Synthesis of amphiphilic, chalcogen-based redox modulators with in vitro cytotoxic activity against cancer cells, macrophages and microbes†
Peng
Du
ab,
Uma M.
Viswanathan
ac,
Khairan
Khairan
ah,
Tomislav
Buric
a,
Nathaniel E. B.
Saidu
b,
Zhanjie
Xu
d,
Benjamin
Hanf
e,
Inga
Bazukyan
c,
Armen
Trchounian
c,
Frank
Hannemann
f,
Ingolf
Bernhardt
e,
Torsten
Burkholz
a,
Britta
Diesel
g,
Alexandra K.
Kiemer
g,
Karl-Herbert
Schäfer
h,
Mathias
Montenarh
b,
Gilbert
Kirsch
d and
Claus
Jacob
*a
aDivision of Bioorganic Chemistry, School of Pharmacy, Saarland University, D-66123 Saarbruecken, Germany. E-mail: c.jacob@mx.uni-saarland.de; Fax: +49 681 302 3464; Tel: +49 681 302 3129
bDivision of Medical Biochemistry and Molecular Biology, Saarland University, D-66424 Homburg, Germany
cDivision of Microbiology, Plants and Microbes Biotechnology, Department of Biology, Yerevan State University, 0025 Yerevan, Armenia
dLaboratoire d'Ingénierie Moléculaire et Biochimie Pharmacologique, SRSMC UMR 7565, Université de Lorraine, 1 Boulevard Arago, 57070 Metz, France
eDivision of Biophysics, Department of Biology, Saarland University, D-66123 Saarbruecken, Germany
fDivision of Biochemistry, Saarland University, D-66123 Saarbruecken, Germany
gDepartment of Pharmacy, Saarland University, Pharmaceutical Biology, Saarbruecken, Germany
hDepartment of Biotechnology, University of Applied Sciences Kaiserslautern, Zweibruecken, Germany
First published on 8th October 2013
Abstract
Several amphiphilic, chalcogen-based redox modulators have been synthesized which exhibit a widespread, yet in some instances also selective, biological activity which is most likely based on their ability to modulate the intracellular redox balance and to interact with cellular membranes and specific proteins.
The last decade has witnessed a growing interest in the development of redox modulating agents, which are able to effectively, yet also selectively, attack cells with a disturbed redox balance, such as diverse cancer cells, macrophages and sclerodermic fibroblasts.1–5 A similar strategy has also been employed to kill various microorganisms, which are vulnerable because of a weak antioxidant defence.1,6–8 In many cases, catalytic selenium and tellurium agents have been at the forefront of these developments, since these compounds exploit the efficiency and selectivity of a chemical catalyst for its intracellular substrate(s) to generate pronounced cytotoxic events in specific cells.4–6,9,10 Such compounds are, for instance, able to induce apoptosis in leukemic B-cells while healthy B-cells of the same patient remain largely unaffected.2,11
Unfortunately, many of the most interesting organoselenium and tellurium agents available to date are only poorly soluble in aqueous media and often unstable – and hence possess a comparably poor bioavailability and drug profile, even in cell culture. While attempting to circumvent some of the problems associated with such unfavourable physico-chemical properties, the idea of amphiphilic redox modulators has emerged, as detergent-like properties may, at least in theory, bestow such molecules with certain additional, quite beneficial aspects. Firstly, such amphiphilic compounds should be fairly soluble in aqueous media. Secondly, amphiphilic structures should cross cell membranes and hence enter cells more easily when compared to more hydrophilic or lipophilic agents. Thirdly, amphiphilic agents should be able to interact strongly with membranes and (hydrophobic parts of) proteins. And finally, such agents may cluster together at certain cellular sites (e.g. at membranes, in hydrophobic pockets) and hence may act synergistically.
