Seitz and M. J. Gait, Nucleic Acids Res., 2011, 40, 2152 CrossRef PubMed.
- C. Avitabile, L. Moggio, G. Malgieri, D. Capasso, S. Di Gaetano, M. Saviano, C. Pedone and A. Romanelli, PLoS One, 2012, 7, e35774 CAS.
- M. Egholm, O. Buchardt, L. Christensen, C. Behrens, S. M. Freier, D. A. Driver, R. H. Berg, S. K. Kim, B. Norden and P. E. Nielsen, Nature, 1993, 365, 566 CrossRef CAS PubMed.
- M. Gaglione, G. Milano, A. Chambery, L. Moggio, A. Romanelli and A. Messere, Mol. BioSyst., 2011, 7, 2490 RSC.
- A. Manicardi, E. Fabbri, T. Tedeschi, S. Sforza, N. Bianchi, E. Brognara, R. Gambari, R. Marchelli and R. Corradini, ChemBioChem, 2012, 13, 1327 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of miR-509-3p and PNAs; annealing procedure; UV and CD studies; cell line, constructs and transfections; EMSA. See DOI: 10.1039/c3md00257h |
| This journal is © The Royal Society of Chemistry 2014 |