Surprisingly, only few suitable amphiphilic redox-modulating agents have been reported in the literature so far, and most of these substances have not been studied comprehensively in cell culture.12,13 Recently, some relevant chalcogen compounds have emerged in this context in the literature, confirming that the synthesis of such structures is realistic.13
We have therefore decided to synthesize a series of amphiphilic selenium and tellurium compounds with an anionic head group and hydrocarbon ‘tails’ of different tail lengths in order to explore the various aspects – and possible benefits – of such agents in the context of biological activity, selectivity and mode(s) of action. The compounds ultimately selected and subsequently synthesized as part of this study are shown in Fig. 1. The design of these compounds combines aspects of previously studied selenium and tellurium compounds (e.g. aryl substituents for enhanced stability) with typical features of an amphiphilic agent.13 As for the synthesis of such compounds, once the most promising synthetic avenue was selected (see Fig. 1), the preparation of these molecules has been fairly straightforward (see the ESI†). In many instances, good yields (up to 63%) were possible even without further optimization.
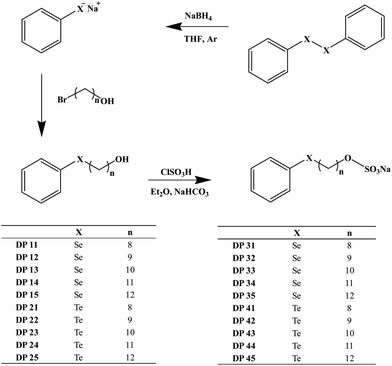 | ||
| Fig. 1 Chemical structures of compounds selected for this study. The most successful synthetic route is provided (see text and ESI for details†). | ||
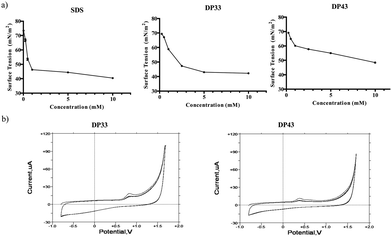
Once synthesized and chemically characterized, the two most interesting physico-chemical properties associated with these compounds from a biological perspective (i.e. the amphiphilic and redox properties) have been confirmed using surface tension measurements and cyclic voltammetry. The results, illustrated exemplarily for DP33 and DP43 in Fig. 2 and summarized in Table 1, confirm that the compounds selected indeed possess amphiphilic properties, with Critical Micelle Concentrations (CMCs) in water in the low millimolar range (Fig. 2a). As expected, the CMC values of these compounds generally decrease with increasing hydrocarbon chain length, and compounds DP35 and DP45, respectively, show the lowest CMC values of the selenium and tellurium compounds studied. Under these conditions, the selenium-containing compound DP35 has a CMC of 0.7 mM and the tellurium-containing compound DP45 of just 0.3 mM, which is comparable to the CMC of sodium dodecylsulfate (SDS) under these conditions (Table 1). The latter is used here as a benchmark anionic surfactant and has a CMC of 0.9 mM under the experimental conditions used. Interestingly, there is no major difference in CMC values between the selenium and tellurium analogues.
| Compound | X | n | Yield (%) | CMC (mM) | E pa (mV) | Lysis RBCs (%) at 100 μM | IC50 (μM) HCT116 | IC50 (μM) RAW 264.7 |
|---|---|---|---|---|---|---|---|---|
| SDS | — | — | — | 0.9 | — | 9.51 ± 1.31 | >100 | >100 |
| 31 | Se | 8 | 64 | 2.6 | 472.3 | 1.24 ± 0.18 | >100 | >100 |
| 32 | Se | 9 | 73 | 2.5 | 783.8 | 2.73 ± 0.63 | >100 | >100 |
| 33 | Se | 10 | 77 | 1.2 | 819.4 | 7.94 ± 1.81 | >100 | >100 |
| 34 | Se | 11 | 67 | 1.1 | 822.2 | 63.41 ± 4.02 | >100 | >100 |
| 35 | Se | 12 | 81 | 0.7 | 826.5 | 66.80 ± 2.55 | >100 | >100 |
| 41 | Te | 8 | 63 | 5.0 | 306.1 | 3.09 ± 0.07 | 5.99 ± 1.16 | 3.95 ± 1.16 |
| 42 | Te | 9 | 80 | 4.8 | 363.1 | 20.44 ± 0.90 | 4.99 ± 1.46 | 4.67 ± 1.01 |
| 43 | Te | 10 | 77 | 0.9 | 374.5 | 45.35 ± 1.35 | 7.47 ± 0.74 | 5.32 ± 1.20 |
| 44 | Te | 11 | 81 | 0.5 | 385.9 | 53.35 ± 3.35 | 9.73 ± 0.34 | 7.84 ± 1.22 |
| 45 | Te | 12 | 82 | 0.3 | 385.9 | 67.61 ± 2.04 | 19.84 ± 1.26 | 4.96 ± 1.41 |
At the same time, these compounds are redox active and exhibit an electrochemical behaviour more or less typical of mono-selenides and tellurides (Fig. 2b). Here, the oxidation potentials Epa are typically in the range of +472 to +827 mV vs. the standard silver/silver chloride electrode (SSE) for the selenides and +306 to +386 mV vs. SSE for the tellurides. In contrast to the CMC values, the Epa values therefore do differ significantly between the selenium and tellurium analogues, in line with previous reports pointing towards generally lower Epa values for tellurium compounds compared to their selenium analogues. Interestingly, the Epa values within the respective selenium and tellurium series do seem to increase with increasing hydrocarbon ‘tail’ length; yet these increases are in the range of a couple of tens of millivolts only and hence minor in comparison.
While it is unlikely that such amphiphilic agents form micelles under physiological conditions – the concentrations generally employed in cell culture are too low for micellation and the complexity of the biological ‘buffer’ also interferes with micelle formation – they should still be able to interact rather strongly with – hydrophobic parts of – biomolecules surrounding the cell or being present therein. Within this context, and in analogy to the action of SDS, interactions with cellular membranes and with proteins are of particular interest.14 We have therefore briefly explored the potential interactions of some of our compounds with the membrane of red blood cells (RBCs) and/or whole RBCs with a representative protein, i.e. haemoglobin (Hb) (see also the ESI†).
RBCs were chosen to study compound–membrane interactions as they represent intact cells rather than just liposomes. RBCs therefore enable the study of direct effects of compounds on the cell membrane, yet do not possess the kind of signalling usually associated with dividing cells.14 We know, for instance, that redox modulators can trigger secondary, indirect responses in dividing cells, hence complicating investigations (see also below).9,15,16Fig. 3 shows the pronounced effect of the selenium and tellurium containing agents on the integrity of the RBCs. This effect is concentration dependent (see Fig. S1 in the ESI†). Rather modest concentrations of compounds such as DP35, DP44 and DP45 cause significant lysis of the cell membrane, as determined by the haemoglobin release assay. Compound DP45 is the most ‘active’ in this assay, with 67.6% lysis at 100 μM of the compound tested. This compound has also the lowest CMC value (around 300 μM), indicating that the activity on the RBC cell membrane is apparently dominated by the amphiphilic character of the compounds and is less dependent on the nature of the chalcogen involved. Indeed, there is a dramatic increase in lysis with increasing hydrocarbon chain length in the selenium as well as tellurium series, from a couple of percents in the case of short tails (DP31, DP41) to almost 70% in the case of the longest tails (DP35, DP45). There is only a slight increase in lysis when switching from selenium to tellurium (66.8% lysis in the case of DP35 and 67.6% in the case of DP45). Therefore the impact of compounds such as DP34, DP35, DP44 and DP45 on the cell membrane is driven primarily by the amphiphilic character of these agents, and is possibly slightly enhanced by the presence of tellurium. Although speculative at this time, one may envisage that the primary interaction of these compounds is indeed with the phospholipid bilayer directly, while a smaller, secondary, chalcogen-driven effect is perhaps due to yet to be specified interactions with – probably cysteine containing – membrane proteins. A similar behaviour apparently dominated by amphiphilicity rather than redox activity has also been found in the case of nematodes, whose cuticula or epidermis may be the target of such compounds (see below).
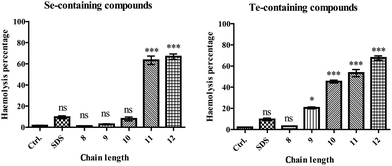 | ||
| Fig. 3 Interactions of amphiphilic compounds with possible biochemical target molecules. Membrane interactions were recorded with subsequent lysis of RBCs (at a compound concentration of 100 μM). The chain lengths refer to n, the number of carbon atoms in the hydrophobic tail, as defined in Fig. 1. Lysis of RBCs increases with increasing tail length from n = 8 (DP31 and DP41) to n = 12 (DP35 and DP45). Data are presented as mean ± SD, and one-way ANOVA test (Dunnett) is used: p < 0.05 (*), p < 0.01 (**) or p < 0.001 (***). | ||
In the next step, we have turned our attention to possible (non-covalent) interactions with proteins. Here, Hb has been used as the initial choice as this protein occurs commonly in RBCs, exhibits distinct signals in CD and, as a metalloprotein, also accounts for possible chalcogen–metal interactions (the iron–sulfur cluster containing protein Adx has been used as a further control). Indeed, the studies employing circular dichroism (CD) in order to determine possible interactions of the amphiphilic agents with Hb indicate rather pronounced effects of these compounds on the secondary structure of such proteins. At 50 μM concentrations, most of the compounds studied showed some impact on the Hb structure, which was comparable to that of SDS at the same concentration. The underlying interaction(s) were not particularly specific and seemed to be dominated by the amphiphilic character of the molecules (see Fig. S2 in the ESI†). SDS and DP31 had pronounced effects on the structure of Hb, while the impact of DP41 was less apparent. Interactions of such compounds with the protein structure could also be observed in the case of the reference protein Adx, where DP41 was slightly more potent than DP31 (see the ESI†).
Encouraged by these results, which clearly support the notion of possible multiple interactions of such compounds with various types of biomolecules, we have turned our attention towards potential biological targets. In the first part of the evaluation of biological activity, two different epithelial human cell lines have been chosen: human colon cancer cells (HCT116 cells, ATCC® CCL-247™) represent a well established cancer cell model, while human retinal pigment epithelia cells (ARPE-19, ATCC® CRL-2302™) provide a suitable and often acceptable control as they exhibit many features of non-cancerous, primary cells, yet do not result in the various practical and ethical issues associated with using primary human colon cells obtained, for instance, from biopsies. ARPE-19 cells are particularly suited as control cells when the activity of redox modulating agents is investigated, as such epithelial cells are fairly resilient towards oxidative stress.15 Hence screening compounds against HCT116 and ARPE-19 cells in tandem often allows a first but certainly preliminary glance at cytotoxicity against human cells and also a comparison of a cancer cell line with ‘normal’ cells.2,15–17
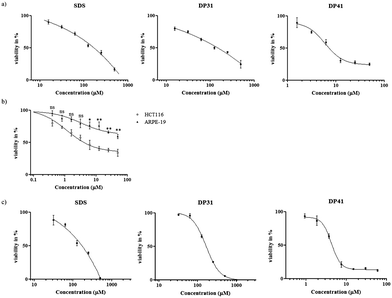
Fig. 4 shows the results obtained for a set of amphiphilic compounds against HCT116 and ARPE-19 cells (for IC50 values see Table 1). While the selenium-containing compounds and SDS were more or less inactive against HCT116 cells at the concentrations used, all of the tellurium containing compounds showed considerable cytotoxic activity against these cancer cells with IC50 values in the range of 5 to 20 μM. Interestingly, a small but consistent and statistically significant, ultimately around 3–4-fold, increase in IC50 values was observed when the hydrocarbon tail length was increased, from 6.0 μM in the case of DP41 to 19.8 μM in the case of DP45. In line with the notable absence of any observable cytotoxicity associated with the selenium compounds (DP31 to DP35) up to a concentration of 100 μM, this trend in IC50 values obviously counts against (purely) amphiphilic (inter-)actions of such compounds.
Here, one must bear in mind, of course, that the IC50 values (below or around 20 μM) are considerably lower than the concentrations employed for lysis of RBCs (100 μM), which in turn are lower than the CMCs (300 μM in the case of DP45). It is therefore likely that most of the most dramatic amphiphilic effects of such compounds only come to bear at concentrations which are 10-fold or even higher than the ones required to kill a human cell. In the case of the most cytotoxic compound DP42, the IC50 value in HCT116 cells is around 1000 times lower than the CMC of this compound.
In fact, while longer chain lengths may promote interactions with cell membranes (as seen in the case of RBCs), they may also prevent compounds from ultimately crossing such membranes, i.e. from leaving the membrane again and entering the cytosol. In the case of one of the most active compounds, DP41, we have therefore used Energy-dispersive X-ray spectroscopy (EDX) to confirm the presence of tellurium inside (washed) HCT116 cells (see Fig. S3 in the ESI†). While it is not possible to confirm the exact location and chemical state of tellurium inside these cells using this method, EDX nonetheless confirms that the compound has crossed the cell membrane and indeed is ultimately present inside the cell.
Amazingly, the IC50 values of some of these tellurium agents were consistently lower in HCT116 cells when compared to the IC50 values of the same compounds in ARPE-19 cells (the latter were usually around 50 μM), pointing towards a high and, in this case, also selective activity in the HCT116 cell line. While it is premature to speculate why such tellurium compounds may be particularly active in HCT116 cells – and not to the same extent in ARPE-19 cells, and why the selenium analogues are not active in HCT116 cells, these findings are in excellent agreement with previous results obtained for organotellurium compounds in such cell lines.10 They also confirm once more the significantly higher cytotoxicity associated with tellurium compounds when compared to their selenium analogues.1,3,4,10,17 As for the possible underlying biochemical causes of such high activity and selectivity (for tellurium as well as for HCT116 cells), a specific interaction of tellurium compounds with cellular targets, such as individual components of the intracellular thiolstat, or burst of reactive oxygen species (ROS) is most likely.1,2,4,5,10,15,16,18–22 Indeed, there are numerous reports that selenium as an antioxidant seems to be unable to induce a ROS generating chemistry, which may also explain the rather low activity of the selenium compounds in our assays.1,3,23,24
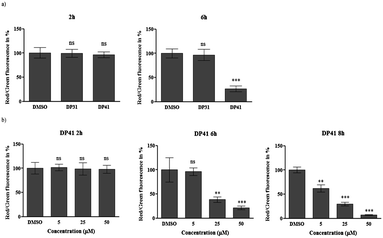
We have therefore investigated this possible ‘redox link’ between a tellurium compound and apoptosis in HCT116 cells in more detail.15 In the first instance, fluorescent staining of intracellular superoxide anion radicals (O2˙−) was performed using dihydroethidium (DHE) as a fairly specific probe for this particular radical.15 As expected, initial results confirm a sharp increase in intracellular O2˙− levels in response to compounds such as DP41. At a concentration of 50 μM and within 40 min of application, this tellurium compound causes a significant increase of O2˙− levels (in our preliminary experiments, the O2˙− concentration measured by this method almost doubles). Similar increases in intracellular ROS levels have already been observed for quinone-containing organotellurium agents, and ultimately may lead to cell death via apoptosis.5,10,15 Indeed, our subsequent investigation of the effects of DP41 on the mitochondrial membrane potential ΔΨM using JC-1 as a dual colour red/green fluorescent reporter dye revealed a significant, time- and concentration-dependent decrease of ΔΨM in response to this tellurium compound (see Fig. 5 for a graphic evaluation of fluorescent data). Such a loss of mitochondrial membrane potential may form an important part of apoptotic processes resulting in cell death. It should be emphasized, however, that any – causal – relationship(s) between O2˙− levels on the one side and ΔΨM, on the other, are not immediately obvious and require further investigation. Considering the timing of these events, i.e. less than 1 h for the increase in O2˙− levels and evidently more than 2 h for the decrease in ΔΨM, it appears that the increase in ROS occurs prior to mitochondrial damage. This in turn points towards a redox event as the initial cause of activity and counts against a decisive amphiphilic interaction of compounds such as DP41 with the mitochondrial membrane. Indeed, the selenium-analogue of DP41, i.e. compound DP31, did not cause any significant increases in O2˙− levels and also did not cause a major decrease in ΔΨM, hence supporting the notion of a tellurium-specific activity which is linked to the generation of intracellular ROS. DP41 differs from previously used quinone-containing organotellurium compounds, and the build-up of O2˙− occurs in the absence of a radical-generating quinone moiety. It may not be caused directly by the redox chemistry of DP41, but by a more indirect, secondary event, possibly mediated by cellular processes, such as ER stress.
While the initial results obtained in human RBCs, HCT116 and ARPE-19 cells are rather instructive, they may be due to specific interactions which occur – perhaps solely – in those cell types. We have therefore also considered the impact of such selenium and tellurium compounds on cultured RAW 264.7 macrophages (ATCC® TIB-71™). Macrophages were selected as an additional cell system because these immune cells naturally produce high levels of ROS and hence should provide a suitable target for compound-induced redox modulation.1 The results obtained in RAW 264.7 cells are summarized in Table 1 and shown for DP31 and DP41 in Fig. 4c. These results confirm the considerable cytotoxicity of DP41 (IC50 = 3.95 μM). Indeed, it seems that the macrophages are even slightly more sensitive to DP41 when compared to the HCT116 cells (IC50 = 5.99 μM), which may be due to the high ROS levels present naturally in and near such immune cells. A similar activity of tellurium-based redox modulators on macrophages has been observed previously.1 While the precise cause of such activity is speculative at this time, it should be noted that the selenium analogue of DP41, i.e. DP31, as well as SDS, are both considerably less active against RAW 264.7 cells with IC50 values of over 100 μM. These findings support further the notion of a tellurium-based, redox modulating activity of compounds such as DP41, which is notably absent in the case of the corresponding selenium compounds or SDS. The specific activity of DP41, together with the low concentrations of this compound required, once more count against an amphiphilic interaction as the main cause of (cytotoxic) activity in proliferating human cell lines.
The results obtained in isolated or cultured human cells may only provide a partial picture of the various biological activities and biochemical mode(s) of action associated with these compounds. It is known, for instance, that many chalcogen-based compounds also exhibit a pronounced activity against various bacteria and some redox sensitive parasites. Therefore initial screens for antibiotic activity against Staphylococcus aureus and Escherichia coli were performed, which point towards some activity of the tellurium compounds when used at higher micromolar concentrations, which exceed the activity of the selenium analogues and SDS. DP41, which was among the most cytotoxic compounds in the case of cultured human cells, was also the most active compound against E. coli with an IC50 of around 550 μM. Nonetheless, antibacterial activity was weak when compared to the cytotoxicity against human cells.
When DP31 and DP41 were tested in the context of the agricultural nematode Steinernema feltiae, a rather different picture emerged. S. feltiae is a parasitic nematode often used in initial toxicity screens, as this organism is easy to cultivate and also fairly reliable in producing representative and reproducible results.25 The nematicidal activities associated with DP31, DP41 and SDS are shown in Fig. S4 in the ESI.† SDS is only weakly active against S. feltiae (LD50 > 400 μM), counting against a particular sensitivity of this organism towards surfactants. Perhaps surprisingly, therefore, DP41 and especially DP31 are quite active against this organism. Interestingly, the selenium compound DP31 (LD50 = 30 μM) is even more active when compared to its tellurium analogue, DP41 (LD50 = 78 μM). The exact causes for this pronounced activity of DP31 – compared to DP41 – are still unclear. One may speculate that the selenium compound is taken up and metabolized to toxic metabolites more readily, as most organisms possess pathways for the metabolism of selenium compounds, but not for tellurium compounds. Indeed, the results obtained for our compounds in the four human cell models, the bacteria and nematodes confirm that such agents are not merely globally toxic but possess some selectivity which may result from their ability to enter cells and to act on particular cellular targets, be it on specific proteins or on a local or global redox state.
Ultimately, our studies have shown that it is possible to synthesize a range of selenium- and tellurium-containing surfactants with comparable ease. As expected, these molecules combine redox activity with amphiphilic properties and hence exhibit several advantages when compared to traditional redox modulators or surfactants. The compounds reported here are easy to handle, fairly soluble in aqueous solutions and endowed with considerable biological activity against a range of important therapeutic targets, such as certain cancer cells, macrophages, bacteria and a representative nematode. In fact, the activity determined so far compares well with that of so-called ‘multifunctional’ redox modulators which combine a selenium or tellurium redox moiety with a quinone.1,26 While the latter require the presence of a radical generating quinone for adequate activity in the high nanomolar to low micromolar range, the compounds discussed here exhibit a similar, slightly lower activity yet do not require the presence of a cytotoxic quinone moiety. At the same time, the tellurium compounds, which are often more active than their selenium counterparts, show activity not only against HCT116 cancer cells, but also against RAW 264.7 macrophages, S. feltiae and E. coli.
This raises the question, why such compounds are active, and possibly even show some selectivity. Our initial experiments conducted to explore the underlying biochemical mode(s) of action point towards a combination of two activities. On the one hand, there is clearly an amphiphilic, probably non-covalent and disruptive interaction of these agents with membranes and proteins; on the other hand, the presence of tellurium (and less so selenium) seems to directly or indirectly enable these compounds to affect the cellular redox balance (as seen for O2˙− levels) and maybe also cause a covalent modification of key proteins of the cellular thiolstat. While the amphiphilic events seem to occur only at higher concentrations (50 to 100 μM and above) and do not discriminate significantly between the presence of selenium and tellurium, the redox interactions seem to be more or less specific for tellurium and occur at lower concentrations (10 to 50 μM in cultured human cells).
Extensive future studies are obviously required to investigate further the exact underlying biochemical mode(s) of action and to identify possible intracellular targets (such as specific organelles, membranes or proteins). At this point, the intracellular pathways triggered or influenced by such compounds also need to be mapped out in more detail. Ultimately, it will also be necessary to produce a wider range of such compounds, including some sulfur-containing analogues, and to screen for further activities and selectivity, also in order to derive reliable structure–activity relationships. Our initial results point towards a particularly promising spectrum of activities associated with the tellurium compounds, especially compound DP41, which may be considered as a lead compound emerging from these studies. As the structure of this compound provides considerable scope for modifications, and the synthesis of derivatives is now straightforward, a wider spectrum of additional compounds based on this initial lead appears possible. Here, it will be interesting to see if the presence of a quinone helper group will further enhance activity and/or selectivity, as has already been observed for a previous generation of such redox modulating compounds.1,2,4,5,9,26
In the future, such compounds will be evaluated extensively for possible anticancer and antimicrobial activity. Cells and organisms which naturally produce high amounts of ROS (such as certain cancer or immune cells), or exhibit a weak antioxidant defence (such as certain parasites, including nematodes and Plasmodium falciparum), will obviously form the prime targets of such redox modulating agents.6 In any case, our findings bode well for the further development of such amphiphilic redox modulators as lead structures for the treatment of a range of human diseases and for possible agricultural applications.
Acknowledgements
The authors acknowledge financial support from Saarland University, the Landesforschungsfoerderungsprogramm Saarland (T/1-14.2.1.1.-LFFP 12/23) and the BMBF (grant number 01DK12002). The authors would like to thank Dr Josef Zapp from Saarland University and Ms Veronique Poddig from the University Lorraine for NMR measurements.Notes and references
- M. Doering, B. Diesel, M. C. H. Gruhlke, U. M. Viswanathan, D. Manikova, M. Chovanec, T. Burkholz, A. J. Slusarenko, A. K. Kiemer and C. Jacob, Tetrahedron, 2012, 68, 10577–10585 CrossRef CAS PubMed.
- N. Lilienthal, C. Prinz, A. A. Peer-Zada, M. Doering, L. A. Ba, M. Hallek, C. Jacob and M. Herling, Leuk. Lymphoma, 2011, 52, 1407–1411 CrossRef CAS PubMed.
- V. Jamier, L. A. Ba and C. Jacob, Chem.–Eur. J., 2010, 16, 10920–10928 CrossRef CAS PubMed.
- W. K. Marut, N. Kavian, A. Servettaz, C. Nicco, L. A. Ba, M. Doering, C. Chereau, C. Jacob, B. Weill and F. Batteux, J. Invest. Dermatol., 2012, 132, 1125–1132 CrossRef CAS PubMed.
- M. Doering, L. A. Ba, N. Lilienthal, C. Nicco, C. Scherer, M. Abbas, A. A. P. Zada, R. Coriat, T. Burkholz, L. Wessjohann, M. Diederich, F. Batteux, M. Herling and C. Jacob, J. Med. Chem., 2010, 53, 6954–6963 CrossRef CAS PubMed.
- S. Mecklenburg, S. Shaaban, L. A. Ba, T. Burkholz, T. Schneider, B. Diesel, A. K. Kiemer, A. Roseler, K. Becker, J. Reichrath, A. Stark, W. Tilgen, M. Abbas, L. A. Wessjohann, F. Sasse and C. Jacob, Org. Biomol. Chem., 2009, 7, 4753–4762 CAS.
- C. Jacob, Biochem. Soc. Trans., 2011, 39, 1247–1253 CrossRef CAS PubMed.
- T. Schneider, A. Baldauf, L. A. Ba, V. Jamier, K. Khairan, M. B. Sarakbi, N. Reum, M. Schneider, A. Roseler, K. Becker, T. Burkholz, P. G. Winyard, M. Kelkel, M. Diederich and C. Jacob, J. Biomed. Nanotechnol., 2011, 7, 395–405 CrossRef CAS PubMed.
- S. Shaaban, R. Diestel, B. Hinkelmann, Y. Muthukumar, R. P. Verma, F. Sasse and C. Jacob, Eur. J. Med. Chem., 2012, 58, 192–205 CrossRef CAS PubMed.
- T. Schneider, Y. Muthukumar, B. Hinkelmann, R. Franke, M. Doring, C. Jacob and F. Sasse, MedChemComm, 2012, 3, 784–787 RSC.
- N. Lilienthal, A. A. Peer-Zada, L. A. Ba, H. Liu, C. Jacob, M. Hallek and M. Herling, Onkologie, 2010, 33, 240–240 Search PubMed.
- L. B. Xing, S. Yu, X. J. Wang, G. X. Wang, B. Chen, L. P. Zhang, C. H. Tung and L. Z. Wu, Chem. Commun., 2012, 48, 10886–10888 RSC.
- P. Han, N. Ma, H. Ren, H. Xu, Z. Li, Z. Wang and X. Zhang, Langmuir, 2010, 26, 14414–14418 CrossRef CAS PubMed.
- T. Schneider, L. A. Ba, K. Khairan, C. Zwergel, N. D. Bach, I. Bernhardt, W. Brandt, L. Wessjohann, M. Diederich and C. Jacob, MedChemComm, 2011, 2, 196–200 RSC.
- N. E. B. Saidu, R. Touma, I. Abu Asali, C. Jacob and M. Montenarh, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830, 2214–2225 CrossRef CAS PubMed.
- C. Busch, C. Jacob, A. Anwar, T. Burkholz, L. A. Ba, C. Cerella, M. Diederich, W. Brandt, L. Wessjohann and M. Montenarh, Int. J. Oncol., 2010, 36, 743–749 CAS.
- L. A. Ba, M. Doring, V. Jamier and C. Jacob, Org. Biomol. Chem., 2011, 8, 4203–4216 Search PubMed.
- K. K. Bhasin, E. Arora, A. S. Grover, Jyoti, H. Singh, S. K. Mehta, A. K. K. Bhasin and C. Jacob, J. Organomet. Chem., 2013, 732, 137–141 CrossRef CAS PubMed.
- C. Jacob, E. Battaglia, T. Burkholz, D. Peng, D. Bagrel and M. Montenarh, Chem. Res. Toxicol., 2012, 25, 588–604 CrossRef CAS PubMed.
- C. Scherer, C. Jacob, M. Dicato and M. Diederich, Phytochem. Rev., 2009, 8, 349–368 CrossRef CAS.
- F. H. Fry and C. Jacob, Curr. Pharm. Des., 2006, 12, 4479–4499 CrossRef CAS.
- N. M. Giles, N. J. Gutowski, G. I. Giles and C. Jacob, FEBS Lett., 2003, 535, 179–182 CrossRef CAS.
- C. A. Collins, F. H. Fry, A. L. Holme, A. Yiakouvaki, A. Al-Qenaei, C. Pourzand and C. Jacob, Org. Biomol. Chem., 2005, 3, 1541–1546 CAS.
- G. I. Giles, F. H. Fry, K. M. Tasker, A. L. Holme, C. Peers, K. N. Green, L. O. Klotz, H. Sies and C. Jacob, Org. Biomol. Chem., 2003, 1, 4317–4322 CAS.
- B. Czepukojc, U. M. Viswanathan, A. Raza, S. Ali, T. Burkholz and C. Jacob, Phosphorus, Sulfur Silicon Relat. Elem., 2013, 188, 446–453 CrossRef CAS.
- F. H. Fry, A. L. Holme, N. M. Giles, G. I. Giles, C. Collins, K. Holt, S. Pariagh, T. Gelbrich, M. B. Hursthouse, N. J. Gutowski and C. Jacob, Org. Biomol. Chem., 2005, 3, 2579–2587 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3md00204g |
| This journal is © The Royal Society of Chemistry 2014 |